Pain is inherently linked to motor processes, but the interactions between pain and motor processes in the human brain are not yet fully understood. Using electroencephalography, we show that pain reduces movement-preparatory brain activity. Further results indicate that this effect is not pain specific but independent of the modality of stimulation.
Keywords: pain, motor preparation, electroencephalography, readiness potential, event-related desynchronization
Abstract
The protective function of pain depends on appropriate motor responses to avoid injury and promote recovery. The preparation and execution of motor responses is thus an essential part of pain. However, it is not yet fully understood how pain and motor processes interact in the brain. Here we used electroencephalography to investigate the effects of pain on motor preparation in the human brain. Twenty healthy human participants performed a motor task in which they performed button presses to stop increasingly painful thermal stimuli when they became intolerable. In another condition, participants performed button presses without concurrent stimulation. The results show that the amplitudes of preparatory event-related desynchronizations at alpha and beta frequencies did not differ between conditions. In contrast, the amplitude of the preparatory readiness potential was reduced when a button press was performed to stop a painful stimulus compared with a button press without concomitant pain. A control experiment with nonpainful thermal stimuli showed a similar reduction of the readiness potential when a button press was performed to stop a nonpainful thermal stimulus. Together, these findings indicate that painful and nonpainful thermal stimuli can similarly influence motor preparation in the human brain. Pain-specific effects on motor preparation in the human brain remain to be demonstrated.
NEW & NOTEWORTHY Pain is inherently linked to motor processes, but the interactions between pain and motor processes in the human brain are not yet fully understood. Using electroencephalography, we show that pain reduces movement-preparatory brain activity. Further results indicate that this effect is not pain specific but independent of the modality of stimulation.
pain is inherently linked to motor processes. The preparation and execution of motor responses are essential for the protective function of pain (Melzack and Casey 1968). Moreover, physical exercise (Naugle et al. 2012) and the stimulation of motor areas in the brain (Mylius et al. 2012; Nguyen et al. 2011) can alleviate pain. Vice versa, pain critically influences motor behavior (Bank et al. 2013; Hodges and Tucker 2011), which likely contributes to the pathology of chronic pain (Hodges and Smeets 2015). Consequently, the relationship between pain and motor processes increasingly attracts attention (Morrison et al. 2013; Piedimonte et al., 2017; Sullivan 2008; Tabor et al. 2017; Vogt and Sikes 2009; Wiech and Tracey 2013).
The mechanisms underlying interactions between pain and motor processes in the brain are not yet fully understood. Anatomical studies have shown direct nociceptive projections to cingulate motor areas (Dum et al. 2009). Moreover, pain (Apkarian et al. 2005) and movement preparation and execution (Geyer et al. 2012) activate extended networks of brain areas that overlap and interact in cingulate and frontal premotor areas (Misra and Coombes 2015; Perini et al. 2013). Interactions between pain and motor processes can be further disentangled by using electroencephalography (EEG) that records a typical sequence of movement-related responses. During movement preparation, a slow negative wave termed the readiness potential is observed (Brunia et al. 2012; Colebatch 2007; Shibasaki and Hallett 2006). It starts as early as 2,000 ms before movement onset and is most consistently observed during the last 500 ms over contralateral sensorimotor areas (Brunia et al. 2012; Colebatch 2007; Shibasaki and Hallett 2006). In addition, movement preparation is associated with decreases of neuronal oscillations at alpha (8–13 Hz) and beta (14–30 Hz) frequencies termed event-related desynchronization (Cheyne 2013; Pfurtscheller and Lopes da Silva 1999; van Wijk et al. 2012). These decreases also begin ~2,000 ms before movement onset and are strongest during the last 500 ms over contralateral sensorimotor regions (Cheyne 2013; Pfurtscheller and Lopes da Silva 1999; van Wijk et al. 2012). However, whether and how pain influences these movement-preparatory phenomena has remained largely unknown so far.
In the present study, we used EEG to investigate one specific aspect of the two-way interaction between pain and motor processes, i.e., whether and how pain affects motor preparation in the human brain. Specifically, we hypothesized that pain is inherently linked to motor preparation in the human brain that might manifest as a pain-induced change of the preparatory readiness potential and/or preparatory event-related desynchronization. The results of an ecologically valid motor task in which movements stopped painful stimuli reveal that the amplitude of the preparatory readiness potential was reduced compared with a motor task without concomitant pain. However, comparable results were found when movements ended nonpainful stimuli, suggesting a modality-unspecific effect of pain on motor preparation in the human brain.
