Abstract
Zinc (Zn) is an essential trace element that plays important roles in the immune system. There is little known about the role of trace elements in allergic diseases, and previous reports have shown conflicting results. The aim of this study was to investigate the relationship between serum Zn levels and total or allergen-specific immunoglobulin E (IgE) levels. The initial candidates for this study were those who participated in the 5th Korean National Health and Nutrition Examination Survey 2010 (n = 8,958), and 1,867 adults who had serum total and allergen specific-IgE levels measured were included. Upon adjusting for covariates, mean total IgE, Dermatophagoides farinae and dog-specific IgE levels increased significantly as the Zn levels decrease from the highest to the lowest quartile (p = 0.009, 0.004, and < 0.001, respectively). The multiple logistic regression analyses showed significant negative linear correlations between serum Zn levels and total, D. farinae-, cockroach-, and dog-specific IgE levels (p-value for linear trend = 0.004, 0.006, 0.027, and < 0.001, respectively). This study demonstrated that total/allergen specific IgE and Zn levels are significantly inversely related.
Introduction
Type I hypersensitivity clinically manifests in a variety of allergic diseases such as atopic dermatitis, asthma, allergic rhinitis, allergic conjunctivitis, food allergies and specific types of urticaria1. Immunoglobulin E (IgE) has a central role in type I hypersensitivity, which reflects the sensitization of mast cells by allergen-specific IgE antibodies bound to their high-affinity receptors (FcεRI)2.
Zinc (Zn) is an essential trace element that plays important roles in the immune system, from contributing to the skin barrier to gene regulation in lymphocytes3. Zn is essential for FcεRI-mediated cytokine production in mast cell and transcription of IL-6 and TNF-α mRNA4. Zn is also known to function as an antioxidant and stabilize cell membranes3. Zn deficiency affects about 2.2 billion people around the world and as much as 25% of the world’s population is at risk5. A Korean study of 245 pregnant women showed that low Zn status is highly prevalent (76.3%)6. The clinical manifestations of Zn deficiency include alopecia, acrodermatitis enteropathica, diarrhea, emotional disorders, weight loss, dysfunction of cell-mediated immunity and neurological disorders5,7.
There is little known about the role of trace elements in allergic diseases and previous reports have shown conflicting results. There have been some reports of association between decreased Zn levels and asthma and atopic dermatitis8–10, whereas other studies have not shown a significant association11,12. There have also been a few reports on the effects of Zn supplementation in asthma and atopic dermatitis7,13,14. To date, there has been no study of the association of IgE levels with Zn levels. The aim of this study was to investigate the relationship between serum Zn levels and total or allergen-specific IgE levels using the Korea National Health and Nutrition Examination Survey (KNHANES) databases.
Methods
Study design and database
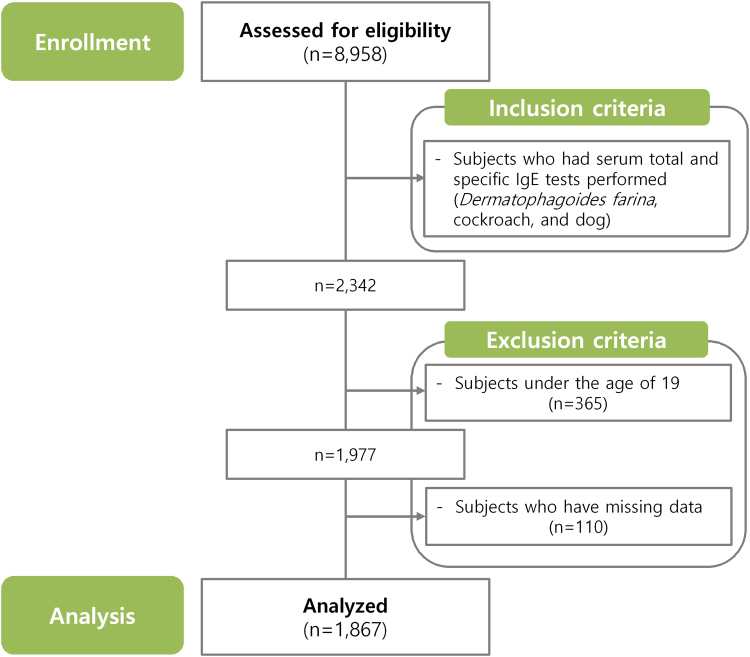
This study was a retrospective cross-sectional study using the 5th KNHANES conducted by the Korea Centers for Disease Control and Prevention from 2010 January to 2012 December. However, since serum Zn and IgE tests were conducted only in 2010, the database from 2010 January to 2010 December was utilized in this study. Selection of the household units and participants for the 5th KNHAES was made based on a complex, multi-stage, probability sampling design. KNHANES collects a number of variables regarding the participants’ demographic, social, health and nutritional statuses using three component surveys: the health interview, the health examination and the nutrition survey. The surveys collect detailed information on anthropometric measures, health behaviors, socioeconomic status, healthcare utilization, quality of