Abstract
Polygalacturonases (PGs), an important industrial enzyme group classified under depolymerases, catalyze the hydrolytic cleavage of the polygalacturonic acid chain through the introduction of water across the oxygen bridge. In order to produce and increase the concentration of this enzyme group in fermentation processes, a new approach called microparticle cultivation, a promising and remarkable method, has been used. The aim of this study was to increase the PG activity of Aspergillus sojae using aluminum oxide (Al2O3) as microparticles in shake flask fermentation medium. Results indicated that the highest PG activity of 34.55 ± 0.5 U/ml was achieved with the addition of 20 g/L of Al2O3 while the lowest activity of 15.20 ± 0.2 U/mL was obtained in the presence of 0.1 g/L of Al2O3. In fermentation without microparticles as control, the activity was 15.64 ± 3.3 U/mL. Results showed that the maximum PG activity was 2.2-fold higher than control. Additionally, smaller pellets formed with the addition of Al2O3 where the lowest pellet diameter was 955.1 µm when 10 g/L of the microparticle was used. Also, it was noticed that biomass concentration gradually increased with increasing microparticle concentration in the fermentation media. Consequently, the PG activity was significantly increased in microparticle-enhanced shake flask fermentation. In fact, these promising preliminary data can be of significance to improve the enzyme activity in large-scale bioreactors.
Keywords: Aluminum oxide, Aspergillus sojae, Morphology engineering, Polygalacturonase, Shake flask fermentation
Introduction
Pectinases or pectinolytic enzymes are an enzyme group which are the most used industrial enzymes to degrade pectin and other pectic substances in the cell walls of plants. Recently, pectinases have gained attention due to their numerous industrial applications in fruits and vegetable processing, wine processing, saccharification of agricultural substrates, extraction of vegetable oil, processing of textile material, tea and coffee processing, processing of animal feed, biobleaching of kraft pulp, and recycling of wastepaper (AlMatar and Makky 2016; Garg et al. 2016). Polygalacturonase (PG) is an important subgroup of pectinases, which are responsible for hydrolysis of α-1,4-glycosidic bonds in polygalacturonic acid in a sequential fashion and are classified under depolymerases (Demir and Tari 2016; Tounsi et al. 2016).
The very common sources for production of industrial enzymes are microbial systems. 50% of industrial enzymes originate from fungi and yeast, 35% of enzymes are of bacterial origin, and the rest of enzymes are either of plant or animal origin (Garg et al. 2016). Among these, fungi, especially the genus Aspergillus, which is used in food fermentation industry for production of fermented foods such as koji, are the most commonly used ones for large-scale production of industrial enzymes. Various Aspergillus species such as A. niger (Finkler et al. 2017), A. flavipes (Wolf-Márquez et al. 2017), A. fumigatus (Anand et al. 2016), A. awamori (Botella et al. 2007), A. oryzae (Meneghel et al. 2014), A. terreus (Sethi et al. 2016), A. sojae (Fratebianchi et al. 2017), etc., have been used for the production of pectinases. In the literature, the production of pectinases are widely performed by Aspergillus niger, which is the main producer of commercial pectinases and is considered as generally recognized as safe (GRAS) allowing the utilization of its metabolites in the food industry (Fratebianchi et al. 2017; Sathya et al. 1998). Additionally, the metabolites obtained from A. sojae and A. oryzae are also considered to be of GRAS status (Jørgensen 2007). In fact these fungi can be considered as potential producers of pectinases for applications in food industry (Fratebianchi et al. 2017) as well.
Submerged fermentation (SmF) is generally a very convenient technique for the production of high cost industrial products and to understand the biochemical and physiological aspects of the microbial metabolite formations, particularly the enzyme synthesis. SmF has also been utilized more commonly because of its advantages such as easy-to-use compared to solid-state fermentation (SSF) (Kumar et al. 2011; Liu et al. 2016a, b). It was reported that the total upstream processing cost of PG production in SmF process is 14% lower than the SSF process per year (Nakkeeran et al. 2012).
In SmF, control of fungal growth has always been an important parameter for bioprocess productivity (Wang et al. 2005). Therefore, apart from methods such as utilization of different bioreactor types and fermentation modes, adjustment of fermentation parameters such as agitation, pH, aeration, temperature, medium composition, inoculum size, etc., and metabolic engineering, some novel approaches have been attempted to regulate the morphology of filamentous microorganisms (Walisko et al. 2015). Microparticle-enhanced cultivation (MPEC) is regarded as a novel and promising approach to overcome some problems such as excessive, and irregular shaped growth and bulk formation, insufficient oxygen transfer and substrate diffusion, insufficient agitation and aeration, and unrepeatable results (Antecka et al. 2016a; Germec et al. 2017; Yatmaz et al. 2016). In addition, the reduction of pellet size up to single hyphal growth during fermentation is a significant issue for product formation. Therefore, microparticles can ensure more precise control of fungal morphology in SmF by eliminating bulk fungal growth (Germec et al. 2017; Yatmaz et al. 2016). For this purpose, recently, various microparticles such as Al2O3 (Antecka et al. 2016b; Germec et al. 2017; Yatmaz et al. 2016), magnesium silicate (talcum, Mg3O·Si4O2·H2O) (Coban and Demirci 2016; Coban et al. 2015a, b; Germec et al. 2017; Yatmaz et al. 2016), iron oxide (FeO·Fe2O3) (Etschmann et al. 2015), and titanium silicate oxide (TiSiO4) (Driouch et al. 2012; Germec et al. 2017) were used.
