Abstract
In all vertebrates studied so far, germ cells are not required for pubertal maturation of the gonadal steroidogenic system, subsequent development of secondary sex characteristics and reproductive behavior. To explore if the absence of germ cells affects puberty or growth in Atlantic salmon, germ cell-free (GCF), dnd knockout and wild type (WT) postsmolts were stimulated to enter puberty. No GCF fish entered puberty, whereas 66.7% (males) and 30% (females) WT fish completed or entered puberty, respectively. Expression of genes related to steroidogenesis (star, cyp17a1, cyp11β, cyp19a1a), gonadal somatic cells (insl3, amh, igf3), oocytes (bmp15), gonadotropin receptors (fshr, lhcgr), and pituitary gonadotropic cells (fshb, lhb, gnrhr4) showed an immature status and failure to up-regulate gonadal sex steroid production in male and female GCF fish was also reflected in low or undetectable plasma sex steroids (11-ketotestosterone, estradiol-17β and testosterone). A gender difference (high in females, low in males) was found in the expression of star and cyp17a1 in GCF fish. No clear difference in growth was detected between GCF and immature WT fish, while growth was compromised in maturing WT males. We demonstrate for the first time in a vertebrate that germ cells are required for pubertal activation of the somatic steroidogenic cells.
Introduction
Teleost fish share many homologies with other vertebrates, including the basic building blocks of the endocrine system regulating reproductive processes via the brain-pituitary-gonad (BPG) axis1–3. Considering gonadal functions at the molecular level, sex steroid hormones and growth factors regulate germ cell maturation but also exert feedback effects on the brain/pituitary level. The gonadal feedback is merged with information from other internal (e.g. nutritional or health status) and external (e.g. photoperiod, temperature, behavior of conspecifics) sources into an integrated output to control the synthesis and release of two pituitary gonadotropins, follicle-stimulating hormone (Fsh) and luteinizing hormone (Lh). However, it is uncertain how different cell types in the gonad communicate during the initiation of puberty. Germ cell-free (GCF) mice still up-regulate gonadal steroid hormone production during puberty4 and GCF fish display secondary sex characteristics5–7, suggesting that germ cells are not essential for gonadal sex steroid production and the subsequent development of secondary sex characteristics. To our knowledge, there are no studies in vertebrates showing a link between germ cells and the pubertal up-regulation of steroidogenic activity.
Gonadectomy studies in tilapia have shown that the gonads exert growth promoting effects8. While it is known that Sertoli cells – a somatic cell type in the testis - express growth hormone (Gh) in both tilapia and Japanese eel, studies in tilapia have shown that growth hormone receptors (ghr) are expressed by germ cells8,9, and might respond to Gh by producing Igf family members10. Moreover, sex steroid hormone signaling may be involved in this growth promoting effect of the gonads8. However, it is not clear if Gh-producing Sertoli cells or Igf-producing germ cells, or the joint activity of these cell types are responsible for the growth promoting effects of the gonads. The GCF Atlantic salmon model11, obtained by knocking out dead end (dnd), a well-known germ cell-specific gene in fish11–16, appears to be an excellently suited model to investigate the role of germ to soma communication regarding both gonadal sex steroid production and growth promoting effects.
It is yet unknown if germ cells affect both growth and maturation in salmon. To elucidate this, we induced maturation in one year old wild-type (WT) and GCF Atlantic salmon11,17,18, and subsequently followed growth and maturation in these fish for one full year in a common garden experiment, to study potential effects of the absence of germ cells on growth and pubertal maturation. Unexpectedly, sexual maturation was observed exclusively in WT fish, and the pubertal up-regulation sex steroid production was blocked in both female and male GCF salmon. Growth was similar in sterile mutant and immature WT fish of both sexes, but was as expected compromised in maturing WT males.
Results and Discussion
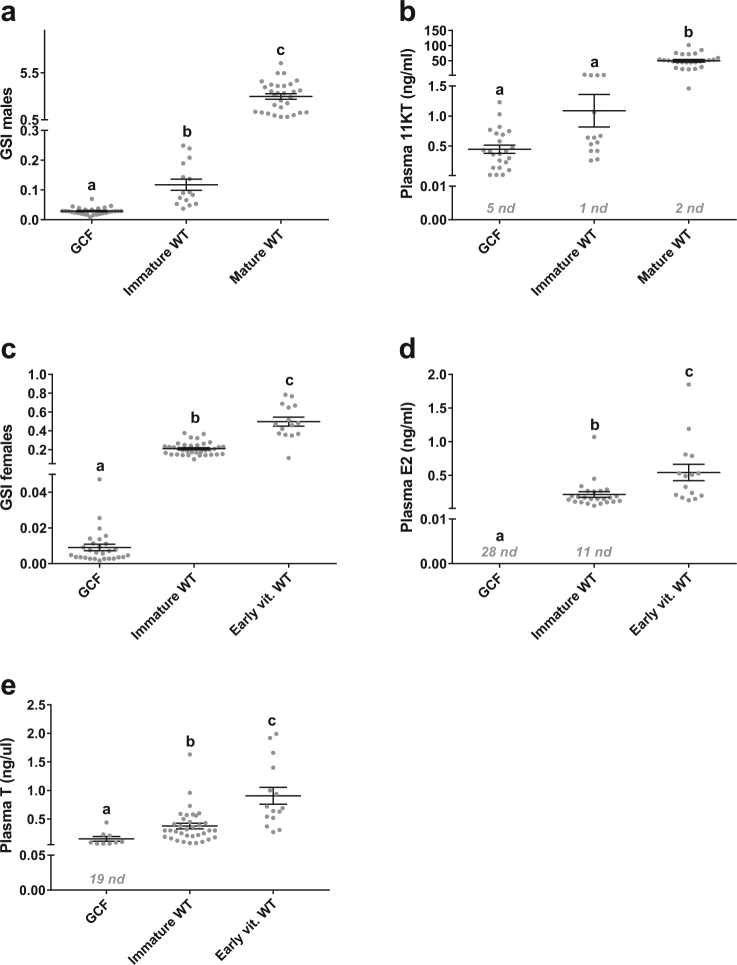
Complete loss of germ cells in all vertebrates studied so far resulted in sterility, while other gonadal functions, such as sex steroid production, remain intact4, followed by development of secondary sex characteristics5–7. We have explored the phenotype of dnd knockout and hence GCF, sterile male salmon11, exposed to an established postsmolt maturation regime, usually inducing sexual maturation in 50–90% of the males in different families of Atlantic salmon17–19. In our study, 66.7% of the WT males (n = 45) but none of the GCF males (n = 28) were maturing in response to a maturation-inducing regime. Furthermore, we included females in the assessment of puberty. Although salmon females subjected to the postsmolt maturation regime are not recruited into the accelerated gonadal growth that is evident in males, 30% of the WT females (n = 50) responded to the maturation regime by showing a more advanced (early vitellogenic) stage of ovarian development associated with elevated GSI and plasma E2 levels (Fig. 1c,d). While these more advanced females may have matured earlier than females not subjected to the postsmolt maturation-inducing regime, full maturation did not occur during the experiment, in contrast to the observation made in males. Full female maturation is potentially too demanding metabolically to be triggered at this body size. In any case, none of the GCF females (n = 28) showed any sign of maturation (Fig. 1c,d).