life, biochemical profiles using fasting blood serum and urine, measures for dental health, vision, hearing and bone density, X-ray test results, food intake and dietary behavior. Blood and urine tests are performed in participants 10 years of age or older. Other details about the 5th KNHANES have been described previously15. The initial candidates for this study were those who participated in the 5th KNHANES 2010 (n = 8,958). We selected subjects who had serum total and allergen-specific IgE (Dermatophagoides farinae, cockroach and dog) tests performed (n = 2,342). Then, we excluded the subjects under the age of 19 (n = 365) and those who had missing data (n = 110). Finally, a total of 1,867 subjects were included in this study (Fig. 1). This study was approved by the Korean Ministry of Health and Welfare (2010 – 02CON-21-C) and was conducted per the principles of the Declaration of Helsinki.
Figure 1.
Flowchart of the study.
Anthropometric measurements
Height (cm) was measured by medical stadiometer (Seca 225, Seca, German) with the subject facing directly forward with the shoes off, feet together, arms by the sides, and the heels, buttocks, and upper back in contact with the wall. Weight (kg) were measured by digital medical weight scale (GL-6000–20, G-Tech International co., South Korea) while they were wearing light clothes. Results were expressed by rounded to the second decimal place and recorded by a trained examiner. To maintain the accuracy of the measurements, the instruments have been replaced with newly calibrated devices every year. Body mass index (BMI) was calculated by dividing weight (kg) by height squared (m2). Metabolic syndrome was defined as having at least three of the following; (1) central obesity: waist circumference ≥ 90 cm (Asian male), ≥ 85 cm (Asian female), (2) dyslipidemia: triglyceride ≥ 150 mg/dl, (3) dyslipidemia: high-density lipoprotein (HDL) < 40 mg/dL (male), < 50 mg/dL (female), (4) blood pressure ≥ 130/85 mmHg (or treated for hypertension), (5) fasting plasma glucose ≥ 100 mg/dl16,17.
Variables
The population was divided according to residential area: rural or urban. The study subjects’ socioeconomic factors were surveyed by a self-administered questionnaire. The household income was estimated by standardization methods for classifications based on the national standard income level. The household income was categorized into four quartiles with very low (0 to 25th percentile), low (25th to 50th percentile), medium (50th to 75th percentile) and high (75th to 100th percentile). All subjects completed a survey to determine their smoking history (never, former or current) and alcohol consumption habits (glasses per day in a recent month). Comorbidities, including atopic dermatitis, asthma and allergic rhinitis, were identified with a self-reported questionnaire about past diagnoses. Other details have been described elsewhere15.
Biochemical analysis
Peripheral blood samples were obtained after an 8-h fast, and then were immediately processed and transported to a central laboratory (Neodin Medical Institute, Seoul, South Korea). All blood samples were analyzed within 24 h of collection. Serum Zn levels were determined by inductively coupled plasma mass spectrometry (ICP-MS, PerkinElmer Inc., Waltham, MA, USA). In previous studies, low serum zinc levels were analyzed using the 1st tertile or quartile18,19. In our subjects, the median and 25th quartile serum Zn levels were 134.0 µg/dL and 117.0 µg/dL, respectively, so the authors defined hypozincemia as a plasma Zn level below 120 µg/dL for comparison of baseline and clinical characteristics. Total serum IgE and allergen-specific IgE (D. farina, cockroach and dog) levels were examined by immunoradiometric assay (1470 WIZARD gamma-Counter, PerkinElmer Inc.). An increase in serum total IgE levels was defined as levels greater than 100 kU/L, and subjects with allergen-specific IgE levels of 0.35 kU/L or more were defined as being sensitized to that specific allergen20.