There are many studies on PG production in the literature using solid-state and submerged fermentation with intra or extracellular microorganisms. A. sojae 20235 is a wild type and extracellular PG producer microorganism. It can be used for SmF after fermentation conditions and medium composition were optimized by using different statistical methods (Heerd et al. 2014). The first study using wild type of A. sojae 20235 resulted in 13.5 U/mL PG activity in SmF fermentation without microparticle (Tari et al. 2007). Then, the maximum PG activity was enhanced to 20.1 U/mL using the same strain and medium (Tokatli et al. 2009). Accordingly, the main objective of this study was not only to improve the fungal morphology of A. sojae by the addition of Al2O3 into the fermentation media but also to increase the PG activity in microparticle-enhanced shake flask fermentation.
Materials and methods
Microorganism and inoculum preparation
Aspergillus sojae ATCC 20235 was supplied by the laboratory of Prof. Dr. Canan TARI, at Izmir Institute of Technology, Izmir, Turkey. Cultures were grown on Petri plates containing yeast malt extract agar (10 g/L malt extract, 4 g/L yeast extract, 4 g/L glucose, and 20 g/L agar) at 30 °C for 7 days. In order to maintain viability, the fungus-grown plates were maintained at 4 °C and sub-cultured monthly. Stock spore cultures were prepared using 20% glycerol and stored at −80 °C (Tokatli et al. 2009). Molasses agar slants were utilized to obtain the spore suspensions for inoculation. For sub-culturing and inoculum preparation, spores were harvested from the slants with 10 mL of 0.02% Tween 80-water solution (Gogus et al. 2006). The resulting suspension solution had about 2.7 × 107 spore per mL.
Microparticle and preparation of microparticle
To test enzyme production using fungal microorganisms, microparticles have been added into the fermentation media prior to inoculation (Kaup et al. 2008). For this purpose, Al2O3 microparticle (≥ 64% Al2O3 powder, Sigma-Aldrich, Seelze, Germany) was used in this study. Before adding into the medium, the microparticles were prepared in 100 mM Na-acetate buffer (pH 4.8). Microparticle concentrations applied were (g/L): 0.05, 0.1, 0.5, 2.5, 5.0, 10, 15, 20, and 25. After autoclaving at 121 °C for 20 min, they were aseptically and separately added into the medium prior to inoculation (Kaup et al. 2008).
Shake flask fermentation
Shake flasks fermentations were carried out in triplicate with 50 mL fermentation media in 250 mL Erlenmeyer using a shaker incubator (CERTOMAT® IS, Goettingen, Germany). The fermentation media consisted of 25 g of glucose, 2.5 g of peptone, 3.2 g of disodium phosphate, and 3.3 g of monosodium phosphate per liter of deionized water (Tari et al. 2007). For the fermentation, 5 mL of microparticle suspension and 45 mL of fermentation media were individually autoclaved and mixed aseptically after cooling to room temperature. Afterwards, the fermentation media (pH 6.4) containing microparticles were inoculated with 10% of prepared-spore suspension. Fermentations were performed at 30 °C and 200 rpm for 96 h. 0.5 mL of samples were collected from each flask daily and analyzed for PG activity, sugar concentration, and pellet size. The fermentation broth was harvested and centrifuged at 7200×g for 15 min, where the supernatant was assessed for the enzyme activity.
PG activity
The PG activity was assayed according to Panda et al. (1999) with slight modifications. 2.4 g/L of polygalacturonic acid was used as substrate and prepared in sodium-acetate buffer (0.1 M, pH 4.8, 26 °C). The enzyme-to-substrate ratio was 1/4 (v/v). The first incubation was performed at 40 °C for 20 min. After adding 1 mL of copper agent as a stopper, the resulting solution was incubated in a boiling water bath for 10 min to stop activity and to accelerate the reaction for the color change and then was swiftly cooled to room temperature. Absorbance values were measured at 500 nm using a spectrophotometer (Thermo Scientific Evolution, Evolution 201, Waltham, MA, USA) and converted to PG activity considering Eq. 1.
| 1 |
where 0.0117 is the slope of curve, 212.15 is the molecular weight of galacturonic acid (µg/mole), 20 is the incubation period (min), 0,1 is the sample amount, and 1000 is the dilution rate.
The same mixture to which the same amount of the inactivated crude enzyme (heated in a boiling water same as other test tubes) was added before the reaction was utilized as the control. Deionized water was used as blank. One unit of enzyme activity (U) was defined as the amount of enzyme titer that catalyzes the release of 1 μmol of galacturonic acid per unit volume of culture filtrate per unit time at standard assay conditions. Galacturonic acid (Sigma, St Louis, MO, USA) was used as standard for calibration curve of PG activity.
Residual sugar
To determine the residual glucose concentration in fermentation broth, 3,5-dinitrosalycylic acid method was used (Miller 1959). Absorbance values measured at 575 nm were converted to residual glucose concentration using a glucose standard curve. Deionized water was used instead of samples in blanks.
Biomass
The final biomass concentration on dry basis was determined by gravimetric method. The fermentation broth was filtered via pre-weight filter paper (Whatman No: 1), removed solubles and followed by drying to constant weight at 60 °C for 24 h.
Image analysis
Fungal morphology and pellet diameters were analyzed by a stereo-microscope (Stemi 2000-C, Zeiss, Germany) with an AxioCamERc5 camera (Stemi 2000-C, Zeiss, Göttingen, Germany). Before analysis, the biomass samples were washed with deionized water to remove fermentation broth and residual microparticles from the pellets. After the biomass samples were analyzed and the average size of pellets was determined by measuring their diameters. The image analyses were conducted with a software (the ImageJ 1.50b, National Institutes of Health, USA). Firstly, the pellets of the sample picture were contrasted to be black, and then the program was used to calculate the size of each pellet (Driouch et al. 2012; Yatmaz et al. 2016).
Statistical analysis
All fermentations and analyses were performed in triplicate. The data were evaluated using SAS statistical software (SAS Institute INC., Carry, NC, USA). Duncan’s multiple comparison test was used at a significance level, p < 0.05.