MATERIALS AND METHODS
Participants
Twenty-one healthy human participants (9 men, 12 women; age 27 ± 6 yr, mean ± SD) participated in the main experiment, and 24 participants (9 men, 15 women; age 26 ± 4 yr) took part in a control experiment. Data from one participant and four participants of the main and control experiments, respectively, were excluded because of technical problems during recording and/or poor data quality. Thus the final analysis included 20 participants (9 men, 11 women; age 27.3 ± 6.3 yr) for the main experiment and 20 participants (8 men, 12 women; age 25.8 ± 4.7 yr) for the control experiment. All participants were right-handed as assessed by the Edinburgh Handedness Inventory (Oldfield 1971). All participants gave written informed consent before participation. The study was approved by the local ethics committee and conducted in conformity with the Declaration of Helsinki.
Paradigm
The study included a main and a control experiment (Fig. 1). During both experiments, participants sat comfortably in a dimly lit room. An infrared thermometer was used to ensure that the skin temperature at the beginning of the experiment (main experiment: 31 ± 2.2°C; control experiment: 32 ± 1.8°C) was in the suggested range for thermal sensory testing (Hagander et al. 2000). During the recording, participants were exposed to white noise through headphones to cancel out ambient noise.
Fig. 1.
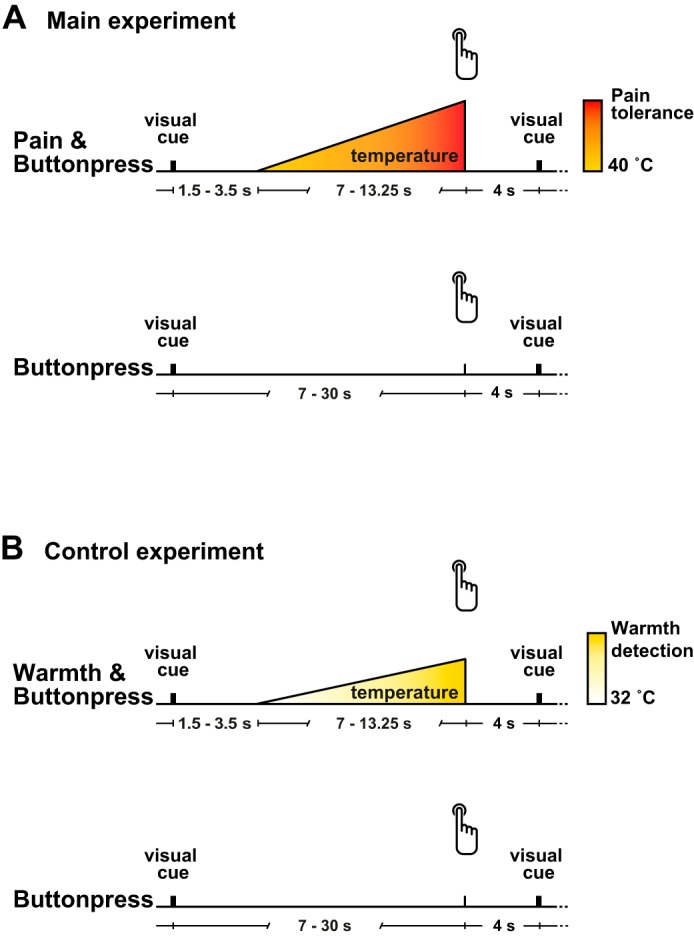
Paradigm. A: main experiment. In the Pain & Buttonpress condition, 60 heat stimuli of increasing intensity were applied to the dorsum of the left hand. Participants were instructed to press a button with the right index finger to stop the stimulation at the maximum pain intensity they were willing to tolerate. In the Buttonpress condition, participants were instructed to perform button presses at about the same interval as in the Pain & Buttonpress condition but without concurrent painful stimulation. B: control experiment. In the Warmth & Buttonpress condition, participants were instructed to interrupt the increasing thermal stimulation when it was perceived as clearly warm. The Buttonpress condition matched that of the main experiment.