Statistical analyses
Continuous variables were presented as means with their respective standard deviation. To analyze the baseline characteristics of subjects, t-tests and chi-square tests and were used for continuous and categorical variables, respectively. Comparisons of mean total and allergen-specific IgE levels according to the quartile of the serum Zn levels were analyzed via analysis of covariates (ANCOVA). Model 1 was non-adjusted; model 2 was adjusted for age and sex; model 3 was adjusted for age, sex, BMI, smoking, drinking, exercise, education, income, metabolic syndrome and vitamin D level. We divided serum Zn levels into 4 groups according to quartile ranges.
We also used multivariable logistic regression analyses to examine the associations between serum Zn levels and total and allergen-specific IgE levels, adjusting for age, sex, BMI, smoking, drinking, exercise, education, income, metabolic syndrome and vitamin D level. Statistical significance was considered present when the P value was less than 0.05. Data were analyzed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
Demographic and clinical characteristics
This study included a total of 1,867 subjects, and baseline demographic and clinical characteristics are summarized in Table 1. Among the 1,867 subjects, 554 subjects were classified as hypozincemia. The hypozincemia group had a lower percentage of males (35.6%, p < 0.001), current smokers (19.1%, p = 0.002), and lower overall alcohol consumption (53.8%, p = 0.002) compared to subjects without hypozincemia. There were significant differences in mean BMI (23.4 ± 3.3 kg/m2, p = 0.025), total cholesterol (184.4 ± 37.8 mg/dL, p = 0.023), triglyceride (123.4 ± 105.6 mg/dL, p = 0.016), HDL (50.2 ± 11.5 mg/dL, p < 0.001), low-density lipoprotein (LDL, 109.1 ± 32.3 mg/dL, p = 0.004) and vitamin D (16.7 ± 5.9 ng/mL, p < 0.001) levels between subjects with hypozincemia and those without hypozincemia. Regarding allergic sensitization, subjects with hypozincemia were most sensitized to D. farina (37.9%), followed by cockroaches (19.0%) and dogs (6.7%). The subjects without hypozincemia showed comparable results (38.4, 20.6 and 4.7%, respectively), and in both groups 44.6% of patients showed increased serum total IgE levels.
Table 1.
Baseline and clinical characteristics of the study population.
| Characteristics | Subjects without hypozincemia (≥120 µg/dL) n = 1,313 | Subjects with hypozincemia (<120 µg/dL) n = 554 | p value | |
|---|---|---|---|---|
| Age, years (SD) | 44.9 (14.5) | 44.8 (15.3) | 0.870 | |
| Male, n (%) | 721 (54.9) | 197 (35.6) | <0.001 | |
| BMI, kg/m2 (SD) | 23.7 (3.4) | 23.4 (3.3) | 0.025 | |
| House income | 0.942 | |||
| Very low (0~25%), n (%) | 205 (15.6) | 88 (15.9) | ||
| Low (25~50%), n (%) | 357 (27.2) | 151 (27.3) | ||
| Moderate (50~75%), n (%) | 373 (28.4) | 150 (27.1) | ||
| High (75~100%), n (%) | 378 (28.8) | 165 (29.8) | ||
| Region | 0.996 | |||
| Urban, n (%) | 614 (46.8) | 259 (46.8) | ||
| Rural, n (%) | 699 (53.2) | 295 (53.2) | ||
| Smoking | 0.002 | |||
| Non-smoker, n (%) | 661 (50.3) | 340 (61.4) | ||
| Ex-smoker, n (%) | 276 (21.0) | 108 (19.5) | ||
| Current smoker, n (%) | 376 (28.6) | 106 (19.1) | ||
| Alcohol consumption (at least once a month), n (%) | 807 (61.5) | 298 (53.8) | 0.002 | |
| Asthma, n (%) | 36 (2.7) | 15 (2.7) | 0.967 | |
| Atopic dermatitis, n (%) | 40 (3.0) | 15 (2.7) | 0.692 | |
| Allergic rhinitis, n (%) | 201 (15.3) | 80 (14.4) | 0.632 | |
| Metabolic syndrome, n (%) | 306 (23.3) | 112 (20.2) | 0.145 | |
| Systolic blood pressure, mmHg (SD) | 117.4 (16.7) | 116.4 (16.7) | 0.210 | |
| Diastolic blood pressure, mmHg (SD) | 75.2 (10.5) | 74.6 (10.2) | 0.283 | |
| Fasting glucose | 0.249 | |||
| Within normal range (<100 mg/dL), n (%) | 1003 (76.4) | 404 (72.9) | ||