Results and discussion
The main objective of this study was to examine the effect of Al2O3 microparticles on PG production of A. sojae in shake flask fermentation. The PG activity was monitored for 4 days in all fermentation experiments with and without Al2O3 microparticles.
Effect of Al2O3 on PG enzyme
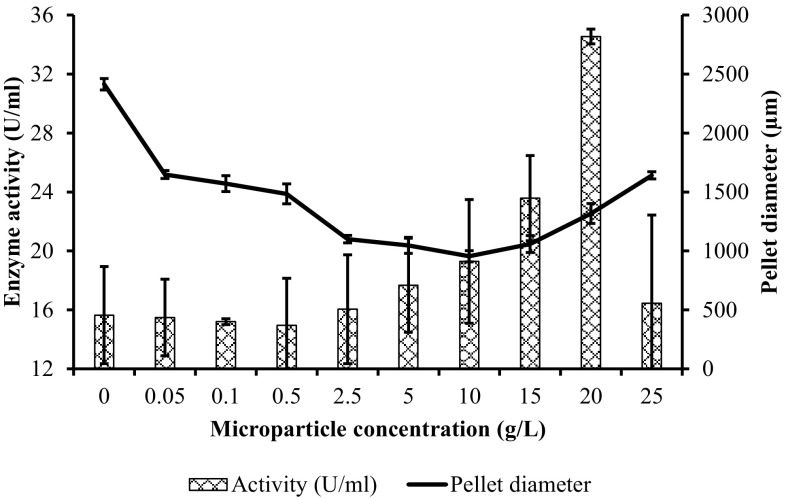
In this study, 9 different concentrations of Al2O3 mentioned above were experimented along with a control study (no-added microparticles) in triplicate. According to the results, when fermentation media were not supplemented with any microparticle, PG activity was determined as 15.64 ± 3.3 U/mL (Fig. 1). In experiments containing 0.05 and 0.1 g/L of Al2O3, the enzyme activity was found to be lower compared to the control study (Fig. 1). The lowest enzyme activity (14.95 ± 3.2 U/mL) was detected in fermentations with 0.5 g/L microparticles. On the other hand, when 2.5, 5, 10, 15, 20, and 25 g/L concentrations of Al2O3 were used, enzyme activities were increased by 1.03, 1.13, 1.23, 1.54, 2.21, and 1.05-fold compared to the control study, respectively. The highest PG activity was observed when 20 g/L of Al2O3 was utilized in the fermentation media, which yielded 34.55 ± 0.5 U/mL enzyme activity as shown in Fig. 1. Result showed that the increase of the microparticle concentration has not been always increased the enzyme activity. But, appropriate amount of microparticle addition into the medium improved the enzyme activity. The maximum PG activity (20.1 U/mL) determined in a study conducted by Tokatli et al. (2009), who investigated the modeling of A. sojae 20,235, was more than 40% lower than the current results (34.55 ± 0.5 U/mL). Similarly, compared to another study using the same strain, our results were 2.56 times higher than their maximum activity (13.5 U/mL) (Tari et al. 2007).
Fig. 1.
Relationship between enzyme activity and pellet diameter with the increasing microparticle concentration
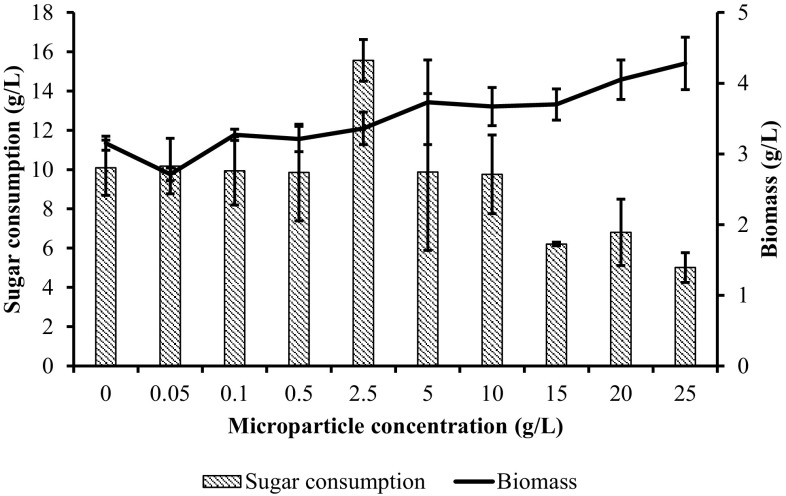
Enzyme versus sugar consumption
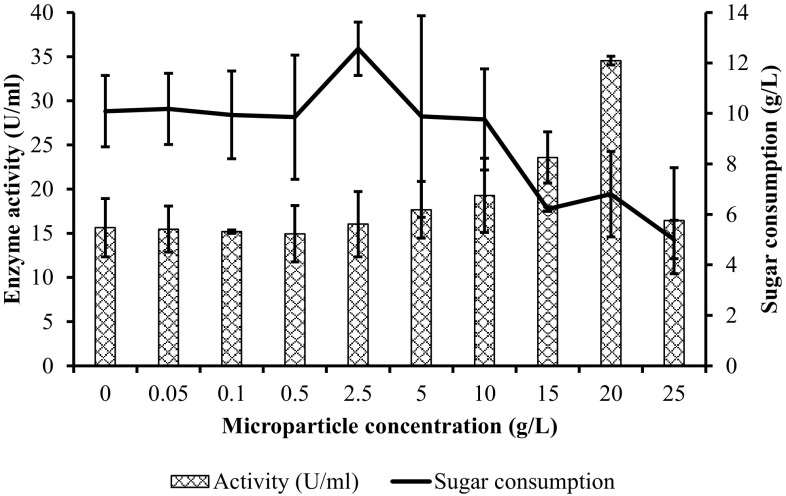
The relationship between enzyme activity and sugar consumption (difference between initial and final sugar concentration) with increasing microparticle concentration is shown in Fig. 2. Results indicated that no correlation was observed between PG activity and sugar consumption. It is well known that microorganisms can be used carbon sources for growing themselves and produced some by-products instead of target product. However, although the highest sugar consumption was observed when microparticle concentration was 2.5 g/L (p < 0.05), the PG activity stayed at 16.04 ± 3.7 U/mL, which was slightly higher compared to control fermentation without microparticles (p > 0.05). The lowest sugar consumption was detected with the addition of 25 g Al2O3/L microparticle and the enzyme activity was relatively high compared to the fermentation without microparticles (p > 0.05) (Fig. 2). Besides, the sugar consumption was relatively same with the addition of 0.05, 0.1, and 0.5 g/L Al2O3 microparticles as well as enzyme production in comparison with control fermentation (p > 0.05) (Fig. 2). Additionally, maximum PG activity was obtained with the consumption of 6.8 g/L glucose in microparticle-enhanced shake flask fermentation (p < 0.05) (Fig. 2 and Table 1). Accordingly, the results demonstrated that high sugar consumption did not mean more PG production and it has no effect on PG activity.