Figure 1.
GSI and plasma sex steroid levels in GCF and WT salmon. Gonadosomatic index (GSI: (gonad weight/body weight) × 100) in Atlantic salmon males (a) and females (c), plasma 11-ketotestosterone (11-KT) in males (b) and plasma estradiol-17β (E2) (d) and testosterone (T) in females (e) at the final sampling Feb 2nd 2016. Data are shown as mean with SEM, n = 28 (GCF), 35 (Immature WT females), 15 (Early vit. WT), 15 (Immature WT males) and 28–30 (Mature WT). Significant differences between groups are indicated by different letters. GCF; germ cell-free, vit; vitellogenic, WT; wild type, nd; not detected (nd = 0.01 ng/ml (11-KT and E2), nd = 0.05 ng/ml (T)).
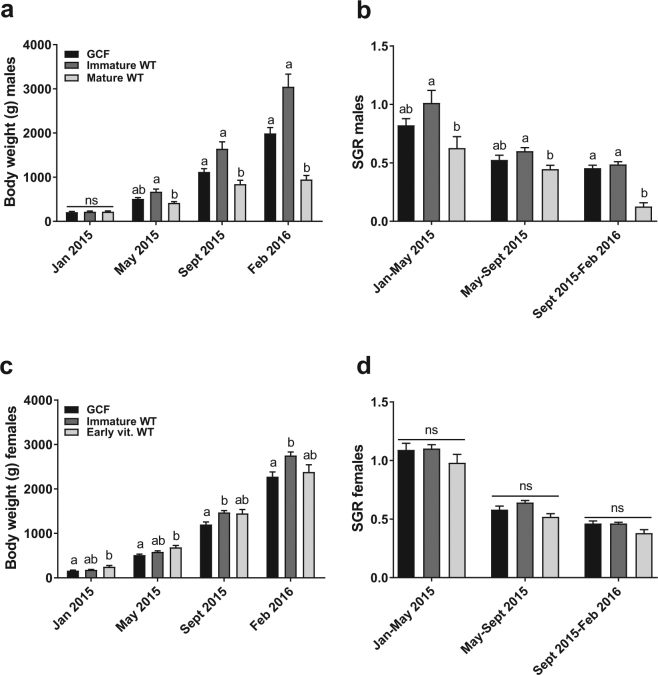
In all GCF males, the plasma concentration of 11-KT was low or undetectable before the start of the maturation regime and did not increase during the experiment (Fig. 1b, Supplementary Figure S1). The low but detectable 11-KT levels in GCF males may reflect the activity of an extratesticular source, which previously has been suggested for castrated salmon20. While 11-KT levels did not differ between immature WT and GCF males, GSI levels were significantly higher in immature WT compared to GCF males (Fig. 1a), probably reflecting the lack of germ cells in GCF fish. It has been observed in tilapia that surgical removal of the gonads reduced somatic growth8. We observed a bimodal growth pattern in salmon WT males, where maturing individuals grew significantly slower than their immature counterparts (Fig. 2a,b). This finding agrees with previous observations that growth was compromised in maturing salmon21. GCF males grew faster than the maturing WT males and at the same rate as the immature WT group (Fig. 2a,b). The latter observations agree with findings in zebrafish, where GCF (dnd-knockdown) males had the same body weight as control males22. There was, however, a non-significant tendency of increased growth in the immature WT compared to the GCF salmon males from January to May. Investigating a larger group of fish may clarify if GCF salmon males grow slower than immature WT males.
Figure 2.
Growth of GCF and WT salmon. Growth of Atlantic salmon males (a,b) and females (c,d) shown as body weight (a,c) and specific growth rate (SGR) (b,d) throughout one year. Data are shown as mean with SEM, n = 21–28 (GCF), 34–35 (Immature WT females), 15 (Early vit. WT), 15 (Immature WT males) and 26–30 (Mature WT). Significant differences between groups are indicated by different letters. ns; not significant, GCF; germ cell-free, vit; vitellogenic, WT; wild type.
The initiation of puberty in females is accompanied by an increase in pituitary fshb, plasma E2 and a transition from the “oildrop” immature stage to the beginning of vitellogenesis, characterized by primary yolk vesicles in the ovary23. Histological analysis revealed both immature (Fig. 3a; 70%) and early vitellogenic (Fig. 3b; 30%) females in the WT fish exposed to the maturation regime. In the WT group, early vitellogenic females showed higher E2 compared to immature animals, while both WT groups had higher E2 levels than the GCF females (Fig. 1d). A similar pattern was observed regarding the GSI, which was low in GCF females, higher in the immature WT group and highest in the early vitellogenic WT fish (Fig. 1c). This shows that a significant proportion of the WT females were entering puberty in response to the maturation regime, while none of the GCF females showed any signs of puberty. Histological analysis of GCF ovaries showed that the GCF stromal tissue contained cell groups with a distinct morphology (Fig. 3c,d). These cells seemed to form small glands. A single nucleolus was present in the large and round nucleus, which was oriented towards the basal compartment of the cell. Much of the cytoplasmic volume was occupied by small vacuoles (Fig. 3d). Overall, the morphology of the cells is reminiscent of hyperplastic adrenal steroidogenic cells24. We assume that these cells are derived from the steroidogenic theca cells in the salmon ovary.
Figure 3.