| Impaired fasting glucose (100~125 mg/dL), n (%) | 238 (18.1) | 112 (20.2) | ||
| Elevated fasting glucose(≥126 mg/dL), n (%) | 72 (5.5) | 38 (6.9) | ||
| Total cholesterol, mg/dL (SD) | 188.6 (36.9) | 184.4 (37.8) | 0.023 | |
| Triglyceride, mg/dL (SD) | 138.3 (127.7) | 123.4 (105.6) | 0.016 | |
| HDL, mg/dL (SD) | 48.0 (11.1) | 50.2 (11.5) | <0.001 | |
| LDL, mg/dL (SD) | 113.8 (31.6) | 109.1 (32.3) | 0.004 | |
| Increased serum total IgE, n (%) | 585 (44.6) | 247 (44.6) | 0.990 | |
| Sensitization to specific allergen | ||||
| Dermatophagoides farinae, n (%) | 504 (38.4) | 210 (37.9) | 0.846 | |
| Cockroaches, n (%) | 271 (20.6) | 105 (19.0) | 0.406 | |
| Dogs, n (%) | 62 (4.7) | 37 (6.7) | 0.085 | |
| Serum Zn, µg/dL (SD) | 149.6 (24.7) | 105.4 (10.9) | <0.001 | |
| Vitamin D, ng/mL (SD) | 18.6 (6.6) | 16.7 (5.9) | <0.001 | |
BMI, body mass index; HDL, high-density lipoprotein; IgE, immunoglobulin E; LDL, low-density lipoprotein; SD, standard deviation; Zn, zinc.
Association between serum Zn levels and allergic sensitization
The 25th percentile, median, and 75th percentile value of serum Zn levels were 31.0, 82.0, and 250.0 kU/L, respectively. Estimated mean serum total and allergen-specific IgE levels between Q1 and Q4 both before and after adjusting for covariates are shown in Table 2. Before adjusting for covariates, total and dog-specific IgE levels tended to increase as Zn levels decreased from Q4 to Q1, and these associations were statistically significant (p = 0.039 and < 0.001, respectively). Upon adjusting for covariates (models 2 and 3), the negative correlation between serum Zn levels and total and allergen-specific IgE levels was more pronounced. As Zn levels decreased from Q4 to Q1, mean total IgE, D. farinae and dog-specific IgE levels increased significantly (model 2: p = 0.035, 0.010, and < 0.001, respectively; model 3: p = 0.009, 0.004, and < 0.001, respectively). However, the relationship between cockroach-specific IgE levels and serum Zn levels was not statistically significant, either before or after adjusting for covariates.
Table 2.
Association between serum zinc levels and allergic sensitization.
| Serum Zn level | p value | |||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |||
| Total IgE (U/mL) | Model 1 | 111.6 (93.8–132.7) | 93.8 (78.9–111.6) | 80.3 (66.0–97.6) | 83.9 (71.4–98.5) | 0.039 |
| Model 2 | 110.8 (93.1–131.9) | 94.3 (79.7–111.5) | 78.9 (65.4–95.3) | 84.5 (72.5–98.5) | 0.035 | |
| Model 3 | 111.4 (93.9–132.1) | 92.0 (77.6–109.0) | 76.7 (63.7–92.3) | 79.1 (68.4–91.6) | 0.009 | |
| Dermatophagoides farinae | Model 1 | 0.30 (0.23–0.40) | 0.23 (0.17–0.31) | 0.17 (0.13–0.23) | 0.23 (0.18–0.30) | 0.052 |
| Model 2 | 0.32 (0.24–0.42) | 0.23 (0.17–0.31) | 0.17 (0.13–0.22) | 0.21 (0.16–0.28) | 0.010 | |
| Model 3 | 0.33 (0.25–0.45) | 0.23 (0.17–0.31) | 0.17 (0.13–0.22) | 0.19 (0.15–0.25) | 0.004 | |
| Cockroach | Model 1 | 0.11 (0.088–0.137) | 0.10 (0.09–0.12) | 0.09 (0.07–0.12) | 0.09 (0.08–0.12) | 0.721 |
| Model 2 | 0.11 (0.089–0.138) | 0.10 (0.09–0.12) | 0.09 (0.07–0.11) | 0.09 (0.08–0.12) | 0.633 | |
| Model 3 | 0.11 (0.092–0.136) | 0.10 (0.08–0.11) | 0.09 (0.07–0.11) | 0.09 (0.08–0.11) | 0.429 | |
| Dog | Model 1 | 0.04 (0.035–0.051) | 0.03 (0.03–0.04) | 0.03 (0.02–0.03) | 0.03 (0.02–0.03) | <0.001 |
| Model 2 | 0.04 (0.036–0.052) | 0.03 (0.03–0.04) | 0.03 (0.02–0.03) | 0.03 (0.02–0.03) | <0.001 | |
| Model 3 | 0.04 (0.036–0.053) | 0.03 (0.03–0.04) | 0.03 (0.02–0.03) | 0.02 (0.02–0.03) | <0.001 | |
IgE, immunoglobulin E; Q1, 1st quartile; Q2, 2nd quartile; Q3, 3rd quartile; Q4, 4th quartile. *Model 1: not adjusted; Model 2: adjusted for age and sex; Model 3: adjusted for age, sex, body mass index, smoking, alcohol consumption, income, metabolic syndrome and serum vitamin D level.
