Abstract
Hexane, dichloromethane and methanol extracts of the roots of Piper sarmentosum Roxb. were screened for toxicity towards Sitophilus oryzae (L.), Rhyzopertha dominica (F.), and Plodia interpunctella (Hübner) and the hexane extract exhibited the highest mortality percentage. Bioassay-guided fractionation of the hexane extract resulted in the isolation of asaricin 1, isoasarone 2, and trans-asarone 3. Asaricin 1 and isoasarone 2 were the most toxic compounds to Sitophilus oryzae, Rhyzopertha dominica, and Plodia interpunctella. Sitophilus oryzae and Rhyzopertha dominica exposed to asaricin 1 and isoasarone 2 required the lowest median lethal time. Insecticidal activity of trans-asarone 3 showed consistent toxicity throughout the 60 days towards all three insects as compared to asaricin 1 and isoasarone 2. Asaricin 1 and isoasarone 2 at different doses significantly reduced oviposition and adult emergence of the three insects in treated rice. Trans-asarone 3 had lowest toxicity with highest LC and LT values in all tested insects relative to its mild oviposition inhibition and progeny activity. Moreover, asaricin 1 and isoasarone 2 significantly inhibited acetylcholinesterase in comparison with trans-asarone 3 and the control. Acetylcholinesterase inhibition of Rhyzopertha dominica and Plodia interpunctella by asaricin 1 and isoasarone 2 were lower than that of Sitophilus oryzae, which correlated with their higher resistance.
Introduction
Storage pests have a direct effect on the reduction of the quantity and quality of grain during postharvest storage. The damages brought upon stored grains and their related grain products by insects could be as high as 20–30% in tropical countries such as Malaysia1–3. Sitophilus oryzae (L.), Rhyzopertha dominica (F.), and Plodia interpunctella (Hübner) are considered as main pests of stored food4. These insects can infest a wide range of stored grains such as wheat and corn. The adults of S. oryzae and R. dominica are known to attack and consume the intact grains while their larvae feed on the kernel and develop inside of it5,6. Plodia interpunctella, also known as the Indian moth larva, during its adult stage does not cause much damage as compared to its larval stage7. P. interpunctella is an external feeder. The larva continuously produces a silk web around the surface and inside of the food and feeds within the web8.
Synthetic insecticides are commonly used to protect stored grains and their related products against pests. The mechanism of toxicity of most insecticides such as organophosphorus and carbamate compounds is based on the inhibition of acetylcholinesterase9–11. In insects, acetylcholinesterase (AChE) hydrolyzes the neurotransmitter acetylcholine (ACh) to terminate neuronal excitement at the postsynaptic membrane12. Repeated usage of synthetic insecticides has resulted in poisoning non-targeted organisms, residual contamination and the increased resistance in insect pests13–15. These problems have warranted the need for developing alternative strategies which include using ecofriendly products in particular plant derived compounds16–18.
Plants offer an alternative source of insect-control agents as they contain a wide range of bioactive compounds, many of which are selective and have little or no harmful effects on non-targeted organisms and the environment unlike synthetic insecticides16,19,20. Plant extracts have been employed in many traditions as insecticides even before the advent of synthetic insecticides. Their potential in controlling insect growth has been used to store grains21,22. For example, farmers in India used neem leaves and seed to control stored grain pests23,24 and in some traditions in East Africa, dried leaves of Ocimum plants are added to foodstuff to protect against pests25.
Piper sarmentosum Roxb. is mainly distributed in tropical and sub-tropical regions of the world. It has an aromatic odour and a pungent taste26,27. In Malaysia, it is known as ‘kadok’28 and often used for traditional treatment of a variety of ailments such as cough, headache, fungal dermatitis and pleurisy27. Since the genus Piper is an important source of secondary metabolites which exhibit insecticidal activity29, hence, the metabolites have potential as pest control agents30. For example, the extracts and chemical constituents of P. sarmentosum are known to demonstrate larvicidal activity against mosquitoes31–33. However, to the knowledge of the author, there is no report on its potential insecticidal activity against storage pests. Hence, this prompted us to investigate the insecticidal activity of the hexane, dichloromethane and methanol extracts of P. sarmentosum against three important stored rice pests, namely S. oryzae, R. dominica, and P. interpunctella. Subsequently, a bioassay-guided fractionation of the active extract was conducted in order to isolate and characterize the compounds which were responsible for its toxicity towards S. oryzae, R. dominica, and P. interpunctella. Activity levels of the isolated compounds on the inhibition of AChE and its target site were monitored using biochemical assays.
Results
Insecticidal activity with treated grains
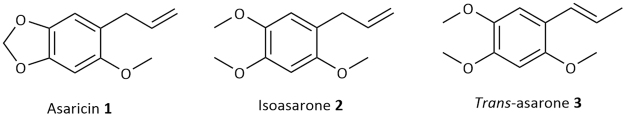
The toxicity potential of the hexane (HE), dichloromethane (DE) and methanol extracts (ME) from the roots of P. sarmentosum against S. oryzae, R. dominica, and P. interpunctella are presented in Table 1. Based on the results obtained, the hexane extract exhibited the highest percentage of mortality for all three storage pests at a concentration level of 500 µg/mL over an exposure period of 72 hours (Table 1). Hence, HE was subjected to toxicity-guided fractionation which gave faction 2 (F2) as the active fraction (Table 1). Potent toxicity of F2 led to the isolation and characterization of asaricin 1, isoasarone 2, and trans-asarone 3 as the active constituents (Fig. 1).
Table 1.
Percentage of mortality of S. Oryzae, R. dominica adults and P. interpunctella larvae 72 hours after exposure to 500 µg/mL of extracts and fractions.
| Tested extracts and fractions* | % Mortality (mean ± SE) after 72 hours** | ||
|---|---|---|---|
| S. oryzae | R. dominica | P. interpunctella | |
| HE | 100.0 ± 0.0 a | 87.8 ± 3.9 b | 59.1 ± 4.6 b |
| DE | 9.6 ± 3.9 c | 7.1 ± 1.7 d | 4.3 ± 2.4 d |
| ME | 76.8 ± 3.9 b | 57.4 ± 6.1 c | 32.1 ± 4.4 c |
| F2 | 100.0 ± 0.0 a | 100.0 ± 0.0 a | 82.8 ± 6.8 a |
*HE (Hexane extract), DE (dichloromethane extract), ME (Methanol extract), F2 (Fraction two out of eight fractions of the hexane extract).
**Mean ( ± SE) followed by the same letters in a row indicate no significant difference (p < 0.05) according to the Tukey test.
Fractions with no activity were not presented.
Figure 1.
Isolated phenylpropanoids; asaricin 1, isoasarone 2, and trans-asarone 3.
Evaluation of lethal concentration of the compounds
Asaricin 1 and isoasarone 2 showed high toxicity against all three tested insects. The LC50 of S. oryzae was estimated to be 4.7 µg/mL for asaricin 1 and 5.6 µg/mL for isoasarone 2 which considered the lowest LC50 among the tested insects (Table 2). Similarly lowest LC95 value was observed for S. oryzae in response to asaricin 1 (13.6 µg/mL) followed by isoasarone 2 (14.3 µg/mL). P. interpunctella was more tolerant to the toxicity of asaricin 1 and isoasarone 2 with LC95 value of 35.9 and 37.7 µg/mL respectively.
Table 2.