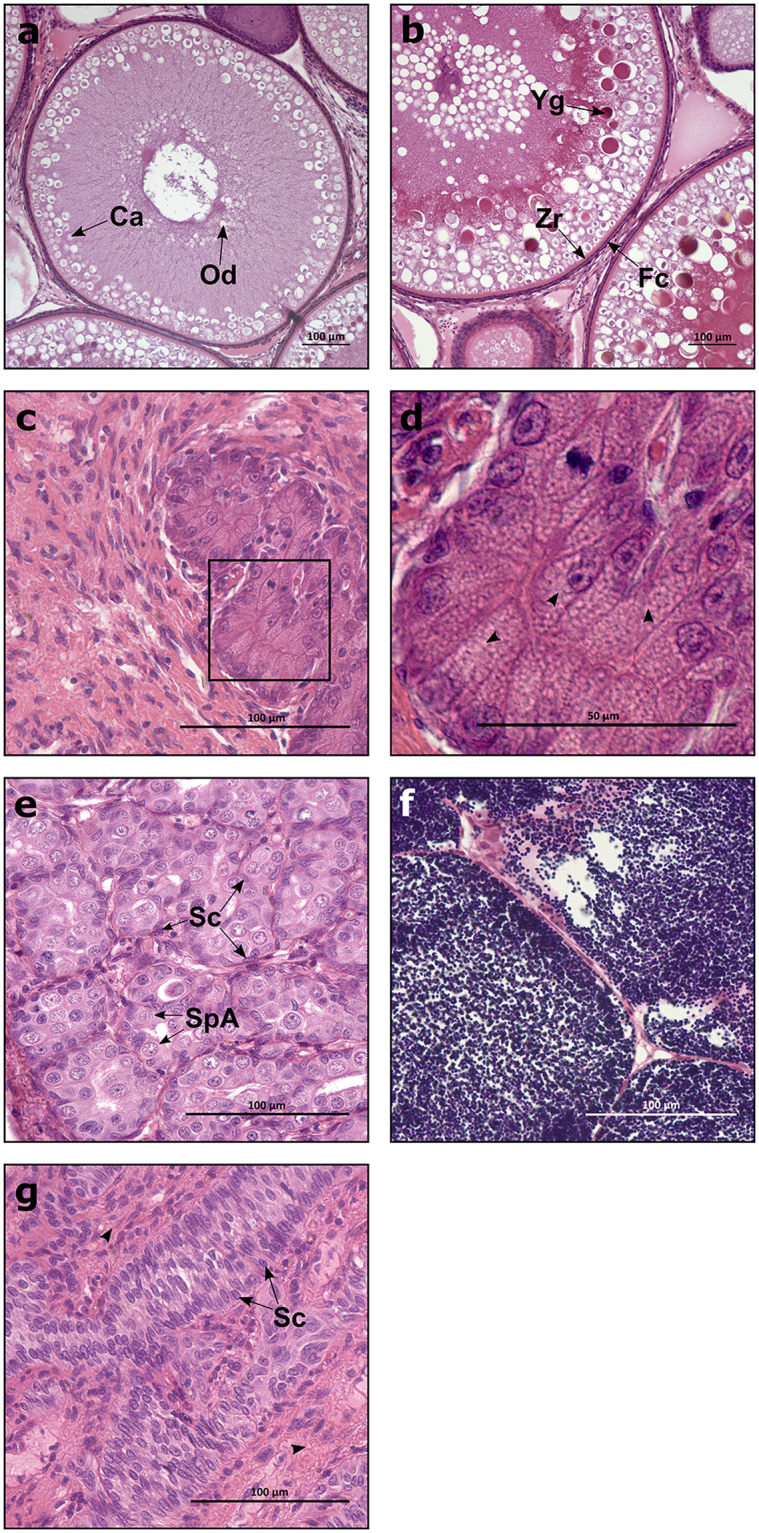
Gonadal tissue from GCF and WT salmon. Histological images of Atlantic salmon ovaries (a–d) and testis (e–g). (a) Immature (oildrop stage) oocytes. (b) Early vitellogenic oocytes. (c) GCF ovary. (d) GCF ovary with lipid vacuoles (arrowheads), magnified from the marked area in (c). (e) Immature testis. (f) Mature testis containing mostly spermatozoa. (g) GCF testis with interstitial area (arrowheads). GCF, germ cell-free; Ca, cortical alveoli; Od, oil drop; Yg, yolk granule; Zr, zona radiata; Fc, follicle cell; SpA, spermatogonia A; Sc, Sertoli cell. Scale bar = 100 µm (a–c, e–g), 50 µm (d).
Few studies have investigated the effects of the absence of germ cells on body growth in female fish. In Mozambique tilapia (Oreochromis mossambicus) females, gonadectomy retarded somatic growth8. Loss of germ cells due to heat treatment of Nile tilapia (Oreochromis niloticus) augmented growth in females, however, it was not clear whether granulosa cells had been affected or not25. This seems relevant because an increase in temperature induced apoptosis in granulosa cells in mammals26, which may result in secondary germ cell loss. In the present experiment, body weight was measured at 4 time points over a 1.5-year period, with fish size increasing from around 50 to 2500 g. The early vitellogenic WT were heavier than the GCF females at the start of the experiment (Fig. 2c). Over time, this weight difference disappeared, although the immature WT group became heavier than GCF females (Fig. 2c). Nevertheless, no significant differences were detected in SGR between any female groups at any time point in this study (Fig. 2d), suggesting that there are no negative effects on growth in GCF salmon females. Since we could not follow the female fish into full maturity in the present study, we cannot exclude a growth effect at later stages. However, in commercial production, females are harvested before rapid ovarian growth starts. Therefore, we do not expect a negative effect on growth due to loss of germ cells.
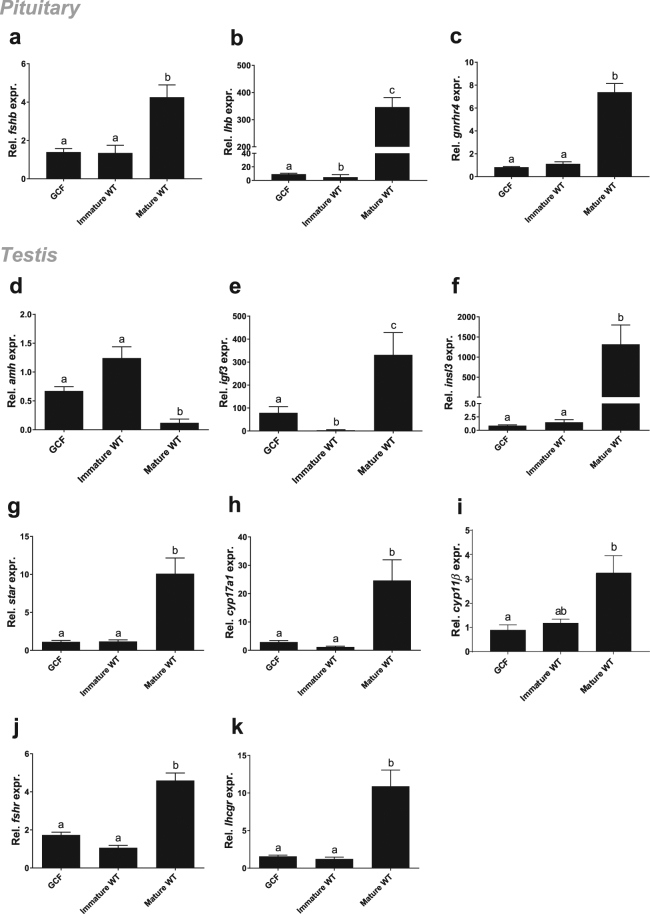
What molecular signature accompanies the inability of gonadal tissue in GCF fish to activate sex steroid production? To pursue this question, we analysed the expression of selected genes in both gonad and pituitary samples from GCF and WT fish. In testis tissue, we measured transcript levels of anti-müllerian hormone (amh, Fig. 4d) and insulin-like growth factor 3 (igf3, Fig. 4e), as these are Sertoli cell factors and markers of immature and maturing testis, respectively27. In GCF testis, amh was expressed at the same level as in immature WT males, indicating an immature status of the Sertoli cells in the GCF testis (Fig. 4d). The igf3 transcript levels were significantly higher in GCF testis compared to immature WT, but lower than in mature WT (Fig. 4e). This may indicate that Sertoli cells in GCF males show some degree of maturation. Sertoli cells were rather abundant in GCF testis and Sertoli cell number in the GCF tubuli seemed to increase with time, comparing the morphology of the tubuli between 1 and 2.5-year-old GCF males (Fig. 3g 11). Since Igf3 also stimulated Sertoli cell proliferation in zebrafish28, elevated Igf3 signalling might contribute to an autocrine loop stimulating Sertoli cell proliferation, leading to Sertoli cell-rich but germ cell-free tubuli (Fig. 3g). Up-regulation of igf3 in maturing compared to immature WT males seems to be in line with reports in zebrafish that Fsh-stimulated Igf3 release by Sertoli cells promotes spermatogenesis29. Further, we measured the expression of insulin-like peptide 3 (insl3), which is a Leydig cell-derived stimulator of spermatogonial differentiation in zebrafish30. Moreover, insl3 transcript levels are exquisitely sensitive to Fsh in zebrafish31. The transcript level of insl3 in GCF testes was low and not different from immature WT, but was up-regulated in mature WT testes. This suggests that both immature and GCF males experience low Fsh stimulation, in strong contrast to mature males (Fig. 4f).