Fig. 2.
Relationship between enzyme activity and sugar consumption with the increasing microparticle concentration
Table 1.
Morphology type for different microparticle concentrations
| Microparticle concentration (g/L) | Pellet diameter (µm) | Fungal morphology |
|---|---|---|
| 0.00 | 2414a ± 48 | Big pellets + filamentous |
| 0.05 | 1648b ± 33 | Big pellets |
| 0.1 | 1571b ± 67 | Big pellets + pellets |
| 0.5 | 1484bc ± 84 | Pellets |
| 2.5 | 1100d ± 31 | Pellets + small pellets |
| 5 | 1047d ± 69 | Pellets + small pellets |
| 10 | 955d ± 47 | Small pellets |
| 15 | 1058d ± 72 | Small pellets |
| 20 | 1317c ± 85 | Small pellets + filamentous |
| 25 | 1641b ± 30 | Big pellets |
Effect of diameter on enzyme activity
Average diameters of pellets ranged from 2414 µm to 955 µm, which decreased about 2.53-fold (p < 0.05) through microparticle additions (Table 1). While the highest pellet diameter (2414 µm) was observed in control, the lowest pellet diameter (955 µm) was obtained when 10 g/L of Al2O3 was used (Fig. 1 and Table 1). It was clearly seen that pellet size reduced in the range of 0.05–10 g/L of Al2O3 compared to control when more microparticles were added. However, it increased above the concentrations of 10 g/L. The average pellet sizes of cells taken from fermentations conducted with 15, 20 and 25 g/L of microparticles were determined as 1058, 1317 and 1641 µm. On the other hand, the highest PG activity was obtained using 20 g/L of Al2O3 microparticles (Table 1). These results indicated that the microparticle concentration was a critical parameter to promote the PG activity. Many researchers reported that the most efficient Al2O3 concentration was 15 g/L for various metabolites and strains (Antecka et al. 2016b; Coban and Demirci 2016; Coban et al. 2015b; Kaup et al. 2008). Kaup et al. (2008) investigated the influence of microparticle-enhanced cultivation on chloroperoxidase production by Calsariomyces fumago with the addition of Al2O3 and reported that the activity was increased about 5-fold by addition of 15 g Al2O3/L compared to control fermentation. Furthermore, the pellet diameter was reduced up to 40-fold by application of microparticles. Coban et al. (2015b) and Coban and Demirci (2016) improved A. ficuum phytase production and Rhizopus oryzae lactic acid production by 2.0- and 2.3-fold, respectively, with the addition of 15 g/L Al2O3 compared to the control fermentations. In A. ficuum phytase production, the average fungal pellet radius decreased from 800 to 500 µm by the addition of 15 g/L Al2O3 (Coban et al. 2015b). Similarly, while the laccase production of Pleurotus sapidus was increased 2.0-fold by the addition of 15 g/L Al2O3, it was enhanced 3.5-fold by Cerrena unicolor with the same amount of Al2O3. The pellet radius of Cerrena unicolor decreased from 2 to 1 mm or decreased from 6 to 4 mm with the addition of 15 g/L Al2O3 (Antecka et al. 2016b). In another study, the pellet radius of recombinant Aspergillus sojae was reduced from 1747.8 to 774.1 µm in glucose medium and reduced from 1428.2 to 530.9 µm in carob extract medium with the addition of 1 and 15 g/L Al2O3 microparticles, respectively (Yatmaz et al. 2016).