LC50 and LC95 of asaricin 1, isoasarone 2, and trans-asarone 3 against S. oryzae, R. dominica and P. interpunctella after 72 hours exposure.
| Compounds* | Tested Insects** | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S. oryzae | R. dominica | P. interpunctella | ||||||||||
| LC50 (µg/mL) (95% C.I.)*** | LC95 (µg/mL) (95% C.I.)*** | F**** | p | LC50 (µg/mL) (95% C.I.)*** | LC95 (µg/mL) (95% C.I.)*** | F**** | p | LC50 (µg/mL) (95% C.I.)*** | LC95 (µg/mL) (95% C.I.)*** | F**** | p | |
| Asaricin 1 | 4.70 (3.6 to 5.6) | 13.60 (10.5 to 21.6) | 15.2 | <0.05 | 10.60 (8.1 to 11.9) | 18.60 (15.8 to 30.3) | 8.4 | <0.05 | 17.37 (13.4 to 20.3) | 35.90 (29.1 to 57.1) | 15.3 | <0.05 |
| Isoasarone 2 | 5.60 (4.5 to 6.6) | 14.30 (11.5 to 20.8) | 11.3 | <0.05 | 8.70 (7.16 to 10.6) | 28.10 (20.3 to 49.2) | 10.8 | <0.05 | 15.76 (12.4 to 19.1) | 37.70 (29.1 to 61.5) | 11.5 | <0.05 |
| Trans-asarone 3 | 258.90 (218.7 to 303.1) | 670.20 (531.1 to 967.7) | 25.0 | <0.05 | 396.48 (328.1 to 474.7) | 1283.56 (959.6 to 2072.3) | 32.1 | <0.05 | 432.42 (387.3 to 485.2) | 1795.70 (1425.1 to 2444.6) | 4.05 | <0.05 |
*Asaricin 1, isoasarone 2 and trans-asarone 3 represent phenylpropanoids isolated from P. sarmentosum hexane active fraction (F2).
**Test performed on S. oryzae and R. dominica two weeks old adults and P. interpunctella third instar larvae.
***LC50 and LC95 values significant difference (p < 0.05) is based on non-overlap of the 95% confidence interval (C.I.).
****Since Chi square goodness of fit test is not significant (p > 0.15), no heterogeneity factor is used in the calculation of fiducial limits.
Evaluation of lethal time of the compounds
The lethal time (LT50 and LT95) by each isolated compound studied on S. oryzae, R. dominica, and P. interpunctella was presented in Table 3. Smaller LT50 and LT95 values of asaricin 1 and isoasarone 2 indicated both compounds had faster action than trans-asarone 3. However, S. oryzae and R. dominica exposed to asaricin 1 and isoasarone 2 had lower LT50 values ranging from 15.7 hours to 18.4 hours as compared to P. interpunctella (46.9 hours). Trans-asarone 3 showed the highest LT50 (≥38.9 hours) and LT95 values (≥79.2 hours). By comparing of 95% confidence interval there was no significant difference (p > 0.05) between the LT values observed from asaricin 1 and isoasarone 2. P. interpunctella had the significant highest LT values as compared to S. oryzae and R. dominica.
Table 3.
LT50 and LT95 of asaricin 1, isoasarone 2, and trans-asarone 3 against S. oryzae, R. dominica and P. interpunctella after 72 hours exposure.
| Compounds | Tested Insects* | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S. oryzae | R. dominica | P. interpunctella | ||||||||||
| LT50 (hours) (95% C.I.)** | LT95 (hours) (95% C.I.)** | F*** | p | LT50 (hours)(95% C.I.)** | LT95 (hours)(95% C.I.)** | F*** | p | LT50 (hours) (95% C.I.)** | LT95 (hours) (95% C.I.)** | F*** | p | |
| Asaricin 1 | 15.7 (8.6 to 19.6) | 34.2 (22.5 to 51.2) | 7.8 | <0.05 | 18.4 (11.1 to 27.8) | 39.8 (29.8 to 57.7) | 12.1 | <0.05 | 46.9 (28.7 to 62.2) | 91.5 (63.1 to 167.3) | 9.9 | <0.05 |
| Isoasarone 2 | 17.3 (9.5 to 23.4) | 39.3 (27.3 to 56.8) | 12.8 | <0.05 | 19.6 (11.2 to 28.6) | 48.3 (31.2 to 61.5) | 10.5 | <0.05 | 45.3 (34.4 to 51.8) | 88.1 (69.6 to 159.6) | 13.2 | <0.05 |
| Trans-asarone 3 | 38.9 (28.8 to 43.5) | 79.2 (58.2 to 103.4) | 28.4 | <0.05 | 48.7 (31.6 to 57.2) | 88.9 (68.9 to 129.2) | 30.3 | <0.05 | 52.7 (39.4 to 82.9) | 92.7 (88.1 to 134.9) | 18.8 | <0.05 |
*Test performed on S. oryzae and R. dominica two weeks old adults and P. interpunctella their instar larvae.
**LT50 and LT95 values significant difference (p < 0.05) is based on non-overlap of the 95% C.I.
***Since Chi square goodness of fit test is not significant (p > 0.15), no heterogeneity factor is used in the calculation of fiducial limits.
Contact toxicity
The results clearly demonstrated that asaricin 1, isoasarone 2, and trans-asarone 3 did not show potent contact toxicity against stored grain insect pest even at the highest concentration of 200 μg/mL (Fig. 2). The efficacy in respect to the toxicity of asaricin 1, isoasarone 2, and trans-asarone 3 towards S. oryzae and R. dominica adults and P. interpunctella larvae was relatively weak up to 48 hours monitoring after the treatment. Trans-asarone 3 were not significantly different as compared to the control, S. oryzae (F = 3.3, p < 0.05), R. dominica (F = 3.8, p < 0.05) and P. interpunctella (F = 3.8, p < 0.05).
Figure 2.
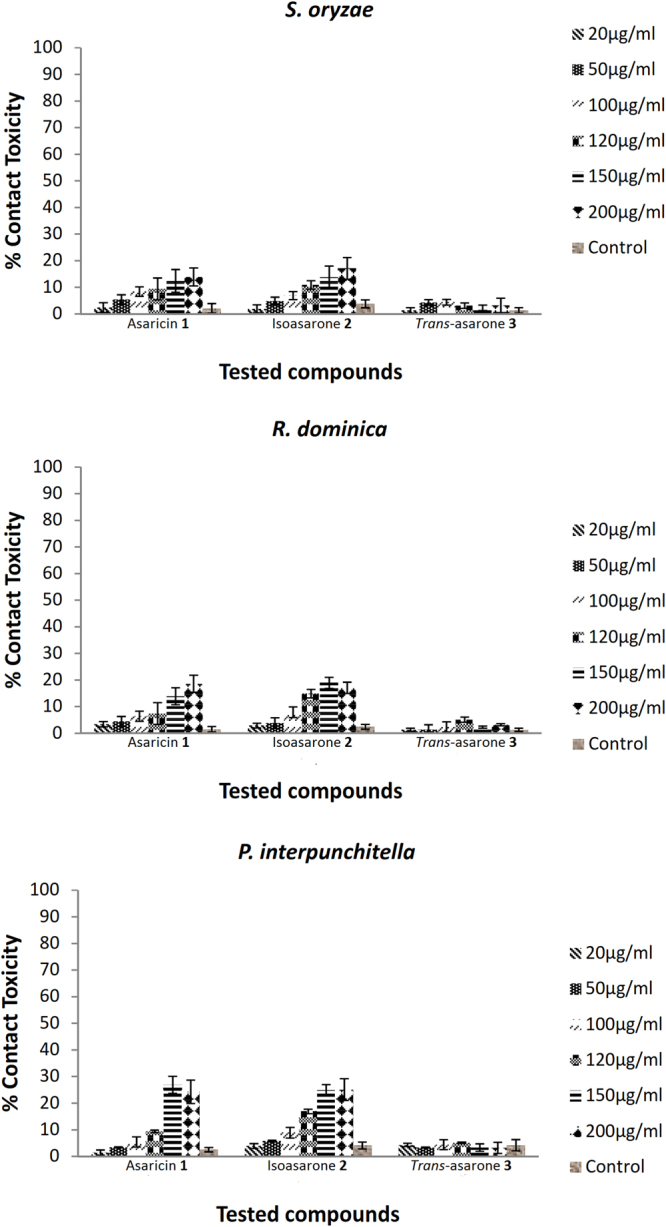
Percentage of contact toxicity of asaricin 1, isoasarone 2, and trans-asarone 3 against S. oryzae and R. dominica adults and P. interpunctella larvae. Data were expressed as mean ± SEM.