Figure 4.
Gene expression in pituitary and testis from GCF and WT males. Expression of fshb (a), lhb (b), gnrhr4 (c), amh (d), igf3 (e), insl3 (f), star (g), cyp17a1 (h), cyp11β (i), fshr (j), and lhcgr (k) relative to ef1a, in Atlantic salmon male pituitaries (a–c) and testes (d–k), measured by qPCR. Data are shown as mean with SEM, n = 21–22 (GCF), 8–10 (Immature WT) and 8–9 (Mature WT). Significant differences between groups are indicated by different letters. GCF; germ cell-free, WT; wild type.
To investigate if changes in the expression of steroidogenesis-related genes could explain the low 11-KT plasma levels in GCF males, we measured steroidogenic acute regulatory protein (star), cytochrome p450 17a1 (cyp17a1) and 11-beta-hydroxylase (cyp11β) transcript levels in testis tissue. StAR facilitates the rapid, gonadotropin-stimulated translocation of cholesterol to the inner mitochondrial membrane in steroidogenic cells, where the rate-limiting step of steroid production takes place, cholesterol side-chain cleavage32,33. Cyp17a is involved in converting i) pregnenolone to 17-hydroxypregnenolone, ii) progesterone to 17α-hydroxyprogesterone and iii) 17α-hydroxyprogesterone to androstenedione34, an essential step towards androgen production. Cyp11β is essential for the conversion of testosterone but predominantly androstenedione35 to 11beta-hydroxylated androgens36. The transcript level of star was similarly low in GCF and immature males but clearly up-regulated in mature WT testis tissue (Fig. 4g). The changes in cyp17a1 and cyp11β transcript levels showed patterns similar to that of star (Fig. 4h,i). The lower levels of star, cyp17a1 and cyp11β suggest that expression of two key genes in androgen biosynthesis, and the gene required for 11beta-hydroxylation of androgens, failed to be up-regulated in GCF testis. This is in line with low 11-KT plasma levels found in GCF males. Moreover, analysis of pituitary transcripts fshb, lhb and gnrhr4 revealed low levels in GCF and immature males, which became significantly up-regulated in mature WT males (Fig. 4a–c). The same pattern emerged from quantifying fshr and lhcgr transcripts in testis tissue (Fig. 4j,k). At present, we have no explanation for the significantly lower lhb expression in the pituitary of immature WT compared to GCF fish (Fig. 4b). Taken together, this data set suggests that GCF testes fail to produce a signal that seems required to enable the brain-pituitary system to respond to the stimulatory photoperiod/temperature cues. Consequently, pituitary sensitivity to Gnrh and testicular sensitivity to gonadotropins remain in a prepubertal status. In the absence of data on circulating gonadotropins, we can infer from our data on testicular transcript levels that a gonadotropic stimulation did not occur in GCF males. In this regard, it will be interesting to study in future experiments for example, if the GCF testis can respond to exogenous gonadotropin.
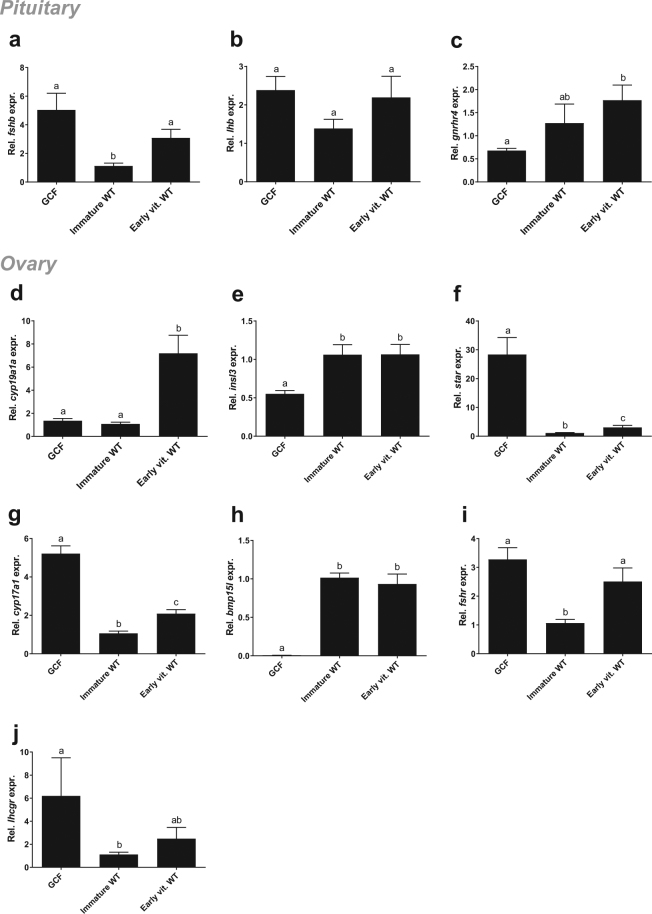
In ovarian tissue, we measured the expression of selected genes expressed by somatic cells, including granulosa cells, aromatase (cyp19a1a), required for estrogen production in the ovary37. As expected (Fig. 1d), cyp19a1a was significantly higher expressed in early vitellogenic WT ovaries compared to both GCF and immature WT ovaries (Fig. 5d), indicating an immature status of the GCF ovary. Furthermore, we measured the ovarian mRNA level of insl3, which was significantly lower in GCF ovaries compared to both immature and early vitellogenic WT ovaries (Fig. 5e). Limited information exists on Insl3 function in fish ovaries, however, in mammals INSL3 may have a regulatory role in maintaining thecal androgen production38. In contrast to the GCF testis, the GCF ovary displayed clearly elevated transcript levels of star and cyp17a1, suggesting that substrate availability for the cholesterol side-chain cleavage and the production of androstenedione were not limiting factors (Fig. 5f,g), implying that an initial and an intermediate step of the steroidogenic pathway towards estrogen production were not inhibited in GCF females. We were not able to measure androstenedione, which is a precursor of both testosterone and estrogen, however, we were able to measure testosterone that, similar to E2 (Fig. 1d), was found to be low or undetected in GCF females (Fig. 1e). Elevated transcript levels of fshb (Fig. 5a) and its receptor fshr (Fig. 5i), in conjunction with elevated levels of steroidogenesis-related genes star and cyp17a1 (Fig. 5f,g) would all be in line with a high level of gonadotropic stimulation of the GCF ovaries. Female mice devoid of germ cells due to knockout of dazl, showed increased levels of plasma Fsh4, pointing at a conserved trait between salmon and mouse. Moreover, elevated circulating gonadotropin levels are characteristic of postmenopausal primates, where the germ cell-depleted ovary is unable to sustain estrogen production39. In coho salmon, Fsh stimulated the expression of star and cyp17a1, while cyp19a1a remained unaffected40. Likewise, we observed no upregulation of cyp19a1a in our mutants compared to immature WT fish. Although Fsh up-regulated E2 release via a cAMP-dependent mechanism in brook trout41, this does not necessarily involve an increase in cyp19a1a transcript levels. In our study, the very low E2 plasma levels in GCF salmon females suggest that the potentially elevated circulating Fsh levels could neither induce a noticeable E2 production, probably due to the very low aromatase activity, nor that an up-regulation of cyp19a1a took place. In the GCF ovary, glandular structures were observed that were composed of cells showing small lipid vacuoles in their cytoplasm (Fig. 3c,d), a feature typical of steroidogenic cells. However, the high number of these vacuoles is unusual and may reflect a strong gonadotropic stimulation. An accumulation of these lipid droplets has also been observed in steroidogenic cells of the adrenal cortex in genetically modified mice showing a high level of ACTH stimulation42. However, in this model the star gene was missing while GCF ovaries show elevated star transcript levels that despite the also elevated cyp17a1 transcript levels, still did not result in elevated androgen production. Hence, unravelling the exact mechanism responsible for the steroidogenic failure in the GCF ovary requires further research.