Effect of serum Zn levels on the odds ratios for allergic sensitization
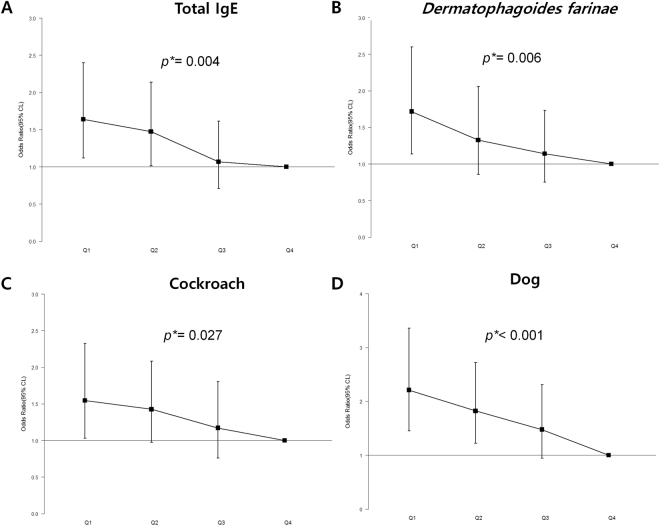
Multivariable logistic regression analyses were performed for quartiles based on serum Zn levels to examine the association of serum Zn level with both total and allergen-specific IgE levels. Upon adjustment for age, sex, BMI, smoking, drinking, exercise, education, income, metabolic syndrome and vitamin D level, the trend analyses showed significant negative linear correlations between serum Zn levels and total, D. farina-, cockroach-, and dog-specific IgE levels (p-value for linear trend = 0.004, 0.006, 0.027, and < 0.001, respectively) (Fig. 2).
Figure 2.
Effects of serum Zn levels on the odds ratios for allergic sensitization. CI, confidence interval; IgE, immunoglobulin E; Q1, 1st quartile; Q2, 2nd quartile; Q3, 3rd quartile; Q4, 4th; Q, quartile. *p-value for linear trend.
Discussion
This is the first study to evaluate the association between serum IgE levels and Zn levels in a general adult population of 1,867. Our study demonstrates that study subjects with increased total and allergen-specific IgE levels had significantly lower Zn levels. The trend analysis also showed significant negative linear correlations between total, D. farinae-, cockroach-, and dog-specific IgE levels and serum Zn levels.
Several conditions have been reported to be associated with Zn levels. Zn deficiency is clinically manifested in alopecia, acrodermatitis enteropathica, diarrhea, emotional disorders, weight loss, dysfunction of cell-mediated immunity and neurological disorders5,7. Recently, a study reported the associations between serum Zn levels and metabolic syndrome. They showed Serum Zn levels were negatively associated with elevated fasting glucose and positively associated with elevated triglycerides in men. They also showed that Zinc levels have a decreasing trend with increasing numbers of metabolic syndrome components in women with metabolic syndrome19. Therefore, we have included previously known factors in our analyses which may be associated with serum Zn levels. After adjusting the covariates, our results demonstrated that decreased serum Zn levels are associated with increased total IgE levels and allergic sensitization, including sensitization to D. farinae, cockroach and dog. There have been several reports investigating the relationship between Zn levels and allergic diseases, including allergic asthma and atopic dermatitis. Most recently, Nazila et al. reported that mean serum Zn levels in patients with allergic asthma were significantly lower than in healthy control subjects, although no correlation was found between Zn levels and disease severity10. In a different study on atopic dermatitis, it was reported that erythrocyte Zn levels, which have been reported to be a better measure of mild Zn deficiency, were significantly lower in patients with atopic dermatitis when compared to in healthy control subjects9. On the other hand, David et al., who first suggested the association between serum zinc levels and atopic dermatitis in 1984, found no significant difference in a later article11. Although there have been conflicting results, there might be a link between serum Zn levels and allergic sensitization that is characterized by total and specific IgE.