Residual toxicity using LC95 value of each active compound
The mean mortality of S. oryzae, R. dominica and P. interpunctella were recorded during the 60 days of bioassay (Fig. 3). During the 60 days of residual activity test, the mean mortality of S. oryzae (F = 3.6, p < 0.05), R. dominica (F = 4.6, p < 0.05) and P. interpunctella (F = 4.4, p < 0.05) were significantly higher than the control after treated with asaricin 1, isoasarone 2 and trans-asarone 3. The efficacy of asaricin 1 and isoasarone 2 with their relative LC95 against S. oryzae was consistent during the first 30 days and slowly decreased and this pattern continued till last day of the assay. On the other hand, toxicity efficacy of trans-asarone 3 towards S. oryzae with high LC95 value of 670.2 μg/mL was consistent till the 60th day. R. dominica and P. interpunctella response to residual effects of asaricin 1, isoasarone 2, and trans-asarone 3 were similar and followed almost the same pattern as S. oryzae. Asaricin 1 and isoasarone 2 showed consistent toxicity against tested insects during the first 30 days according to the mortality rate. Then, the insecticidal activity reduced and by the 60th day, it was at the lowest point (Fig. 3).
Figure 3.
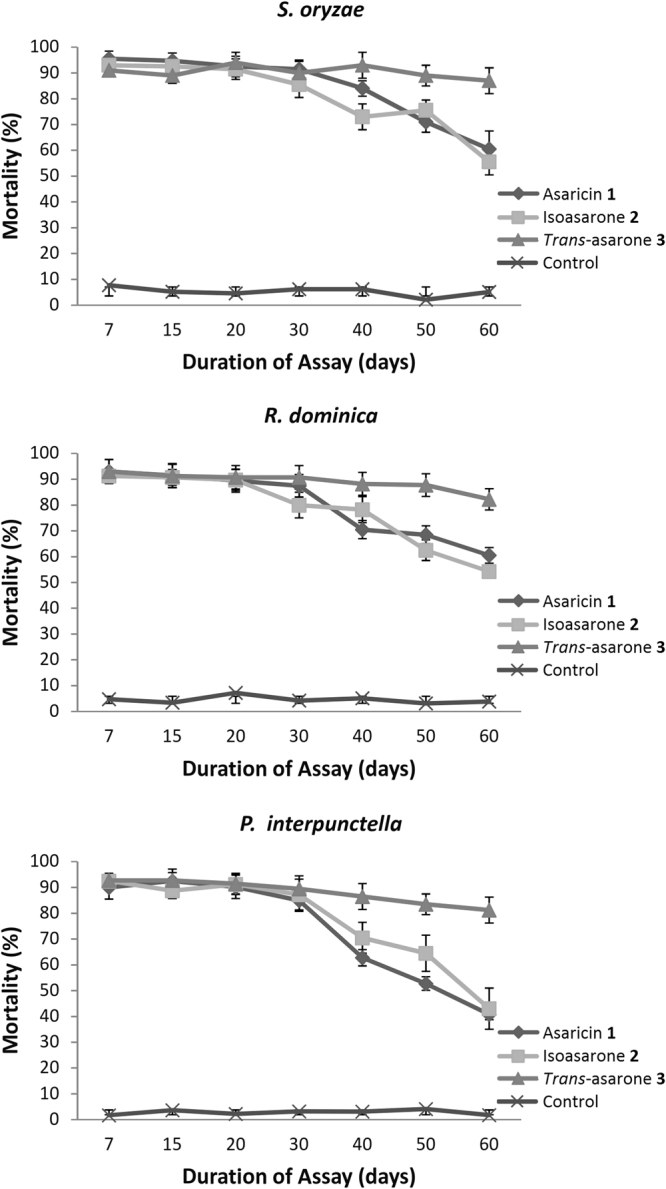
Mean (±SEM) mortality percentage of S. oryzae, R. dominica adult and P. interpunctella larvae on rice grain treated with asaricin 1, isoasarone 2, and trans-asarone 3 by using their relative LC95 value, exposed from 0 to 60 days after treatment. Percentage of mortality for trans-asarone 3 were significant stable as compared to asaricin 1 and isoasarone 2 after 30th days.
Repellency bioassay
Asaricin 1 and isoasarone 2 showed similar repellency activity towards S. oryzae, R. dominica, and P. interpunctella (Table 4). S. oryzae and R. dominica were repelled more by asaricin 1 and isoasarone 2 with mean repellency of 79–85% while P. interpunctella was less repelled with mean repellency of 50–58% after 20 hours. Trans-asarone 3 was less effective in repellency with less than 10% repellency for all tested insects.
Table 4.
Repellency activity of asaricin 1, isoasarone 2, and trans-asarone 3 against S. oryzae, R. dominica and and P.interpunctella.
| Compounds | Insects | Repellency (mean ± SE)* | Mean repellency (%) | |||
|---|---|---|---|---|---|---|
| 5 h | 10 h | 15 h | 20 h | |||
| Asaricin 1 | S. oryzae | 51.2 ± 10.3 a | 67.2 ± 8.5 a | 74.2 ± 5.3 ab | 82.5 ± 6.4 a | 68.6 ± 6.6 a |
| R. dominica | 60.0 ± 7.1 a | 74.5 ± 6.4 a | 81.2 ± 6.3 a | 85.0 ± 7.8 a | 75.1 ± 5.5 a | |
| P. interpunctella | 33.7 ± 4.7 b | 38.7 ± 7.5 b | 45.4 ± 7.2 c | 50.0 ± 7.1 b | 42.7 ± 3.9 b | |
| Isoasarone 2 | S. oryzae | 51.2 ± 7.5 a | 60.0 ± 7.8 a | 69.5 ± 6.2 b | 79.2 ± 8.3 a | 68.1 ± 6.2 a |
| R. dominica | 58.7 ± 13.1 a | 65.0 ± 7.9 a | 72.5 ± 5.5 ab | 79.2 ± 5.1 a | 68.8 ± 4.4 a | |
| P. interpunctella | 31.2 ± 2.5 b | 37.5 ± 6.5 b | 41.2 ± 2.5 c | 58.7 ± 4.8 b | 42.1 ± 5.8 b | |
| Trans-asarone 3 | S. oryzae | NA | NA | NA | NA | NA |
| R. dominica | NA | NA | NA | NA | NA | |
| P. interpunctella | NA | NA | NA | NA | NA | |
*Each data represents the mean of four replicates. Values followed by the same letter are not significantly different according to the Tukey test (p < 0.05).
h: hours after treatment.
NA: below 10% of repellency.
F1 progeny activity
Table 5 shows the effect of F1 progeny activity of asaricin 1, isoasarone 2, and trans-asarone 3 on S. oryzae, R. dominica, and P. interpunctella. Asaricin 1 and isoasarone 2 were highly potent on inhibition of S. oryzae, R. dominica, and P. interpunctella emergence of the adult insects at low dosage as 2 µg/mL. The asaricin 1 and isoasarone 2 at 4 µg/mL concentrations were able to inhibit 100% of the emergence of adult S. oryzae and R. dominica. P. interpunctella were more resistance to asaricin 1 and isoasarone 2 where they only caused 78.98 and 86.37% inhibition of F1 adult emergence at 4 µg/mL. Meanwhile trans-asarone 3 was weak in inhibition of F1 adult emergence as compared to asaricin 1 and isoasarone 2. All the tested isolated compounds were significantly (p < 0.05) different from the controls in their effects.
Table 5.