Figure 5.
Gene expression in pituitary and ovary from GCF and WT females. Expression of fshb (a), lhb (b), gnrhr4 (c), cyp19a1 (d), insl3 (e), star (f), cyp17a1 (g), bmp15l (h), fshr (i) and lhcgr (j) relative to ef1a, in Atlantic salmon female pituitaries (a–c) and ovaries (d–j), measured by qPCR. Data are shown as mean with SEM, n = 21–24 (GCF), 9–10 (Immature WT) and 8–10 (Vitellogenic WT). Significant differences between groups are indicated by different letters. GCF; germ cell-free, vit; vitellogenic, WT; wild type.
The data presented so far suggest that in GCF ovaries, part of the steroidogenic system operates at an elevated level of activity, contrasted by low ovarian aromatase expression and very low E2 plasma levels. Considering germ to somatic cell signaling, however, a possible explanation for low cyp19a1a transcript levels could be the lack of communication, for example via the oocyte-derived Tgf-β family member Bone morphogenetic protein 15 (Bmp15) to surrounding somatic cells. In zebrafish ovaries, loss of Bmp15 resulted in a failure to up-regulate cyp19a1a transcript levels in granulosa cells43. In GCF salmon ovaries, the transcript level of bmp15l was drastically reduced compared to both immature and early vitellogenic WT ovaries (Fig. 5h), suggesting that the germ cells are the main source of this growth factor in salmon ovaries. Thus, the strong reduction in Bmp15 signalling in GCF ovaries may be responsible for the failure to up-regulate cyp19a1a expression, as shown in zebrafish43. These authors moreover suggested that the remaining cyp19a1a expression in bmp15 −/− zebrafish mutants is located in thecal cells, which may have aggregated in the gland-like structures found in the GCF ovaries. Expression of lhb in female pituitaries did not differ between any groups (Fig. 5b), which suggests that there is no feedback mechanism from the GCF ovary. The lhcgr expression in ovaries showed a similar profile, although the GCF group had significantly higher expression than the immature WT group (Fig. 5j). Nevertheless, the GCF group showed a high variation, which calls for a higher n to conclude. Pituitary transcript level of gnrhr4 in females was lowest in the GCF group, and significantly higher in the early vitellogenic group (Fig. 5c). Since the presence of sex steroids are involved in the regulation of gnrhr4 18,44, it is expected that the GCF group, which had low or undetectable levels of E2 (Fig. 1d) and T (Fig. 1e), would have the lowest level of gnrhr4. Taken together, the levels of molecular markers measured in GCF females in this study support that these animals are immature, and that the steroidogenic pathway is inhibited.
There is a clear gender difference in the size and content of gonadal somatic cells, in addition to the presence (males) or absence (females) of the sdY gene45,46, in GCF salmon. The GCF testis showed a high number of Sertoli cells, while the GCF ovary has almost no distinguishable follicular cells in 2.5 years old females, although granulosa-like cells were observed in 1 year old GCF females11. This finding implies a sex-dependent difference in the capacity of the GCF gonad to adhere to its morphogenetic program: the GCF testis still shows spermatogenic tubuli though they are devoid of germ cells and instead filled by Sertoli cells; also, the interstitial area shows no obvious deviations from wild-type immature testicular interstitium. In the GCF ovary, on the other hand, somatic structures like the thecal and granulosa layers evidently cannot form and instead glandular structures appear that are not known from wild type ovarian tissue. The latter, however, may represent an agglomeration of the special theca cells. Hence, in both sexes, the principle steroidogenic cells, special theca and Leydig cells, seem in their cellular identity to be independent of the presence of germ cells. Despite the survival of steroidogenic cells in both sexes, the up-regulation of sex steroid production usually observed during puberty fails to occur in males and is also missing with respect to E2 production in females. However, there are also sex-dependent differences in the response of the steroidogenic system to the GCF status. Finally, we observed no clear effect on body growth in GCF males or females, compared to their immature WT counterparts, while both showed a clear growth advantage compared to maturing wild type males.
Our study shows for the first time in a vertebrate, the Atlantic salmon, that the pubertal activation of gonadal androgen and estrogen production in testis and ovary requires the presence of germ cells. Furthermore, we demonstrate that in the absence of germ cells, there is a gender difference in the expression of some of the key genes (star and cyp17a1) involved in steroidogenesis. Future studies should investigate the molecular mechanisms that are causing the inhibition of steroidogenesis. Moreover, this study confirms our previous finding that the sex differentiation process is comparatively stable in salmon, compared to other fishes, since neither loss of germ cells nor the clear reduction in sex steroid release and the loss of recognizable somatic structures in the GCF ovary lead to a female to male sex change. Finally, body growth is not impaired by the loss of germ cells in salmon.