There have been a few reports about the benefits of Zn supplements in managing allergic diseases. Two animal models show that mice fed with a zinc-deficient diet developed AD-like skin lesions21,22. These reports showed that serum IgE levels and the number of S. aureus on the skin surface were both increased in DS-Nh mice. In a study of children with atopic dermatitis, Zn supplementation for 8 weeks resulted in a significant increase in Zn levels in hair, and significant reductions in eczema area severity index, transepidermal water loss and visual analogue scales for pruritus when compared to healthy control subjects7. In a randomized, placebo-controlled trial in children with asthma, significant improvement in clinical symptoms and spirometry parameters was also observed in Zn supplementation groups14.
The exact role of Zn in allergic diseases remains unclear. Zn affects many aspects of immune system function, and is involved in FcεRI-induced mast cell activation4. FcεRI stimulation has been reported to trigger microtubular formation in a calcium-independent manner23. Zn has been shown to be involved in multiple steps of FcεRI-induced mast cell activation, and is required for degranulation and cytokine production4. Additionally, a complex interaction between Zn, cyclic nucleotide and nitric oxide signaling, inhibitors of κB kinase and inhibition of IL-1 receptor-associated kinase-1 counteracts the production of proinflammatory cytokines24. In light of these findings, Zn released from human mast cells may play a role in the regulation of the inflammation in allergic diseases.
Our study has several limitations. First, we did not analyze the relationship between serum Zn levels and disease, nor between total IgE/specific IgE levels and disease. Second, it remains unclear whether a single measurement of serum Zn levels accurately represents actual Zn status in the general population, and our analyses were not able to analyze causal relationships because of their cross-sectional nature. Prospectively designed studies are necessary to clarify the relationship between serum Zn levels and subsequent allergic sensitization. In spite of these limitations, this study has strength in its use of a comprehensive national database to represent the total Korean population, and in being the first to assess the association between serum Zn levels and allergic sensitization.
In conclusion, our findings demonstrated that increased total and allergen-specific IgE levels are significantly associated with decreased Zn levels. Future studies should clarify the role of Zn in the development of allergic sensitization.
Acknowledgements
This study was supported by a National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (NRF-2015R1C1A2A01054767).
Author Contributions
H.M.S., Y.H.K., and J.H.L. wrote the main manuscript. H.M.S. and J.H.L. prepared Figures 1, 2 and Tables 1, 2. J.S.K., Y.M.P., and J.Y.L. reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wu LC, Zarrin AA. The production and regulation of IgE by the immune system. Nature reviews. Immunology. 2014;14:247–259. doi: 10.1038/nri3632. [DOI] [PubMed] [Google Scholar]
- 2.Gould HJ, et al. The biology of IGE and the basis of allergic disease. Annual review of immunology. 2003;21:579–628. doi: 10.1146/annurev.immunol.21.120601.141103. [DOI] [PubMed] [Google Scholar]
- 3.Shankar AH, Prasad AS. Zinc and immune function: the biological basis of altered resistance to infection. The American journal of clinical nutrition. 1998;68:447s–463s. doi: 10.1093/ajcn/68.2.447S. [DOI] [PubMed] [Google Scholar]
- 4.Kabu K, et al. Zinc is required for Fc epsilon RI-mediated mast cell activation. Journal of immunology (Baltimore, Md. : 1950) 2006;177:1296–1305. doi: 10.4049/jimmunol.177.2.1296. [DOI] [PubMed] [Google Scholar]
- 5.Prasad AS. Discovery of human zinc deficiency: 50 years later. Journal of trace elements in medicine and biology: organ of the Society for Minerals and Trace Elements (GMS) 2012;26:66–69. doi: 10.1016/j.jtemb.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Choi R, et al. A Prospective Study of Serum Trace Elements in Healthy Korean Pregnant Women. Nutrients. 2016;8:749. doi: 10.3390/nu8110749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim JE, Yoo SR, Jeong MG, Ko JY, Ro YS. Hair zinc levels and the efficacy of oral zinc supplementation in patients with atopic dermatitis. Acta dermato-venereologica. 2014;94:558–562. doi: 10.2340/00015555-1772. [DOI] [PubMed] [Google Scholar]