Effect of asaricin 1, isoasarone 2, and trans-asarone 3 against on the adult emergence of three stored product insect pests infesting rice.
| Compounds | Concentrations (µg/mL) | Percentage of reduction in egg number | |||||
|---|---|---|---|---|---|---|---|
| S. oryzae* | R. dominica* | P. interpunctella* | |||||
| Mean number of adult emergence | % IR** | Mean number of adult emergence | % IR** | Mean number of adult emergence | % IR** | ||
| Asaricin 1 | 1 | 19.25 ± 2.01 | 34.25 ± 1.30 d | 16.75 ± 2.17 | 41.10 ± 1.80 c | 28.50 ± 0.86 | 21.50 ± 2.25 d |
| 2 | 2.08 ± 1.04 | 89.25 ± 3.70 b | 1.52 ± 0.76 | 84.50 ± 2.90 b | 11.50 ± 2.36 | 59.25 ± 3.83 c | |
| 4 | 0.00 ± 0.00 | 100.00 ± 0.00 a | 0.00 ± 0.00 | 100.00 ± 0.00 a | 3.80 ± 1.20 | 87.37 ± 3.47 a | |
| Isoasarone 2 | 1 | 15.00 ± 2.64 | 47.50 ± 1.47 c | 15.50 ± 2.53 | 44.70 ± 1.40 c | 21.50 ± 1.52 | 24.60 ± 2.10 d |
| 2 | 1.52 ± 0.76 | 86.00 ± 3.20 b | 1.73 ± 0.86 | 89.34 ± 2.80 b | 11.50 ± 1.52 | 59.50 ± 2.30 c | |
| 4 | 0.00 ± 0.00 | 100.00 ± 0.00 a | 0.00 ± 0.00 | 100.00 ± 0.00 a | 5.40 ± 0.98 | 78.98 ± 4.51 b | |
| Trans-asarone 3 | 1 | 28.00 ± 1.54 | 2.25 ± 0.71 f | 25.30 ± 0.50 | 1.00 ± 0.40 f | 25.50 ± 1.52 | 1.17 ± 1.20 g |
| 2 | 25.00 ± 1.52 | 13.50 ± 2.10 e | 25.75 ± 2.88 | 17.75 ± 0.83 d | 24.50 ± 2.40 | 7.50 ± 0.04 f | |
| 4 | 26.50 ± 1.54 | 8.60 ± 1.02 e | 28.50 ± 1.73 | 9.25 ± 1.04 e | 26.25 ± 2.64 | 14.25 ± 2.50 e | |
*Test performed on S. oryzae and R. dominica two weeks old adults and P. interpunctella their instar larvae.
**% IR (inhibition rate) values followed by a common letter are not significantly different (p < 0.05).
Oviposition deterrent effect
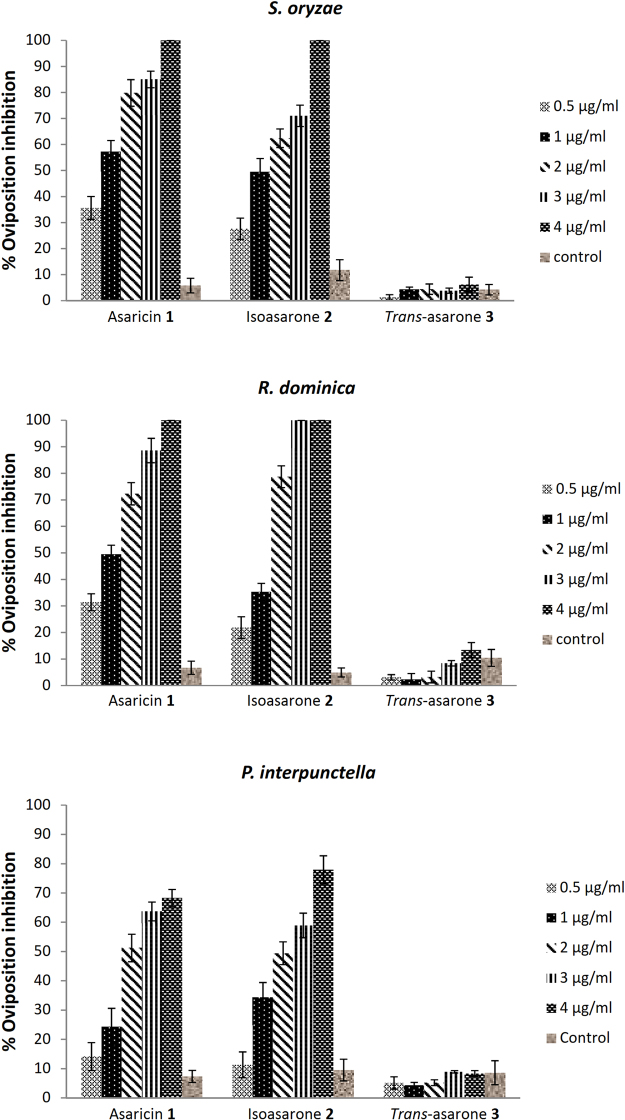
The ovipositional inhibition effect is demonstrated in Fig. 4. The egg deposition was recorded 72 hours after exposure of insects to asaricin 1, isoasarone 2, and trans-asarone 3. Cumulative number of egg laying by the S. oryzae, R. dominica, and P. interpunctella females were highly inhibited by asaricin 1 and isoasarone 2. These results indicated that asaricin 1 and isoasarone 2 significantly suppressed the egg deposition tested insects. Asaricin 1 and isoasarone 2 caused 10 to 35% reduction of egg deposition at the lowest dosage of 0.5 µg/mL in all tested insects. At concentration of 4 µg/mL, asaricin 1 and isoasarone 2 caused 100% ovipositional inhibition in S. oryzae and R. dominica and up to 68–77.9% inhibition in P. interpunctella. Overall, the statistical analysis of proportions of egg laying by insect pests between control and asaricin 1 were found to be statistically significant for S. oryzae (F = 7.6, p > 0.05), R. dominica (F = 5.1, p > 0.05) and P. interpunctella (F = 4.4, p > 0.05) which was similar to isoasarone 2. On the other hand, trans-asarone 3 had weak ovipositional inhibition in all tested insects where it could not reduce the egg laying after treatment. There was no significant differences in trans-asarone 3 and control (F = 3.9, p < 0.05).
Figure 4.
Oviposition inhibition of asaricin 1, isoasarone 2, and trans-asarone 3 on S. oryzae, R.dominica and P. interpunctella.
Cholinesterase Enzymes Inhibitory Activity
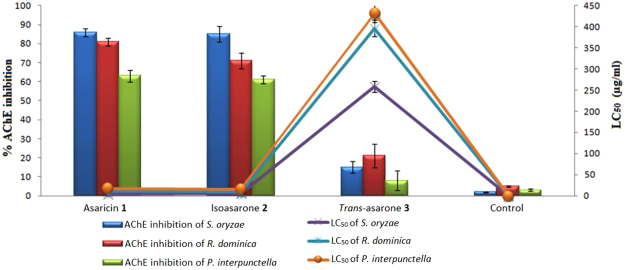
The initial AChE inhibition activity of the three compounds was evaluated at their relative LC50 on the enzyme supernate extract from each insect. Asaricin 1 and isoasarone 2 showed potent AChE inhibition for all three insects. Asaricin 1 and isoasarone 2 which showed high toxicity towards S. oryzae (LC50 value of 4.7 and 5.6 μg/mL respectively) also showed high AChE inhibition on enzyme supernate extracted from S. oryzae. Both asaricin 1 and isoasarone 2 exhibited a slightly lower AChE inhibition of the extracted enzyme from R. dominica and P. interpunctella as compared to that of S. oryzae. The lower AChE inhibition can relate with the relative higher LC value for R. dominica and P. interpunctella. In general, asaricin 1 exhibited the highest acetylcholinesterase inhibition followed by isoasarone 2. Trans-asarone 3 exhibited a lower inhibitory activity of less than 30% as compared to asaricin 1 and isoasarone 2 for all tested insects. The LC50 value and percentage of AChE inhibition had significant negative correlation (Fig. 5). The correlation study between the LC50 and mean percentage of AChE inhibition was significant (p > 0.05). The results of correlation coefficient is presented in Table 6. The correlation study on AChE inhibition of S. oryzae was meaningful and negative for asaricin 1 and isoasarone 2 (correlation coefficient (r) of asaricin 1: −0.920 and isoasarone 2: −0.946) (Table 6). R. dominica and P. interpunctella correlation study was almost similar to S. oryzae.
Figure 5.
Inhibition on acetylcholinesterase enzyme by asaricin 1, isoasarone 2 and trans-asarone 3 in comparison with their relative LC50 value on S. oryzae, R. dominica and P. interpunctella.
Table 6.
Correlation coefficients between AChE inhibition of S. oryzae, R. dominica and P. interpunctella and the relative LC50 of asaricin 1, isoasarone 2, and trans-asarone 3.
| AChE enzymes | Correlation coefficient of inhibitors | ||
|---|---|---|---|
| Asaricin 1 | Isoasarone 2 | Trans-asarone 3 | |
| S. oryzae | −0.920* | −0.946* | −0.866* |
| R. dominica | −0.859* | −0.910* | −0.833* |
| P. interpunctella | −0.953* | −0.938* | −0.863* |
*Represent the meaningful and negative correlation (p > 0.05).