Materials and Methods
Experimental Design
The fish used in this study were reared and sampled at Matre Aquaculture Research Station, Matredal, Norway. GCF (dnd knockout) and WT fish were produced as described in Wargelius et al.11. The eggs used in the experiment were fertilized in Nov 2014, and juveniles were fed from April 2014 and reared under standard conditions until Oct 2014. From Oct 2nd 2014 fish were exposed to a 12 hours dark and 12 hours light regime for a period of 7 weeks, to ensure smoltification. Oct 23rd all fish were pit tagged and placed in common garden tanks with equal amounts of GCF and WT in each tank (11 mm Trovan ID 101 tags, BTS Scandinavia AB, Sweden). Nov 18th the fish were exposed to a continuous light regime and 16 °C to induce postsmolt maturation for a period of 4 months17. Subsequently, fish were transferred to sea water and kept at ambient temperature (~9 °C) and natural photoperiod until termination of the experiment (Feb 2nd 2016). Feeding was done with standard commercial diets. Prior to sampling for length, weight and plasma (Jan 20th (no plasma sampling), May 5th and Sept 25th 2015), the fish were anesthetized with 1 ml/L finquel vet. At the final sampling (Feb 2nd 2016), all fish were anesthetized with 2 ml/L finquel vet and sacrificed by cutting into the medulla oblongata (after sampling of blood). From the sampled fish, one complete gonad and the pituitary were immediately frozen in liquid nitrogen, and stored at −80 °C until further use for RNA isolation and subsequent qPCR analysis. The other gonad was fixed in Bouin’s fixative for 6–24 hours, then transferred into 70% ethanol, and further dehydrated and embedded in paraffin using a Histokinette (Leica TP1020).
Real-time, Quantitative PCR Assays
RNA was extracted from gonad and pituitary tissue using the iPrep™ Trizol® Plus RNA Kit (Thermo Fischer Scientific), according to the manufacturer’s instructions. Purified RNA was then treated with the turbo DNA-free kit (Ambion) to remove trace amounts of contaminating DNA. 500 ng of treated RNA served as input for reverse transcription reaction using the VILO cDNA synthesis kit (Invitrogen) for each sample. Previously published primers and hydrolysis probes specific for Atlantic salmon fshb, lhb 23, gnrhr4 18, fshr, lhcgr 47, igf3, amh 27, cyp19a1a 11 and bmp15l 48 were used in this study. Furthermore, primers and probe sequences for cyp17a1, star and cyp11β were designed online (http://probes.pw.usda.gov/batchprimer3/) and are listed in Supplementary Table S1. The primer sequences for insl3 (Supplementary Table S1) were designed using Primer Express 3.0 (Applied Biosystems); cloning and sequencing of salmon insl3 is described in Supplementary Note S1.
All qPCR assays were performed in duplicates, using 384-well optical plates on an ABI Prism 7700 Sequence Detection System (Applied Biosystems) (male pituitaries) or QuantStudio 5 Real-Time PCR System (ThermoFisher Scientific) (testis, ovaries and female pituitaries) using default settings. One µl of a 1/20 (gonads) or 1/40 dilution (pituitaries) of cDNA was used in a 10 µl Fast Taqman qPCR reaction (ThermoFisher Scientific). In the case of star, cyp17a1, cyp11β and insl3, 1 µl of a 1/160 dilution of cDNA was used in a 10 µl Fast SYBR green qPCR reaction (ThermoFisher Scientific). For each qPCR assay, melting-curve analysis showed that only one product was generated. For each PCR plate, no-template controls were run for each gene. The relative gene expression levels for all genes were calculated using the comparative Ct (or 2−ΔΔCt) method. For each gene, all values were normalized to ef1a and calibrated to the average ΔCt of the immature WT group.
Gonadosomatic Index and Specific Growth Rate
Gonadosomatic index (GSI) was determined as: GSI (%) = gonad weight (g) * 100/total body weight (g). Specific growth rate (SGR) was calculated as: SGR (%) = 100 * ((ln body weightstart − ln body weightend)/number of days.
Steroid Hormone Quantification
Sex steroids (11-ketotestosterone: 11-KT, testosterone: T, and estradiol-17β: E2) were analyzed with ELISA49 and validated for Atlantic salmon, on extracted plasma samples as detailed previously23.
Determination of Sex and Scoring of Sexual Maturity
To determine the sex of each GCF fish, the presence (males) or absence (females) of the sex determining gene sdY 45,46 was measured by PCR11. For the scoring of sexual maturity, all gonads were embedded in paraffin, sectioned and stained with hematoxylin, eosine and saffron (HES). The females were scored as immature (oildrop stage) or early vitellogenic based on histology (Fig. 3a,b), GSI and level of estradiol-17β (E2) (Supplementary Table S2). The males were scored as immature (Fig. 3e) or mature (Fig. 3f) based on GSI and level of 11-ketotestosterone (11-KT) (Supplementary Table S2).
Statistics
Statistical tests were performed using GraphPad Prism 7.02 (GraphPad Software Inc.). All datasets were tested for normal distribution using a D’Agostino & Pearson omnibus normality test. A difference between groups was considered significant when P < 0.05. A nonparametric Kruskal-Wallis with Dunn’s multiple comparisons test was applied to test for significant differences in the following datasets: GSI, plasma steroids, body weight and SGR. For the qPCR datasets, all values were log-transformed to obtain a more normal distribution. In cases where all groups to be compared passed the normality test, a one-way ANOVA with Tukey’s multiple comparisons test was applied on log-values to identify significant differences. In cases where one or more of the groups to be compared did not pass the normality test, we applied Kruskal-Wallis with Dunn’s multiple comparisons test on gene expression values.
Use of Experimental Animals
This experiment was approved by the Norwegian Animal Research Authority (NARA, permit number 5741) and the use of these experimental animals was in accordance with the Norwegian Animal Welfare Act.
Data Availability
The datasets generated and analysed during the current study are included in this published article (and its Supplementary Information Files).
Electronic supplementary material
Acknowledgements
We would like to thank Ivar Helge Matre and Lise Dyrhovden for rearing of the fish, and assisting during sampling. Sven Leininger for screening for dnd mutations in GCF fish and participation on samplings. Anne Torsvik for technical assistance with histological analyses and participation on samplings. Frida Thyri Segafredo for RNA isolation and cDNA synthesis. Grethe Thorsheim and Britt Dae Sværen for assistance during sampling. Jeanette Veivåg and Sara Olausson for performing plasma steroid measurements. This study was funded by the NRC BIOTEK2021 project SALMOSTERILE (221648).