- 8.David TJ, Wells FE, Sharpe TC, Gibbs AC. Low serum zinc in children with atopic eczema. The British journal of dermatology. 1984;111:597–601. doi: 10.1111/j.1365-2133.1984.tb06630.x. [DOI] [PubMed] [Google Scholar]
- 9.Toyran M, et al. Trace element levels in children with atopic dermatitis. Journal of investigational allergology & clinical immunology. 2012;22:341–344. [PubMed] [Google Scholar]
- 10.Nazila A, Reza F, Fahimeh S, Mohamad S, Farahzad JA. Trace Elements Status in Sera of Patients with Allergic Asthma. Reports of biochemistry & molecular biology. 2016;5:20–25. [PMC free article] [PubMed] [Google Scholar]
- 11.David TJ, Wells FE, Sharpe TC, Gibbs AC, Devlin J. Serum levels of trace metals in children with atopic eczema. The British journal of dermatology. 1990;122:485–489. doi: 10.1111/j.1365-2133.1990.tb14725.x. [DOI] [PubMed] [Google Scholar]
- 12.ERTUNÇ V, et al. The serum levels of some important elements and alkaline phosphatase in patients with atopic dermatitis. TURKISH JOURNAL OF MEDICAL SCIENCES. 1996;26:593–596. [Google Scholar]
- 13.Morgan CI, Ledford JR, Zhou P, Page K. Zinc supplementation alters airway inflammation and airway hyperresponsiveness to a common allergen. Journal of inflammation (London, England) 2011;8:36. doi: 10.1186/1476-9255-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghaffari J, Khalilian A, Salehifar E, Khorasani E, Rezaii MS. Effect of zinc supplementation in children with asthma: a randomized, placebo-controlled trial in northern Islamic Republic of Iran. Eastern Mediterranean health journal = La revue de sante de la Mediterranee orientale = al-Majallah al-sihhiyah li-sharq al-mutawassit. 2014;20:391–396. [PubMed] [Google Scholar]
- 15.Kweon S, et al. Data resource profile: the Korea National Health and Nutrition Examination Survey (KNHANES) International journal of epidemiology. 2014;43:69–77. doi: 10.1093/ije/dyt228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). Jama285, 2486–2497 (2001). [DOI] [PubMed]
- 17.Grundy SM, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 18.Kim, N. R., Kim, K. W., Kim, H. N. & Song, S. W. Associations Between Serum Zinc Levels and Mental Health: Findings from the 2010 Korean National Health and Nutrition Examination Survey. Biological trace element research, 10.1007/s12011-017-1051-x (2017). [DOI] [PubMed]
- 19.Seo JA, Song SW, Han K, Lee KJ, Kim HN. The associations between serum zinc levels and metabolic syndrome in the Korean population: findings from the 2010 Korean National Health and Nutrition Examination Survey. PloS one. 2014;9:e105990. doi: 10.1371/journal.pone.0105990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weidinger S, et al. Loss-of-function variations within the filaggrin gene predispose for atopic dermatitis with allergic sensitizations. The Journal of allergy and clinical immunology. 2006;118:214–219. doi: 10.1016/j.jaci.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Makiura M, et al. Atopic dermatitis-like symptoms in HR-1 hairless mice fed a diet low in magnesium and zinc. The Journal of international medical research. 2004;32:392–399. doi: 10.1177/147323000403200407. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi H, et al. Effects of zinc deficient diet on development of atopic dermatitis-like eruptions in DS-Nh mice. Journal of dermatological science. 2008;50:31–39. doi: 10.1016/j.jdermsci.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Nishida K, et al. Fc{epsilon}RI-mediated mast cell degranulation requires calcium-independent microtubule-dependent translocation of granules to the plasma membrane. The Journal of cell biology. 2005;170:115–126. doi: 10.1083/jcb.200501111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakashima-Kaneda K, et al. Regulation of IgE-dependent zinc release from human mast cells. International archives of allergy and immunology. 2013;161(Suppl 2):44–51. doi: 10.1159/000350359. [DOI] [PubMed] [Google Scholar]