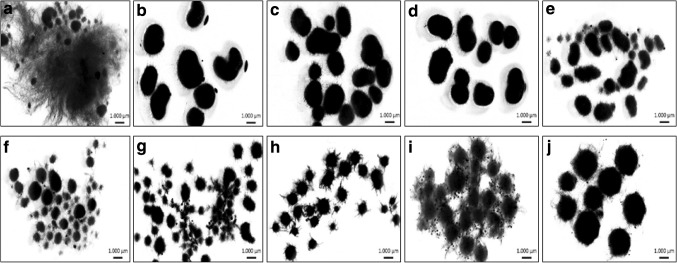
Moreover, pellet diameter variation could be clearly observed from Figs. 1 and 3. The growth profiles of A. sojae in all fermentations were monitored, which changed from big pellets + hyphae to small pellet (Fig. 3). The growth profile of control fermentation was observed as big pellets + hyphae (Fig. 3a). When 0.05 g/L of microparticles was added, the fungal growth was observed in the form of big pellets (Fig. 3b). Nonetheless, the fungal growth was in the form of pellet + small pellets when the microparticle concentrations in shake flask fermentations were 2.5 and 5 g/L (Fig. 3e, f, respectively). Additionally, the growth type of A. sojae was observed in the form of small pellets when the microparticle concentrations were 10 and 15 g/L in microparticle-enhanced cultivations. Indeed, the lowest pellet diameter was measured as 9557 µm when 10 g/L was used as microparticle concentration in MPEC (Table 1). Interestingly, the pellet diameters increased when the microparticle concentrations were 20 and 25 g/L (Fig. 1 and Table 1) and, thus, the fungal growth was also observed in the form of small pellets + hyphae and big pellets, respectively (Figs. 1 and 3i, k). A. ficuum was obtained in the form of smaller pellets with the most effective Al2O3 concentration (15 g/L) in a similar MPEC study (Coban and Demirci 2016). Antecka et al. (2016b) utilized 15 g/L of Al2O3 in SmF and monitored P. sapidus pellets as core–shell-shaped when image analysis was done. In the same study, Cerrena unicolor was employed along with the same amount of Al2O3 and reported that pellets formed as star-shaped, intriguingly (Antecka et al. 2016b). In our previous study, although there were big pellets in the control broth, the fungal morphology of recombinant A. sojae in glucose and carob extract mediums was in the form of smaller pellets than the control when 1 g/L of Al2O3 microparticles was used and the highest mannanase activities were obtained (Yatmaz et al. 2016). To control cell growth and morphology in medium, other methods apart from MPEC have been also used in literature. According to a study, which was purposed to modifying the fungal morphology of Trichoderma reesei Rut C-30, the effect of inoculum size on growth morphology as a classical approach was investigated. They used to be inoculum of 105 and 107 spores/mL for cellulase cultivation and revealed that pulpy growth was obtained with 105 spores/mL while pellet forms occurred using 107 spores/mL. Hereby, they were reported that the average pellet size seems to be inversely proportional to the inoculum size (Domingues et al. 2000). Hama et al. (2015) studied to control the growth of recombinant Aspergillus oryzae via the immobilize cell culture attached biomass support particles. While suspension cells showed a morphology ranging from dispersed free mycelia forms to mycelial clumps, immobilized cells formed a dense biofilm has a thickness of nearly 0.4 mm except for the center region of the matrix. Accordingly, the usage of more microparticles in the fermentation media does not always mean that smaller pellets will be formed. Determine of optimal microparticle concentrations are very important. In general, the more microparticle concentrations resulted in decreasing pellet size. But, if the concentration is higher than the optimal concentration (the highest enzyme produced concentration) for the pellet size diameter, the concentration of microparticles might be shown reverse effect. The mechanism cannot be definitely defined. But, it can be understood from our previous studies, in the use of more microparticles than in the optimum concentration, that the microparticles serve as a bridge between the cells and cause the formation of large particles (Yatmaz et al. 2016).
Fig. 3.
Effect of aluminum oxide on morphology of A. sojae (a Control (0 g/L), b 0.05 g/L, c 0.1 g/L, d 0.5 g/L, e 2.5 g/L, f 5 g/L, g 10 g/L, h 15 g/L, i 20 g/L, k 25 g/L)
Enzyme versus biomass
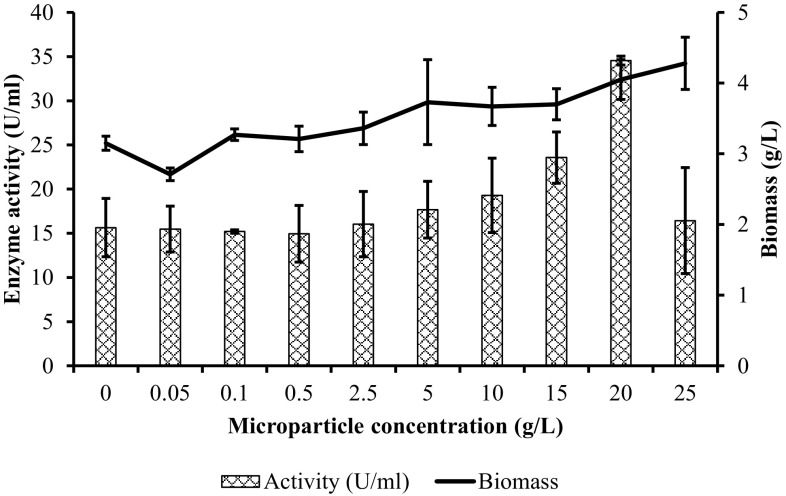
Figure 4 presents the relationship between PG activity and biomass concentration with the increasing microparticle concentrations. Results demonstrated that except using 0.05, 0.1, 0.5, and 25 g/L Al2O3 microparticles, in general, the enzyme activity increased with the increasing biomass concentration. The lowest biomass concentration was measured as 2.71 g/L when the Al2O3 concentration was 0.05 g/L; however, the enzyme activity was nearly the same with control fermentation (p > 0.05). The maximum PG activity was observed when biomass concentration reached 4.05 g/L with the addition of 20 g Al2O3/L (p < 0.05). Additionally, the highest biomass concentration was obtained when 25 g/L of Al2O3 microparticles was used, but the enzyme activity decreased considerably (p < 0.05). Consequently, considering all biomass data, it was seen that addition of Al2O3 had an enhancer effect on PG activity. In a study in which the aim was to increase the chloroperoxidase activity of Caldariomyces fumago, 1.5-fold higher biomass formation was obtained in the presence of 10–15 g/L Al2O3 microparticles when compared to the control (Kaup et al. 2008). Similarly, Gonciarz and Bizukojc (2014) reported that the maximum biomass concentration was reached when talc powder in the range of 10–15 g/L was added to the media. Yang et al. (2016) also investigated the control of a marine-derived fungus Curvularia sp.IFB-Z10 under submerged fermentation using Al2O3 microparticles. It was seen that dry cell weight was increased from 5.14 g/L (control) to 6.79 g/L when 2 g/L Al2O3 microparticles were added. Moreover, the authors found that biomass formation was promoted from 5.48 g/L (control, 120 h) to 7.24 g/L at 144 h in a 5-L stirred bioreactor experiment performed with the addition of 5 g/L talc powder. However, Niu et al. (2015) indicated that dry cell weight was not changed remarkably in their study which enhanced the production of echinocandin using talc powder. They applied addition of different talc powder concentrations within the fermentation media, and reported that the highest biomass concentration was reached by the addition of 30 g/L talcum microparticles. Similarly, enhancement of fructofuranosidase production from Aspergillus niger SKAn1015 in SmF was achieved using microparticles in a variety of concentrations and reported that the biomass formation decreased when microparticle quantity was increased (Driouch et al. 2011). In another study, titanate (TiSiO4) was exploited within the range 0–50 g/L and reported that the lowest biomass was observed in fermentations without microparticle (Driouch et al. 2012).