Author Contributions
A.W., P.F., R.W.S., EA and R.B.E. designed the study. A.W., R.B.E., E.A., K.S., L.K., P.F. and R.W.S. carried out the samplings. P.F was responsible for the maturation-inducing rearing regime applied in this study. L.K. and K.S. set up qPCR assays and analyzed gene expression data. R.W.S., E.A., A.W. and L.K. analyzed histological sections of gonads. J.B cloned and sequenced the salmon insl3 cDNA, and designed the insl3 qPCR primers. B.N. was responsible for the measurements and analysis of plasma sex steroids. L.K., R.W.S. and A.W. wrote the manuscript with the contribution of all authors. All authors approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-12936-w.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zohar Y, Munoz-Cueto JA, Elizur A, Kah O. Neuroendocrinology of reproduction in teleost fish. Gen Comp Endocr. 2010;165:438–455. doi: 10.1016/j.ygcen.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 2.Levavi-Sivan B, Bogerd J, Mananos EL, Gomez A, Lareyre JJ. Perspectives on fish gonadotropins and their receptors. Gen Comp Endocr. 2010;165:412–437. doi: 10.1016/j.ygcen.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 3.Schulz RW, et al. Spermatogenesis in fish. Gen Comp Endocr. 2010;165:390–411. doi: 10.1016/j.ygcen.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 4.McNeilly JR, et al. Loss of oocytes in Dazl knockout mice results in maintained ovarian steroidogenic function but altered gonadotropin secretion in adult animals. Endocrinology. 2000;141:4284–4294. doi: 10.1210/endo.141.11.7764. [DOI] [PubMed] [Google Scholar]
- 5.Slanchev K, Stebler J, de la Cueva-Mendez G, Raz E. Development without germ cells: the role of the germ line in zebrafish sex differentiation. Proc Natl Acad Sci USA. 2005;102:4074–4079. doi: 10.1073/pnas.0407475102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujimoto T, et al. Sexual dimorphism of gonadal structure and gene expression in germ cell-deficient loach, a teleost fish. Proc Natl Acad Sci USA. 2010;107:17211–17216. doi: 10.1073/pnas.1007032107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu W, et al. Complete depletion of primordial germ cells in an All-female fish leads to Sex-biased gene expression alteration and sterile All-male occurrence. BMC genomics. 2015;16:971. doi: 10.1186/s12864-015-2130-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhatta S, et al. Gonads directly regulate growth in teleosts. P Natl Acad Sci USA. 2012;109:11408–11412. doi: 10.1073/pnas.1118704109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miura C, et al. Gh is produced by the testis of Japanese eel and stimulates proliferation of spermatogonia. Reproduction. 2011;142:869–877. doi: 10.1530/REP-11-0203. [DOI] [PubMed] [Google Scholar]
- 10.Le Gac F, Loir M, le Bail PY, Ollitrault M. Insulin-like growth factor (IGF-I) mRNA and IGF-I receptor in trout testis and in isolated spermatogenic and Sertoli cells. Mol Reprod dev. 1996;44:23–35. doi: 10.1002/(SICI)1098-2795(199605)44:1<23::AID-MRD3>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 11.Wargelius A, et al. Dnd knockout ablates germ cells and demonstrates germ cell independent sex differentiation in Atlantic salmon. Sci Rep. 2016;6:21284. doi: 10.1038/srep21284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duan J, et al. Germ cell-specific expression of dead end (dnd) in rare minnow (Gobiocypris rarus) Fish Physiol Biochem. 2015;41:561–571. doi: 10.1007/s10695-015-0029-x. [DOI] [PubMed] [Google Scholar]
- 13.Wang X, et al. The dnd RNA Identifies Germ Cell Origin and Migration in Olive Flounder (Paralichthys olivaceus) Biomed Res Int. 2015;2015:428591. doi: 10.1155/2015/428591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang X, Yue H, Ye H, Li C, Wei Q. Identification of a germ cell marker gene, the dead end homologue, in Chinese sturgeon Acipenser sinensis. Gene. 2015;558:118–125. doi: 10.1016/j.gene.2014.12.059. [DOI] [PubMed] [Google Scholar]
- 15.Li SZ, et al. Molecular characterization and expression pattern of a germ cell marker gene dnd in gibel carp (Carassius gibelio) Gene. 2016;591:183–190. doi: 10.1016/j.gene.2016.07.027. [DOI] [PubMed] [Google Scholar]
- 16.Sun ZH, et al. Sexual dimorphic expression of dnd in germ cells during sex reversal and its requirement for primordial germ cell survival in protogynous hermaphroditic grouper. Comp Biochem Physiol B Biochem Mol Biol. 2017;208-209:47–57. doi: 10.1016/j.cbpb.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 17.Fjelldal PG, Hansen T, Huang TS. Continuous light and elevated temperature can trigger maturation both during and immediately after smoltification in male Atlantic salmon (Salmo salar) Aquaculture. 2011;321:93–100. doi: 10.1016/j.aquaculture.2011.08.017. [DOI] [Google Scholar]
- 18.Melo MC, et al. Salinity and photoperiod modulate pubertal development in Atlantic salmon (Salmo salar) J Endocrinol. 2014;220:319–332. doi: 10.1530/JOE-13-0240. [DOI] [PubMed] [Google Scholar]
- 19.Imsland AK, Handeland SO, Stefansson SO. Photoperiod and temperature effects on growth and maturation of pre- and post-smolt Atlantic salmon. Aquacult Int. 2014;22:1331–1345. doi: 10.1007/s10499-014-9750-1. [DOI] [Google Scholar]
- 20.Lundqvist H, Berglund I, Mayer I, Borg B. Seawater Adaptability in Baltic Salmon, Salmo-Salar, Immature Smolt and Mature Male Parr - Lack of Effect of Springtime Castration. Can J Zool. 1990;68:2181–2184. doi: 10.1139/z90-302. [DOI] [Google Scholar]
- 21.Taranger GL, et al. Control of puberty in farmed fish. Gen Comp Endocr. 2010;165:483–515. doi: 10.1016/j.ygcen.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Wong TT, Zohar Y. Production of reproductively sterile fishby a non-transgenic gene silencing technology. Sci Rep. 2015;5:15822. doi: 10.1038/srep15822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andersson E, et al. Pituitary gonadotropin and ovarian gonadotropin receptor transcript levels: seasonal and photoperiod-induced changes in the reproductive physiology of female Atlantic salmon (Salmo salar) Gen Comp Endocr. 2013;191:247–258. doi: 10.1016/j.ygcen.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Bose HS, Sugawara T, Strauss JF, III, Miller WL. International Congenital Lipoid Adrenal Hyperplasia, C. The pathophysiology and genetics of congenital lipoid adrenal hyperplasia. N Engl J Med. 1996;335:1870–1878. doi: 10.1056/NEJM199612193352503. [DOI] [PubMed] [Google Scholar]