The de-attenuated correlation coefficient was calculated using x represents mean percentage of AChE inhibition, y represents relative LC95 of asaricin 1, isoasarone 2 and trans-asarone 3 against S. oryzae, R. dominica and P. interpunctella.
Discussion
Bioassay-guided fractionation of the active hexane extract resulted in the isolation and characterization of asaricin 1, isoasarone 2, and trans-asarone 3. Asaricin 1 and isoasarone 2 exhibited potent insecticidal activity against S. oryzae, R. dominica and P. interpunctella. The lowest LC50 and LC95 values for S. oryzae were observed for asaricin 1 and isoasarone 2 among the tested insects while P. interpunctella was more resistant to the toxicity of asaricin 1 and isoasarone 2. Based on the LC50 values and their respective 95% confidence intervals, trans-asarone 3 was found to be significantly less effective towards S. oryzae and R. dominica adults and P. interpunctella larvae. The LC50 and LC95 values for all three species were variable and species specific. This result is supported by other reports, which showed great variations in the susceptibility of insects to other biopesticide product34. The percentage of variation in the sensitivity of stored grain insect pests is high in response to toxicity of volatile compounds. Similar results have been reported by several researches on insecticidal activities of other plant extracts35,36. Besides, the results of lethal time (LT50 and LT95) showed fast acting of the asaricin 1 and isoasarone 2 against the S. oryzae, R. dominica and P. interpunctella 37,38. The fast and significant mortality indicated the potent insecticidal activity of asaricin 1 and isoasarone 2.
Furthermore, none of these compounds exhibited potent contact toxicity while asaricin 1 and isoasarone 2 were highly toxic against the pests in treated grain assay. According to previous research some natural compounds or oils express toxicity against stored product pests, such as l-carvone, d-carvone, horseradish oil, and mustard oil, but not all are active against insect pests through contact toxicity39,40. The results suggested that all three compounds act as stomach poison as the toxicity may be due to ingestion and digestion of the compounds in the stomach but not by absorption through insects’ curticle41,42. To confirm this statement, further investigations are needed after the formulation of the compounds. On the other hand, extended period of protection, repellency activities, F1 progeny activity and anti-ovipositional properties of asaricin 1 and isoasarone 2 suggested their excellent grain protectant potential.
Asaricin 1 and isoasarone 2 could provide protection against S. oryzae, R. dominica and P. interpunctella over a relatively long period (over 30 days) according to residual toxicity test. However, insecticidal activity of trans-asarone 3 showed consistent toxicity throughout the 60 days towards the three stored grain pests. This observation suggested that trans-asarone 3 has a longer half-life as compared to asaricin 1 and isoasarone 2. This result may be due to stability of trans-asarone 3. The three compounds have carbon double bonds in the aromatic ring and alkyl side chain. However, trans-asarone 3 has conjugated double bonds while asaricin 1 and isoasarone 2 have isolated double bonds. Conjugated double bond has resonance stabilization, so it is more stable than isolated double bond. Hence, trans-asarone 3 may be have better stability and resistance to oxidative degradation which resulted in longer half-life in comparison to asaricin 1 and isoasarone 2 43,44. Although the insecticidal activity of trans-asarone 3 was the lowest (LC50 value of 258.90–432.42 μg/mL) but since its toxicity effect was consistent during the 60 days, it may be a good option to be used in mixtures with asaricin 1 and isoasarone 2 as it is most probably more stable.
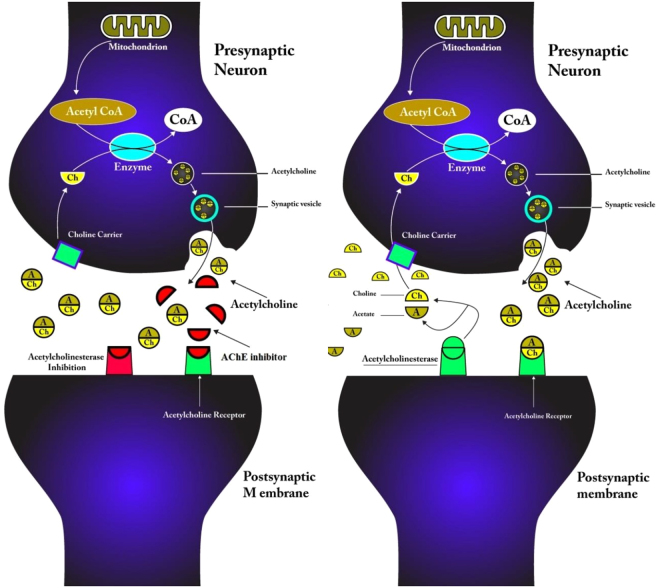
As shown in Fig. 6, the presence of asaricin 1, isoasarone 2 and trans-asarone 3 as acetylcholinesterase inhibitors can cause inhibition of AChE from breaking down to neurotransmitter; acetylcholine, which disrupt or increase the duration of action of the neurotransmitter9,11. Mechanism of action asaricin 1 and isoasarone 2 maybe has similarity with organophosphate insecticides due to their strong AChE inhibition. The inhibition of AChE may has direct effect on bradycardia, bronchoconstriction and prolonged muscle contraction of insects which lead to paralysation and death45. The biochemical assay revealed the possible connection between the AChE inhibition and the toxicity of the three compounds. These results suggested that there was significant and negative relationship between AChE inhibition and LC value where higher inhibition of AChE causes stronger toxicity and results in lower LC50 value. The difference in the AChE inhibition for all three compounds may be due to the insect resistance and different binding modes due to differences in AChE structural features in each species studied46,47.
Figure 6.
Mechanism of action of AChE with the presence of asaricin 1, isoasarone 2 and trans-asarone 3 as insects acetylcholinesterase inhibitors (left) and without the presence of acetylcholinesterase inhibitors (right).
This is the first study conducted on the insecticidal activity and the mechanism of action of the compounds isolated from P. sarmentosum in insect pest control. The results obtained from this study suggested the potential use of P. sarmentosum extracts and its active components in storage pest management. In addition, further investigations regarding the safety issues for mammalian and environmental health, antifeedant activity and formulations will be conducted. Formulations may improve and enhance the insecticidal potency of the compounds.
Materials and Methods
General experimental procedures
General experimental procedures were similar to those described by Hematpoor et al.31.
Plant material
The plant material was collected as previously described31. The voucher specimen (KU 0110) was deposited in the Herbarium of the Department of Chemistry, Faculty of Science, University of Malaya, Kuala Lumpur, Malaysia.
Extraction and Isolation
The roots of P. sarmentosum were extracted as previously described31. The highest percentage of mortality was observed for the hexane extract in a preliminary screening of the potential toxicity of the extracts towards S. oryzae, R. dominica, and P. interpunctella (Table 1). Hence, the hexane extract was subjected to bioassay-guided fractionation and fraction F2 from total of eight fractions was found to exhibit the highest toxicity towards S. oryzae, R. dominica, and P. interpunctella (Table 1). Asaricin 1, isoasarone 2, and trans-asarone 3 were isolated from the active fraction F2 as previously described31.
Structural elucidation of compounds
The structures of the isolated compounds (asaricin 1, isoasarone 2, and trans-asarone 3) were characterized using spectroscopic data as previously described31 and compared with literature values48–50.
Insecticidal activity
Test insects
All tested insects used for the experiments were obtained from colonies cultured in the laboratory at 25 ± 1 °C and 65 ± 5% relative humidity. S. oryzae, R. dominica, and P. interpunctella were reared on rice grains with additional 5% yeast. Two weeks old adults of S. oryzae and R. dominica and 3rd instar-larvae of P. interpunctella were used for the bioassay.