Fig. 4.
Relationship between enzyme activity and biomass concentration with the increasing microparticle concentration
Sugar consumption versus biomass (growth)
The relationship between sugar consumption and biomass concentration with increasing microparticle concentration was shown in Fig. 5, but no correlation was observed. Nonetheless, results illustrated that although the highest biomass concentration in shake flask fermentation with 25 g/L Al2O3 microparticles was detected (p < 0.05), the sugar consumption was minimum (p > 0.05). On the other hand, although 12.56 g/L of sugar in shake flask fermentation with the addition of 2.5 g/L Al2O3 was consumed by A. sojae (p < 0.05), the biomass growth remained at 3.36 g/L (p > 0.05) (Table 1). In addition, the sugar consumption and biomass growth (except for fermentation with 0.05 g/L microparticles) were relatively constant with the increasing microparticle concentration up to 0.5 g/L. Additionally, while the sugar consumption tends to decrease with the increasing microparticle concentration (from 2.5 to 25 g/L), the biomass concentration indicated a reverse relationship (Fig. 5 and Table 1). Because, increasing microparticle concentration in medium means more wall materials for microorganisms to attach them. This caused highest biomass formation in medium. Indeed, the highest PG activity was measured when the biomass concentration and sugar consumption were 4.05 and 6.80 g/L, respectively (Table 1).
Fig. 5.
Relationship between sugar consumption and biomass concentration with increasing microparticle concentration
The main effect of microparticle on morphology was attributed to probably the hindrance of new spore–spore interactions as similarly suggested for hyphal inocula and involves collision-induced aggregate disruption by Kaup et al. (2008) and Driouch et al. (2010). Also, microparticles in the medium force the microorganisms to grow a single cell instead of mycelium. These suspended microparticles in submerged fermentation prevent hyphae from interacting directly with each other and prevent aggregating. This is defined as a physical phenomenon due to the collision of microparticles. However, the mechanism of this physical effect has not yet been fully explained (Kaup et al. 2008). All the previous studies about microparticle applications reported that MPEC was an effective method to control the microbial cell growth in fermentations. Furthermore, the optimum concentration of microparticle enhanced the production of PG. Consequently, microparticles can restrict the excessive growth of fungi and promote the efficiency of any target product by increasing the surface area of the pellets. Also, more repeatable results by addition of microparticles can be obtained due to more homogenous fermentation media, a better agitation and aeration, no excessive cell growth, and further nutrient transfer because of the increase in the surface area of the microorganism.
Conclusion
In this paper, the potential of microparticle usage in SmF was investigated in order to prevent the excessive growth of A. sojae and to provide enhancement of exo-PG activity. It was found that PG activity was increased from 15.20 ± 0.2 to 34.55 ± 0.5 U/mL by the addition of 20 g/L Al2O3 microparticles, with an increase of 2.27-fold. The pellet diameter was significantly reduced by the addition of Al2O3 microparticles in the range of 0.05–25 g/L. It was clearly observed that A. sojae grew in the form of smaller pellets compared to SmF without microparticles. Consequently, the PG activity significantly increased by supplementing a fair amount of Al2O3 microparticles into the fermentation medium and the clumpy fungal growth of A. sojae was considerably prevented.
Acknowledgements
This study was supported by the Scientific and Technological Research Council of Turkey (TUBITAK) Foundation Grant (#115 O 873). Authors also thank to the Akdeniz University Research Foundation.
Compliance with ethical standards
Conflict of interest
All the authors in this study mutually agree for submitting our manuscript to 3 Biotech and declare that they have no conflict of interest in the publication.