- 25.Pandit NP, Bhandari RK, Kobayashi Y, Nakamura M. High temperature-induced sterility in the female Nile tilapia, Oreochromis niloticus. Gen Comp Endocr. 2015;213:110–117. doi: 10.1016/j.ygcen.2015.01.028. [DOI] [PubMed] [Google Scholar]
- 26.Luo M, Li L, Xiao C, Sun Y, Wang GL. Heat stress impairs mice granulosa cell function by diminishing steroids production and inducing apoptosis. Mol Cell Biochem. 2016;412:81–90. doi: 10.1007/s11010-015-2610-0. [DOI] [PubMed] [Google Scholar]
- 27.Melo MC, et al. Androgens directly stimulate spermatogonial differentiation in juvenile Atlantic salmon (Salmo salar) Gen Comp Endocr. 2015;211:52–61. doi: 10.1016/j.ygcen.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 28.Morais RD, et al. Thyroid hormone stimulates the proliferation of Sertoli cells and single type A spermatogonia in adult zebrafish (Danio rerio) testis. Endocrinology. 2013;154:4365–4376. doi: 10.1210/en.2013-1308. [DOI] [PubMed] [Google Scholar]
- 29.Nobrega RH, et al. Fsh Stimulates Spermatogonial Proliferation and Differentiation in Zebrafish via Igf3. Endocrinology. 2015;156:3804–3817. doi: 10.1210/en.2015-1157. [DOI] [PubMed] [Google Scholar]
- 30.Assis LH, et al. INSL3 stimulates spermatogonial differentiation in testis of adult zebrafish (Danio rerio) Cell Tissue Res. 2016;363:579–588. doi: 10.1007/s00441-015-2213-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crespo D, Assis LH, Furmanek T, Bogerd J, Schulz RW. Expression profiling identifies Sertoli and Leydig cell genes as Fsh targets in adult zebrafish testis. Mol Cell Endocrinol. 2016;437:237–251. doi: 10.1016/j.mce.2016.08.033. [DOI] [PubMed] [Google Scholar]
- 32.Stocco DM. Tracking the role of a star in the sky of the new millennium. Mol Endocrinol. 2001;15:1245–1254. doi: 10.1210/mend.15.8.0697. [DOI] [PubMed] [Google Scholar]
- 33.Stocco DM. StAR protein and the regulation of steroid hormone biosynthesis. Annu Rev Physiol. 2001;63:193–213. doi: 10.1146/annurev.physiol.63.1.193. [DOI] [PubMed] [Google Scholar]
- 34.Chishti YZ, Feswick A, Martyniuk CJ. Progesterone increases ex vivo testosterone production and decreases the expression of progestin receptors and steroidogenic enzymes in the fathead minnow (Pimephales promelas) ovary. Gen Comp Endocr. 2014;199:16–25. doi: 10.1016/j.ygcen.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 35.Idler DR, Macnab HC. The biosynthesis of 11-Ketotestosterone and 11β-hydroxytestosterone by Atlantic salmon tissues in vitro. Can J Biochem. 1967;45:581–589. doi: 10.1139/o67-067. [DOI] [PubMed] [Google Scholar]
- 36.Baroiller, J. F. & Guiguen, Y. Endocrine and environmental aspects of sex differentiation in gonochoristic fish. Exs, 177–201 (2001). [DOI] [PubMed]
- 37.Kagawa H, Young G, Adachi S, Nagahama Y. Estradiol-17 beta production in amago salmon (Oncorhynchus rhodurus) ovarian follicles: role of the thecal and granulosa cells. Gen Comp Endocr. 1982;47:440–448. doi: 10.1016/0016-6480(82)90122-8. [DOI] [PubMed] [Google Scholar]
- 38.Glister C, et al. Functional link between bone morphogenetic proteins and insulin-like peptide 3 signaling in modulating ovarian androgen production. Proc Natl Acad Sci USA. 2013;110:E1426–1435. doi: 10.1073/pnas.1222216110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Broekmans FJ, Soules MR, Fauser BC. Ovarian aging: mechanisms and clinical consequences. Endocr Rev. 2009;30:465–493. doi: 10.1210/er.2009-0006. [DOI] [PubMed] [Google Scholar]
- 40.Luckenbach JA, Dickey JT, Swanson P. Follicle-stimulating hormone regulation of ovarian transcripts for steroidogenesis-related proteins and cell survival, growth and differentiation factors in vitro during early secondary oocyte growth in coho salmon. Gen Comp Endocr. 2011;171:52–63. doi: 10.1016/j.ygcen.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 41.Planas JV, Goetz FW, Swanson P. Stimulation of brook trout ovarian steroidogenesis by gonadotropins I and II is mediated by the cyclic adenosine 3′,5′-monophosphate/protein kinase A pathway. Biol Reprod. 1997;57:647–654. doi: 10.1095/biolreprod57.3.647. [DOI] [PubMed] [Google Scholar]
- 42.Miller WL, Bose HS. Early steps in steroidogenesis: intracellular cholesterol trafficking. J Lipid Res. 2011;52:2111–2135. doi: 10.1194/jlr.R016675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dranow DB, et al. Bmp15 Is an Oocyte-Produced Signal Required for Maintenance of the Adult Female Sexual Phenotype in Zebrafish. PLoS genet. 2016;12:e1006323. doi: 10.1371/journal.pgen.1006323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin CJ, et al. Regulation of two forms of gonadotropin-releasing hormone receptor gene expression in the protandrous black porgy fish. Acanthopagrus schlegeli. Mol Cell Endocrinol. 2010;323:137–146. doi: 10.1016/j.mce.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 45.Yano A, et al. An immune-related gene evolved into the master sex-determining gene in rainbow trout. Oncorhynchus mykiss. Curr Biol. 2012;22:1423–1428. doi: 10.1016/j.cub.2012.05.045. [DOI] [PubMed] [Google Scholar]
- 46.Eisbrenner WD, et al. Evidence for multiple sex-determining loci in Tasmanian Atlantic salmon (Salmo salar) Heredity (Edinb) 2014;113:86–92. doi: 10.1038/hdy.2013.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Andersson E, et al. Pharmacological characterization, localization and quantification of expression of gonadotropin receptors in Atlantic salmon (Salmo salar L.) ovaries. Gen Comp Endocr. 2009;163:329–339. doi: 10.1016/j.ygcen.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 48.Kleppe L, et al. bmp15l, figla, smc1bl, and larp6l are preferentially expressed in germ cells in Atlantic salmon (Salmo salar L.) Mol Reprod Dev. 2017;84:76–87. doi: 10.1002/mrd.22755. [DOI] [PubMed] [Google Scholar]
- 49.Cuisset B, et al. Enzyme-Immunoassay for 11-Ketotestosterone Using Acetylcholinesterase as Label - Application to the Measurement of 11-Ketotestosterone in Plasma of Siberian Sturgeon. Comp Biochem Phys C. 1994;108:229–241. doi: 10.1016/0300-9629(94)90089-2. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analysed during the current study are included in this published article (and its Supplementary Information Files).