Insecticidal effects on treated grain
Bioassay was conducted by dissolving 1 mg of each extract (hexane, dichloromethane and methanol) in 1 mL of acetone and introducing them onto 5 g of rice grains using a pipette. The control was only treated with 1 mL of acetone. The treated grains were dried under the fume hood. Twenty unsexed adults of S. oryzae and R. dominica were separately introduced into the respective petri dishes containing 5 g of treated rice. Twenty 3rd instar larvae of P. interpunctella were carefully collected with a fine brush and placed in each petri dish (10 cm diameter × 1.5 cm). All treatments were monitored to obtain the mean percentage of mortality after 72 hours. Experiments were replicated four times. The same technique was used to determine the bioactivity of each fraction obtained from the active extract against the test insects.
Compounds isolated from the active fraction were evaluated for their toxicity towards S. oryzae, R. dominica and P. interpunctella to obtain their lethal concentration values (LC). Five grams of rice grains were treated with different concentrations (w/v) of each compound. Controls were treated with acetone. The treated grains were allowed to dry at room temperature (27 °C). The petri dishes were covered and sealed with parafilm. Mortality was recorded after 24, 48, and 72 hours of exposure.
Contact toxicity
Residual film bioassay technique was used to study the contact toxicity of asaricin 1, isoasarone 2, and trans-asarone 3 1,25. Amount of 1 mg of each compound was dissolved in 1 mL of acetone and used as the stock solution to apply in several dosages to determine their contact toxicity. After a serial dilution, dosages between 20–200 µg/mL were carefully introduced onto filter papers (Whatman No. 1). Controls received only acetone. After drying under a fume hood for 15 minutes, each filter paper was placed at the bottom of a petri dish. Twenty adults of S. oryzae and R. dominica and twenty larvae of P. interpunctella were introduced into each petri dish, respectively. The petri dishes were covered and sealed with parafilm. Mortality of tested insects were recorded after 24 and 48 hours of treatment and percentage of mortality of each compound were calculated using Abbott’s formula51. Abbott’s formula is as follows:
| 1 |
where:
X = percent live in control.
Y = percent live in treatment.
Residual toxicity against storage pests
Residual toxicity against adults of S. oryzae and R. dominica, and larvae of P. interpunctella were assisted by optimization of the method from Islam and Talukder, 200552. The assay was carried out in separate plastic cups for a period of 60 days after 15 g of rice were treated with asaricin 1, isoasarone 2, and trans-asarone 3. The length of exposure and the dosage of each compound were chosen as the concentration required to cause 95% mortality of the individuals. The LC95 values obtained from the earlier study (section 2.5.2) was used to evaluate the persistence of toxicity during the 60 days period. After each treatment was completed, the plastic cups were closed with their caps, secured and held at room temperature (27 °C). The control was only treated with acetone. These bioassays were carried out over a period of 60 days whereby on the 7th day, 30 insects were placed in each plastic cup. After 72 hours, the insects were removed from each cup. Insect’s mortality was recorded and the dead insects were discarded. All cups were tightly closed until the next bioassay. The procedures were repeated on the 15th, 20th, 30th, 40th, 50th and 60th day. All bioassays were replicated 4 times.
Repellency test
Repellency of asaricin 1, isoasarone 2, and trans-asarone 3 was evaluated using area preference methods25. Whatman No. 1 filter papers were cut into halves. One side treated with 100 µL/mL of asaricin 1, isoasarone 2 and trans-asarone 3 respectively using pipette. The other half was treated with acetone as control. The treated and control half discs were air dried under laboratory conditions and full discs re-made using paper tapes to attach together. Each paper was placed in a petri dish and ten S. oryzae was introduced at the centre of the filter paper then the petri dish was closed and sealed with parafilm. Each treatment was replicated four times and the number of S. oryzae present on each side was recorded every 5 hours up to 20 hours. The same procedures were applied for R. dominica and P. interpunctella. The Percentage of Repellency (PR) calculated as follows:
| 2 |
where:
PR = Percentage of repellency.
Nc = Number of insects present on the control strip.
Nt = Number of insects present on the treated strip.
Negative PR value were treated as zero.
F1 progeny activity of asaricin 1, isoasarone 2, and trans-asarone 3
Fifty grams of rice grains were weighed and then three concentrations (1, 2, and 4 μg/mL) of each compound asaricin 1, isoasarone 2, and trans-asarone 3 were individually mixed and specimen were deposited in a 300 mL of plastic containers. The compounds were dissolved in acetone and uniformly spread on rice. Rice treated with acetone only was set as control. Ten pairs of adult insects were introduced into each container. Four replicates of each treatment and untreated controls were laid out in Complete Randomized Design. Adult mortality was counted and recorded after 48 and 72 hours of application. On the 4th day, all insects whether dead or alive, were removed and experiments were left for 35 days to allow for emergence of F1 generation. The number of adults emerged was counted after 35 days and inhibition rate (% IR) in adult emergence was calculated using following formula53.
| 3 |
where:
Cn = number of emerged insects in the control.
Tn = number of emerged insects in the treated container.
Oviposition deterrent activity
The asaricin 1, isoasarone 2, and trans-asarone 3 were tested for their oviposition inhibition activity54. Three concentrations (0.5, 1, 2, 3, and 4 μg/mL) for each compound were prepared by dissolving them in acetone. Twenty undamaged grain of rice were placed in the plastic petri dish and treated separately with different dose of asaricin 1, isoasarone 2, and trans-asarone 3 in uniform manner. The seeds were air dried until all acetone evaporated. For control seeds were dressed in 1 mL acetone only. The treated samples were kept in laboratory condition (27 ± 2 °C and 80 ± 5% RH). Five males and five females were placed on each treatment. After 48 hours the mortality of each insect was observed in each petri dish and all insects (live and dead) were removed. The number of eggs laid on seeds of treated and control seeds were counted after three days of starting the experiment. To evaluate the number of eggs laid for S. oryzae on rice grains samples were recorded after staining the grains to detect the egg plugs55.
| 4 |
where:
Cs = number of eggs laid on the control.
Ts = number of eggs laid in the treated container.
Biochemical assays
The tested insects were respectively subjected to acetylcholinesterase biochemical assay (AChE)56. Each insect was removed and homogenized at 0 °C in 0.05 M-phosphate buffer, pH 7.5 (KH2PO4-NaOH) (Sigma) using a glass homogenizer pestle (5%, w/v, homogenate). Plastic tube covered with ice were used to keep the homogenized mixture. The homogenates were centrifuged at 16,000 rpm for 20 min (5 °C). These supernatants were kept in the ice and used without further purification for studying the acetylcholinesterase and its inhibition. To determine the AChE inhibition activity, procedures from Ellman et al., 1961 were slightly modified and used57,58. 10 μL of enzyme supernatants were transferred to each well of a 96-well microtiter plate using electronic multichannel pipette (Eppendorf, USA) then mixed with 20 μL of tested compounds in solution form and 150 μL of phosphate buffer which were kept at 1–4 °C temperature were added. Asaricin 1, isoasarone 2, and trans-asarone 3 were dissolved in DMSO (Merck, USA) as AChE inhibitor at their LC95 value and 0.1% DMSO (v/v) were added into test well in separated rows. The microtiter plates were incubated for 10 minutes at 25 °C. Next step was followed by adding 20 μL of acetylthiocholine iodide (ACTHI) (Sigma-Aldrich, USA) (0.4 mM) and DTNB (Sigma-Aldrich, USA) (0.3 mM) to enzyme supernatants solution to observe the reaction. During the 30 minutes reaction at room temperature, yellowish or colorless solution was observed. The microtiter plates were sentenced for microplate readers (Synergy H1 Hybrid Multi-Mode Microplate Reader, USA) at 412 nm and results were recorded. Percentage of inhibition calculated based on the mean optical density of enzyme as below:
| 5 |
The resulting data for each selected insect was analyzed using correlation study compare the enzyme expression levels between different insects in response to asaricin 1, isoasarone 2, and trans-asarone 3. All levels of statistical significance were determined at p < 0.05.
Data Analysis
Bioassay data within the range of 5–95% were pooled and subjected to probit analysis59 by using Polo Plus (LeOra Software, Berkeley, CA) to obtain 50% and 95% lethal concentrations (LC50 and LC95). Data on adult mortality were also subjected for determining lethal time required by each isolated compound to kill 50% and 95% (LT50 and LT95). Significant differences in the LC50 and LC95 values were based on non-overlap of 95% confidence intervals60. Abbott’s formula51 was applied to correct the percentage of mortality if the control mortality was more than 5%. The analyses of the data were done using ANOVA followed by Tukey’s test using SAS version 9.1.3, 2002 software. Statistical correlation study was conducted to understand the relation between acetylcholinesterase activity and LC50 dose of asaricin 1, isoasarone 2, and trans-asarone 3 levels of statistical significant were determined at p < 0.05.