References
- AlMatar M, Makky EA. Cladosporium cladosporioides from the perspectives of medical and biotechnological approaches. 3 Biotech. 2016;6(1):4. doi: 10.1007/s13205-015-0323-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand G, Yadav S, Yadav D. Purification and characterization of polygalacturonase from Aspergillus fumigatus MTCC 2584 and elucidating its application in retting of Crotalaria juncea fiber. 3 Biotech. 2016;6(2):201. doi: 10.1007/s13205-016-0517-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antecka A, Bizukojc M, Ledakowicz S. Modern morphological engineering techniques for improving productivity of filamentous fungi in submerged cultures. World J Microbiol Biotechnol. 2016;32(12):193. doi: 10.1007/s11274-016-2148-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antecka A, Blatkiewicz M, Bizukojć M, Ledakowicz S. Morphology engineering of basidiomycetes for improved laccase biosynthesis. Biotech Lett. 2016;38(4):667–672. doi: 10.1007/s10529-015-2019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botella C, Diaz A, De Ory I, Webb C, Blandino A. Xylanase and pectinase production by Aspergillus awamori on grape pomace in solid state fermentation. Process Biochem. 2007;42(1):98–101. doi: 10.1016/j.procbio.2006.06.025. [DOI] [Google Scholar]
- Coban HB, Demirci A. Enhancement and modeling of microparticle-added Rhizopus oryzae lactic acid production. Bioprocess Biosyst Eng. 2016;39(2):323–330. doi: 10.1007/s00449-015-1518-0. [DOI] [PubMed] [Google Scholar]
- Coban HB, Demirci A, Turhan I. Enhanced Aspergillus ficuum phytase production in fed-batch and continuous fermentations in the presence of talcum microparticles. Bioprocess Biosyst Eng. 2015;38(8):1431–1436. doi: 10.1007/s00449-015-1384-9. [DOI] [PubMed] [Google Scholar]
- Coban HB, Demirci A, Turhan I. Microparticle-enhanced Aspergillus ficuum phytase production and evaluation of fungal morphology in submerged fermentation. Bioprocess Biosyst Eng. 2015;38(6):1075–1080. doi: 10.1007/s00449-014-1349-4. [DOI] [PubMed] [Google Scholar]
- Demir H, Tari C. Effect of physicochemical parameters on the polygalacturonase of an Aspergillus sojae mutant using wheat bran, an agro-industrial waste, via solid-state fermentation. J Sci Food Agri. 2016;96(10):3575–3582. doi: 10.1002/jsfa.7543. [DOI] [PubMed] [Google Scholar]
- Domingues FC, Queiroz JA, Cabral JMS, Fonseca LP. The influence of culture conditions on mycelial structure and cellulase production by Trichoderma reesei Rut C-30. Enzyme Microbial Technol. 2000;26(5):394–401. doi: 10.1016/S0141-0229(99)00166-0. [DOI] [PubMed] [Google Scholar]
- Driouch H, Sommer B, Wittmann C. Morphology engineering of Aspergillus niger for improved enzyme production. Biotechnol Bioeng. 2010;105(6):1058–1068. doi: 10.1002/bit.22614. [DOI] [PubMed] [Google Scholar]
- Driouch H, Roth A, Dersch P, Wittmann C. Filamentous fungi in good shape: microparticles for tailor-made fungal morphology and enhanced enzyme production. Bioeng Bugs. 2011;2(2):100–104. doi: 10.4161/bbug.2.2.13757. [DOI] [PubMed] [Google Scholar]
- Driouch H, Hänsch R, Wucherpfennig T, Krull R, Wittmann C. Improved enzyme production by bio-pellets of Aspergillus niger: targeted morphology engineering using titanate microparticles. Biotechnol Bioeng. 2012;109(2):462–471. doi: 10.1002/bit.23313. [DOI] [PubMed] [Google Scholar]
- Etschmann M, Huth I, Walisko R, Schuster J, Krull R, Holtmann D, Wittmann C, Schrader J. Improving 2-phenylethanol and 6-pentyl-α-pyrone production with fungi by microparticle-enhanced cultivation (MPEC) Yeast. 2015;32(1):145–157. doi: 10.1002/yea.3022. [DOI] [PubMed] [Google Scholar]
- Finkler ATJ, Biz A, Pitol LO, Medina BS, Luithardt H, de Lima Luz LF, Krieger N, Mitchell D. Intermittent agitation contributes to uniformity across the bed during pectinase production by Aspergillus niger grown in solid-state fermentation in a pilot-scale packed-bed bioreactor. Biochem Eng J. 2017;121:1–12. doi: 10.1016/j.bej.2017.01.011. [DOI] [Google Scholar]
- Fratebianchi D, Crespo JM, Tari C, Cavalitto S. Control of agitation rate and aeration for enhanced polygalacturonase production in submerged fermentation by Aspergillus sojae using agro-industrial wastes. J Chem Technol Biotechnol. 2017;92(2):305–310. doi: 10.1002/jctb.5006. [DOI] [Google Scholar]
- Garg G, Singh A, Kaur A, Singh R, Kaur J, Mahajan R. Microbial pectinases: an ecofriendly tool of nature for industries. 3 Biotech. 2016;6(1):1–13. doi: 10.1007/s13205-016-0371-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germec M, Yatmaz E, Karahalil E, Turhan İ. Effect of different fermentation strategies on β-mannanase production in fed-batch bioreactor system. 3 Biotech. 2017;7(1):77. doi: 10.1007/s13205-017-0694-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogus N, Tari C, Oncü S, Unluturk S, Tokatli F. Relationship between morphology, rheology and polygalacturonase production by Aspergillus sojae ATCC 20235 in submerged cultures. Biochem Eng J. 2006;32(3):171–178. doi: 10.1016/j.bej.2006.09.023. [DOI] [Google Scholar]
- Gonciarz J, Bizukojc M. Adding talc microparticles to Aspergillus terreus ATCC 20542 preculture decreases fungal pellet size and improves lovastatin production. Eng Life Sci. 2014;14(2):190–200. doi: 10.1002/elsc.201300055. [DOI] [Google Scholar]
- Hama S, Onodera K, Yoshida A, Noda H, Kondo A. Improved production of phospholipase A 1 by recombinant Aspergillus oryzae through immobilization to control the fungal morphology under nutrient-limited conditions. Biochem Eng J. 2015;96:1–6. doi: 10.1016/j.bej.2014.12.013. [DOI] [Google Scholar]