Data availability statement
All data generated or analysed during this study are included in this published article.
Acknowledgements
This work was supported by University Malaya Research Grants (RP001-2012 and PV085/2011A). The funding sources were not involved in the study design, collection, analysis, interpretation of data, writing of the paper or the decision to submit the paper for publication. The authors sincerely thank Ardavan Hematpoor for designing the figure.
Author Contributions
A.H., M.S.A. and K.A. designed the experiments. A.H. performed the experiments. All authors analyzed the data. M.S.A. and K.A. contributed reagents/materials/analysis tools. All authors wrote, read and approved the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rajashekar, Y. & Shivanandappa, T. A novel natural insecticide molecule for grain protection. Julius-Kühn-Archiv, 910–915, 10.5073/jka.2010.425.413 (2010).
- 2.Rojht H, Košir IJ, Trdan S. Chemical analysis of three herbal extracts and observation of their activity against adults of Acanthoscelides obtectus and Leptinotarsa decemlineata using a video tracking system. J. Plant Dis. Protect. 2012;119:59–67. doi: 10.1007/BF03356421. [DOI] [Google Scholar]
- 3.Nakakita, H. Stored rice and stored product insects. (A.C.E. Corporation, Tokyo, 1998).
- 4.Majeed MZ, et al. Biology and management of stored products’ insect pest Rhyzopertha dominica (Fab.) (Coleoptera: Bostrichidae) Int J. Biosci. 2015;7:78–93. [Google Scholar]
- 5.Chanbang Y, Arthur FH, Wilde GE, Throne JE, Subramanyam B. Susceptibility of eggs and adult fecundity of the lesser grain borer, Rhyzopertha dominica, exposed to methoprene. J. Insect Sci. 2008;8:48–48. doi: 10.1673/031.008.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nellie, W. S. C. Development And Competition In Rice Weevil, Sitophilus Oryzae (L.) And Red Flour Beetle, Tribolium Castaneum (Herbst), And Their Responses To Active Compounds In Selected Spices Doctor of Philosophy thesis, Universiti Sains Malaysia, (2016).
- 7.Fasulo, T. R. & Knox, M. A. (University of Florida, University of Florida, 1998).
- 8.Mohandass S, Arthur FH, Zhu KY, Throne JE. Biology and management of Plodia interpunctella (Lepidoptera: Pyralidae) in stored products. J. Stored Prod. Res. 2007;43:302–311. doi: 10.1016/j.jspr.2006.08.002. [DOI] [Google Scholar]
- 9.Čolović MB, Krstić DZ, Lazarević-Pašti TD, Bondžić AM, Vasić VM. Acetylcholinesterase Inhibitors: Pharmacology and Toxicology. Curr. Neuropharmacol. 2013;11:315–335. doi: 10.2174/1570159X11311030006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rajashekar Y, Raghavendra A, Bakthavatsalam N. Acetylcholinesterase Inhibition by Biofumigant (Coumaran) from Leaves of Lantana camara in Stored Grain and Household InsectPests. BioMed Res. Int. 2014;2014:187019. doi: 10.1155/2014/187019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fukuto TR. Mechanism of action of organophosphorus and carbamate insecticides. Environ. Health Perspect. 1990;87:245–254. doi: 10.1289/ehp.9087245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nabeshima T, et al. An amino acid substitution attributable to insecticide-insensitivity of acetylcholinesterase in a Japanese encephalitis vector mosquito. Culex tritaeniorhynchus. Biochem. Biophys. Res. Commun. 2004;313:794–801. doi: 10.1016/j.bbrc.2003.11.141. [DOI] [PubMed] [Google Scholar]
- 13.Cheng S-S, Chang H-T, Chang S-T, Tsai K-H, Chen W-J. Bioactivity of selected plant essential oils against the yellow fever mosquito Aedes aegypti larvae. Bioresour. Technol. 2003;89:99–102. doi: 10.1016/S0960-8524(03)00008-7. [DOI] [PubMed] [Google Scholar]
- 14.Moye JK, Pritsos CA. Effects of Chlorpyrifos and Aldicarb on Flight Activity and Related Cholinesterase Inhibition in Homing Pigeons, Columba livia: Potential for Migration Effects. Bull. Environ. Contam. Toxicol. 2010;84:677–681. doi: 10.1007/s00128-010-0020-2. [DOI] [PubMed] [Google Scholar]
- 15.Arthur FH. Grain protectants: Current status and prospects for the future. J. Stored Prod. Res. 1996;32:293–302. doi: 10.1016/S0022-474X(96)00033-1. [DOI] [Google Scholar]
- 16.Adakole JA, Adeyemi AFF. Larvicidal effects of cymbopogon citratus (lemon grass) extract against Culex quinquefasciatus qularvae (Diptera, culicidae) IJAES. 2012;7:187–192. [Google Scholar]
- 17.Golob, P. & Gudrups, I. The use of spices and medicinals as bioactive protectants for grains. (Food and Agriculture Organization of the United Nations Rome, FAO Agricultural Sciences Bulletin No. 137, 1999).
- 18.Lale, N. E. S. An overview of the use of plant products in the management of stored product Coleoptera in the tropics. Postharvest News and Information6 (1995).
- 19.Koul O, Walia S, Dhaliwal GS. Essential Oils as GreenPesticides: Potential and Constraints. Biopestic. Int. 2008;4:63–84. [Google Scholar]
- 20.Tavares WS, et al. Selective effects of natural and synthetic insecticides on mortality of Spodoptera frugiperda (Lepidoptera: Noctuidae) and its predator Eriopis connexa (Coleoptera: Coccinellidae) J. Environ. Sci. Health. B. 2010;45:557–561. doi: 10.1080/03601234.2010.493493. [DOI] [PubMed] [Google Scholar]
- 21.Grdiša M, Gršić K. Botanical Insecticides in Plant Protection. Agric. Conspec. Sci. 2013;78:85–93. [Google Scholar]
- 22.Belmain SR, Golob P, Andan HF, Cobbinah JR. Ethnobotanicals—future prospects as post-harvest insecticides. Agro Food Ind. Hi Tech. 1999;10:34–36. [Google Scholar]
- 23.Ahmed, S. & Koppel, B. In Proceedings of the American Association for the Advancement of Science Annual Meeting.