- Heerd D, Diercks-Horn S, Fernandez-Lahore M. Efficient polygalacturonase production from agricultural and agro-industrial residues by solid-state culture of Aspergillus sojae under optimized conditions. Springer Plus. 2014;3:742. doi: 10.1186/2193-1801-3-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen TR. Identification and toxigenic potential of the industrially important fungi, Aspergillus oryzae and Aspergillus sojae. J Food Prot. 2007;70(12):2916–2934. doi: 10.4315/0362-028X-70.12.2916. [DOI] [PubMed] [Google Scholar]
- Kaup BA, Ehrich K, Pescheck M, Schrader J. Microparticle-enhanced cultivation of filamentous microorganisms: increased chloroperoxidase formation by Caldariomyces fumago as an example. Biotechnol Bioeng. 2008;99(3):491–498. doi: 10.1002/bit.21713. [DOI] [PubMed] [Google Scholar]
- Kumar S, Sharma H, Sarkar B. Effect of substrate and fermentation conditions on pectinase and cellulase production by Aspergillus niger NCIM 548 in submerged (SmF) and solid state fermentation (SSF) Food Sci Biotechnol. 2011;20(5):1289–1298. doi: 10.1007/s10068-011-0178-3. [DOI] [Google Scholar]
- Liu CT, Erh MH, Lin SP, Lo KY, Chen KI, Cheng KC. Enrichment of two isoflavone aglycones in black soymilk by Rhizopus oligosporus NTU 5 in a plastic composite support bioreactor. J Sci Food Agri. 2016;96(11):3779–3786. doi: 10.1002/jsfa.7569. [DOI] [PubMed] [Google Scholar]
- Liu J-M, Yu T-C, Lin S-P, Hsu R-J, Hsu K-D, Cheng K-C. Evaluation of kojic acid production in a repeated-batch PCS biofilm reactor. J Biotechnol. 2016;218:41–48. doi: 10.1016/j.jbiotec.2015.11.023. [DOI] [PubMed] [Google Scholar]
- Meneghel L, Reis GP, Reginatto C, Malvessi E, da Silveira MM. Assessment of pectinase production by Aspergillus oryzae in growth-limiting liquid medium under limited and non-limited oxygen supply. Process Biochem. 2014;49(11):1800–1807. doi: 10.1016/j.procbio.2014.07.021. [DOI] [Google Scholar]
- Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31(3):426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- Nakkeeran E, Gowthaman MK, Umesh-Kumar S, Subramanian R. Techno-economic analysis of processes for Aspergillus carbonarius polygalacturonase production. J Biosci Bioeng. 2012;113(5):634–640. doi: 10.1016/j.jbiosc.2011.12.021. [DOI] [PubMed] [Google Scholar]
- Niu K, Hu Y, Mao J, Zou S, Zheng Y. Effect of microparticles on echinocandin B production by Aspergillus nidulans. Sheng Wu Gong Cheng Xue Bao. 2015;31(7):1082–1088. [PubMed] [Google Scholar]
- Panda T, Naidu G, Sinha J. Multiresponse analysis of microbiological parameters affecting the production of pectolytic enzymes by Aspergillus niger: a statistical view. Process Biochem. 1999;35(1):187–195. doi: 10.1016/S0032-9592(99)00050-3. [DOI] [Google Scholar]
- Sathya G, Naidu N, Panda T. Application of response surface methodology to evaluate some aspects on stability of pectolytic enzymes from Aspergillus niger. Biochem Eng J. 1998;2(1):71–77. doi: 10.1016/S1369-703X(98)00019-9. [DOI] [Google Scholar]
- Sethi BK, Nanda PK, Sahoo S. Enhanced production of pectinase by Aspergillus terreus NCFT 4269.10 using banana peels as substrate. 3 Biotech. 2016;6(1):1–15. doi: 10.1007/s13205-015-0353-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tari C, Gögus N, Tokatli F. Optimization of biomass, pellet size and polygalacturonase production by Aspergillus sojae ATCC 20235 using response surface methodology. Enzyme Microbial Technol. 2007;40(5):1108–1116. doi: 10.1016/j.enzmictec.2006.08.016. [DOI] [Google Scholar]
- Tokatli F, Tari C, Unluturk SM, Baysal NG. Modeling of polygalacturonase enzyme activity and biomass production by Aspergillus sojae ATCC 20235. J Ind Microbiol Biotechnol. 2009;36(9):1139–1148. doi: 10.1007/s10295-009-0595-y. [DOI] [PubMed] [Google Scholar]
- Tounsi H, Sassi AH, Romdhane ZB, Lajnef M, Dupuy J-W, Lapaillerie D, Lomenech A-M, Bonneu M, Gargouri A, Hadj-Taieb N. Catalytic properties of a highly thermoactive polygalacturonase from the mesophilic fungus Penicillium occitanis and use in juice clarification. J Mol Catal B Enzym. 2016;127:56–66. doi: 10.1016/j.molcatb.2016.02.012. [DOI] [Google Scholar]
- Walisko R, Moench-Tegeder J, Blotenberg J, Wucherpfennig T, Krull R. The taming of the shrew-controlling the morphology of filamentous eukaryotic and prokaryotic microorganisms. In: Krull R, Bley T, editors. Filaments in Bioprocesses. New York: Springer; 2015. pp. 1–27. [DOI] [PubMed] [Google Scholar]
- Wang L, Ridgway D, Gu T, Moo-Young M. Bioprocessing strategies to improve heterologous protein production in filamentous fungal fermentations. Biotechnol Adv. 2005;23(2):115–129. doi: 10.1016/j.biotechadv.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Wolf-Márquez VE, Martínez-Trujillo MA, Osorio GA, Patiño F, Álvarez MS, Rodríguez A, Sanromán MÁ, Deive FJ. Scaling-up and ionic liquid-based extraction of pectinases from Aspergillus flavipes cultures. Biores Technol. 2017;225:326–335. doi: 10.1016/j.biortech.2016.11.067. [DOI] [PubMed] [Google Scholar]
- Yang J, Jiao R-H, Yao L-Y, Han W-B, Lu Y-H, Tan R-X. Control of fungal morphology for improved production of a novel antimicrobial alkaloid by marine-derived fungus Curvularia sp. IFB-Z10 under submerged fermentation. Process Biochem. 2016;51(2):185–194. doi: 10.1016/j.procbio.2015.11.025. [DOI] [Google Scholar]
- Yatmaz E, Karahalil E, Germec M, Ilgin M, Turhan İ. Controlling filamentous fungi morphology with microparticles to enhanced β-mannanase production. Bioprocess Biosyst Eng. 2016;39(9):1391–1399. doi: 10.1007/s00449-016-1615-8. [DOI] [PubMed] [Google Scholar]