- 24.Rajashekar Y, Bakthavatsalam N, Shivanandappa T. Botanicals as Grain Protectants. Psyche. 2012;2012:13. [Google Scholar]
- 25.Obeng-Ofori D, Reichmuth CH, Bekele AJ, Hassanali A. Toxicity and protectant potential of camphor, a major component of essential oil of Ocimum kilimandscharicum, against four stored product beetles. Int. J. Pest Manag. 1998;44:203–209. doi: 10.1080/096708798228112. [DOI] [Google Scholar]
- 26.Damsud T, Adisakwattana S, Phuwapraisirisan P. Three new phenylpropanoyl amides from the leaves of Piper sarmentosum and their α-glucosidase inhibitory activities. Phytochem. Lett. 2013;6:350–354. doi: 10.1016/j.phytol.2013.04.001. [DOI] [Google Scholar]
- 27.Chan EWC, Wong SK. Phytochemistry and pharmacology of three Piper species. An update. 2014;IJP1:534–544. [Google Scholar]
- 28.Zainal Ariffin SH, et al. Intrinsic anticarcinogenic effects of Piper sarmentosum ethanolic extract on a human hepatoma cell line. Cancer Cell Int. 2009;9:6–6. doi: 10.1186/1475-2867-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zoubiri S, Baaliouamer A. Potentiality of plants as source of insecticide principles. J. Saudi Chem. Soc. 2014;18:925–938. doi: 10.1016/j.jscs.2011.11.015. [DOI] [Google Scholar]
- 30.Chansang U, et al. Mosquito larvicidal activity of aqueous extracts of long pepper (Piper retrofractum vahl) from Thailand. J Vector Ecol. 2005;30:195–200. [PubMed] [Google Scholar]
- 31.Hematpoor A, et al. Inhibition and Larvicidal Activity of Phenylpropanoids from Piper sarmentosum on Acetylcholinesterase against Mosquito Vectors and Their Binding Mode of Interaction. PLoS ONE. 2016;11:e0155265. doi: 10.1371/journal.pone.0155265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Intirach J, et al. Chemical Constituents and Combined Larvicidal Effects of Selected Essential Oils against Anopheles cracens (Diptera: Culicidae) Psyche. 2012;2012:11. [Google Scholar]
- 33.Chaithong U, et al. Larvicidal effect of pepper plants on Aedes aegypti (L.) (Diptera: Culicidae) Journal of Vector Ecology. 2006;31:138–144. doi: 10.3376/1081-1710(2006)31[138:LEOPPO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 34.Shaaya E, Kostjukovski M, Eilberg J, Sukprakarn C. Plant oils as fumigants and contact insecticides for the control of stored-product insects. J. Stored Prod. Res. 1997;33:7–15. doi: 10.1016/S0022-474X(96)00032-X. [DOI] [Google Scholar]
- 35.Koul O. Biological activity of volatile di-n-propyl disulfide from seeds of neem, Azadirachta indica (Meliaceae), to two species of stored grain pests, Sitophilus oryzae (L.) and Tribolium castaneum (Herbst) J. Econ. Entomol. 2004;97:1142–1147. doi: 10.1093/jee/97.3.1142. [DOI] [PubMed] [Google Scholar]
- 36.Huang Y, Tan JMWL, Kini RM, Ho SH. Toxic and antifeedant action of nutmeg oil against Tribolium castaneum (Herbst) and Sitophilus zeamais Motsch. J. Stored Prod. Res. 1997;33:289–298. doi: 10.1016/S0022-474X(97)00009-X. [DOI] [Google Scholar]
- 37.Muhamad R, Dzolkhifli O. Evaluation of three bioassays for detecting resistance in cocoa mirid, Helopeltis theivora. Resistant Pest Management Newsletter. 1995;7:18–19. [Google Scholar]
- 38.Busvine, J. R. Recommended methods for measurement of pest resistance to pesticides. (Food and Agriculture Organization of the United Nations, 1980).
- 39.Kim S-I, Park C, Ohh M-H, Cho H-C, Ahn Y-J. Contact and fumigant activities of aromatic plant extracts and essential oils against Lasioderma serricorne (Coleoptera: Anobiidae) J. Stored Prod. Res. 2003;39:11–19. doi: 10.1016/S0022-474X(02)00013-9. [DOI] [Google Scholar]
- 40.Tripathi AK, Prajapati V, Kumar S. Bioactivities of l-carvone, d-carvone, and dihydrocarvone toward three stored product beetles. J. Econ. Entomol. 2003;96:94–1601. doi: 10.1093/jee/96.3.990. [DOI] [PubMed] [Google Scholar]
- 41.Shukla E, Thorat LJ, Nath BB, Gaikwad SM. Insect trehalase: Physiological significance and potential applications. Glycobiology. 2015;25:357–367. doi: 10.1093/glycob/cwu125. [DOI] [PubMed] [Google Scholar]
- 42.Asano N. Glycosidase inhibitors: update and perspectives on practical use. Glycobiology. 2003;13:93R–104R. doi: 10.1093/glycob/cwg090. [DOI] [PubMed] [Google Scholar]
- 43.Ouellette, R. J. & Rawn, J. D. Organic Chemistry: Structure, Mechanism, and Synthesis. (Elsevier Science, 2014).
- 44.Bhardwaj A, et al. Larvicidal and Structure–Activity Studies of Natural Phenylpropanoids and Their Semisynthetic Derivatives against the Tobacco Armyworm Spodoptera litura (Fab.) (Lepidoptera: Noctuidae) Chem. Biodivers. 2010;7:168–177. doi: 10.1002/cbdv.200800345. [DOI] [PubMed] [Google Scholar]
- 45.Nair VP, Hunter JM. Anticholinesterases and anticholinergic drugs. Contin. Educ. Anaesth. Crit. Care Pain. 2004;4:164–168. doi: 10.1093/bjaceaccp/mkh045. [DOI] [Google Scholar]
- 46.Massoulie J, Bon S. The Molecular Forms of Cholinesterase and Acetylcholinesterase in Vertebrates. Ann. Rev. Neurosci. 1982;5:57–106. doi: 10.1146/annurev.ne.05.030182.000421. [DOI] [PubMed] [Google Scholar]
- 47.Arpagaus M, Fournier D, Toutant J-P. Analysis of acetylcholinesterase molecular forms during the development of Drosophila melanogaster. Evidence for the existence of an amphiphilic monomer. Insect Biochem. 1988;18:539–549. doi: 10.1016/0020-1790(88)90005-4. [DOI] [Google Scholar]
- 48.Tanimori S, Watanabe K, Kirihata M. Synthesis of cinnamyl-sesamol derivatives. Res. Chem. Intermed. 2009;35:909–917. doi: 10.1007/s11164-009-0073-1. [DOI] [Google Scholar]
- 49.Santos BVdO, Chaves EVLd-CMCdO, Gray AI. Phenylalkanoids from piper Marginatumfn2. Phytochemistry. 1998;49:1381–1384. doi: 10.1016/S0031-9422(98)00100-9. [DOI] [Google Scholar]
- 50.Patra A, Mitra AK. Constituents of Acorus calamus: Structure of Acoramone. Carbon-13 NMR Spectra of Cis- and Trans-Asarone. J. Nat. Prod. 1981;44:668–669. doi: 10.1021/np50018a007. [DOI] [Google Scholar]
- 51.Abbott WS. A Method of Computing the Effectiveness of an Insecticide. J. Econ. Entomol. 1925;18:265–267. doi: 10.1093/jee/18.2.265a. [DOI] [Google Scholar]
- 52.Islam MS, Talukder FA. Toxic and residual effects of Azadirachta indica, Tagetes erecta and Cynodon dactylon seed extracts and leaf powders towards Tribolium castaneum. JPDP. 2005;112:594–601. [Google Scholar]
- 53.Tapondjou LA, Adler C, Bouda H, Fontem DA. Efficacy of powder and essential oil from Chenopodium ambrosioides leaves as post-harvest grain protectants against six-stored product beetles. J. Stored Prod. Res. 2002;38:395–402. doi: 10.1016/S0022-474X(01)00044-3. [DOI] [Google Scholar]
- 54.Chudasama JA, Sagarka NB, Sharma S. Deterrent effect of plant extracts against Callosobruchus maculatus on stored cowpea in Saurashtra (Gujarat, India) IJANS. 2015;7:187–191. [Google Scholar]
- 55.Holloway GJ. The potency and effect of phytotoxins within yellow split-pea (Pisum sativum) and adzuki bean (Vigna angularis) on survival and reproductive potential of Sitophilus oryzae (L.) (Coleoptera: Curculionidae) Bulletin of Entomological Research. 1986;76:287–295. doi: 10.1017/S0007485300014759. [DOI] [Google Scholar]
- 56.WHO. Techniques to detect insecticide resistance mechanisms (field and laboratory manual) 42 (World Health Organization, Geneva, Switzerland, 1998).
- 57.Ellman GL, Courtney KD, Andres V, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 58.Swale, D. R. et al. Inhibitor profile of bis(n)-tacrines and N-methylcarbamates on acetylcholinesterase from Rhipicephalus (Boophilus) microplus and Phlebotomus papatasi. Pest Biochem. Physiol. 106, 10.1016/j.pestbp.2013.1003.1005, 10.1016/j.pestbp.2013.03.005 (2013). [DOI] [PMC free article] [PubMed]
- 59.Finney, D. J. Probit analysis. (Cambridge, England: Cambridge University Press, 1971).
- 60.Kljajić P, Perić I. Effectiveness of wheat-applied contact insecticides against Sitophilus granarius (L.) originating from different populations. J. Stored Prod. Res. 2007;43:523–529. doi: 10.1016/j.jspr.2007.03.001. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.