Main experiment.
The main experiment (Fig. 1A) comprised three conditions: the Pain & Buttonpress condition, the Buttonpress condition, and the Pain condition. In the Pain & Buttonpress condition, painful heat stimuli were applied with increasing intensity and participants were asked to stop the stimulation by pressing a button when it became intolerable. In the Buttonpress condition, participants performed button presses without concomitant painful stimulation. In the Pain condition, painful heat stimuli identical to the Pain & Buttonpress condition were applied but only passively perceived, i.e., participants did not perform button presses to stop the stimulation. The Pain condition is not further analyzed here as it does not add to the central question of the present study, i.e., the influence of pain on motor preparation. As the durations of the stimuli in the Pain condition were taken from the Pain & Buttonpress condition, the latter condition was always performed first, followed by the Buttonpress and Pain conditions. Painful heat stimuli were applied to the dorsum of the left hand with a thermode (TSA-II, Medoc), and button presses were performed with the index finger of the right hand. After the Pain & Buttonpress condition, the thermode was slightly displaced in the lateral-medial direction to avoid skin damage. Each condition included 60 trials. Stimulation was controlled with MATLAB (MathWorks, Natick, MA) and the Psychophysics Toolbox (http://psychtoolbox.org/).
In the Pain & Buttonpress condition (Fig. 1A, top), a heat stimulus with increasing intensity was applied and participants were instructed to stop the stimulation at the maximum pain intensity they were willing to tolerate by pressing the button. Thus, by definition, the participants stopped the stimulation at pain tolerance level, ensuring that they experienced pain before the button press. A black fixation cross was displayed at the center of a computer monitor. Each trial started when the black fixation cross turned green for 1 s. After a randomly varied interval between 1.5 and 3.5 s, the stimulation temperature increased from a baseline of 40°C with a changing rate of 0.8°C/s until the participants stopped the stimulus increase by pressing the button. Participants on average stopped the stimulation at a mean maximum temperature of 47.1 ± 0.9°C and 10.0 ± 1.2 s after the start of the temperature increase. After the button press, the temperature decreased back to the baseline with a cooling rate of 8°C/s. The next trial started 4 s after the button press. The mean latency between button presses, i.e., the mean trial duration, was 17.4 ± 1.6 s. A comparison of the peak temperatures of the first and second halves of the condition did not indicate habituation or sensitization effects (47.1°C for both halves, P = 0.8, paired t-test).
In the Buttonpress condition (Fig. 1A, bottom), participants were instructed to press the button at an interval resembling the interval between two button presses in the Pain & Buttonpress condition. Again, each trial started when the black fixation cross turned green for 1 s and ended 4 s after the button press. Pilot experiments indicated that participants tended to decrease the interval between button presses over the course of the experiment. Therefore, when the interval between button presses was shorter than 7 s for more than two consecutive trials, a red cross was presented for 1 s after the button press to instruct the participants to increase the interval between button presses. Moreover, in these cases, up to 15 additional trials were performed, aiming at a total number of 60 trials with a duration >7 s for each participant. The mean latency between button presses for this condition was 19.0 ± 10.2 s.
As in the Pain & Buttonpress and Buttonpress conditions no speeded responses to sensory stimuli were performed, reaction times were not assessed. Moreover, in the Pain & Buttonpress condition no single-trial pain ratings were obtained, so that the relationship between pain intensity and motor preparatory brain activity was not quantitatively assessed.
Control experiment.
To test for the pain specificity of the results, a control experiment with a nonpainful warmth stimulus was performed (Fig. 1B). Apart from the nonpainful warmth stimulus, the control experiment was identical to the main experiment. The control experiment thus included a Warmth & Buttonpress condition, a Buttonpress condition, and a Warmth condition. The Warmth condition was not further analyzed as it does not add to the central question of the present study, i.e., the influence of pain/warmth on motor preparation.
In the Warmth & Buttonpress condition (Fig. 1B, top), participants were instructed to stop a thermal stimulus with increasing intensity by pressing the button as soon as it was perceived as clearly warm. The temperature increased from a baseline of 32°C. The changing rate was adjusted for each participant during a preliminary training session to ensure a trial duration comparable to the main experiment, i.e., longer than 7 s (changing rate 0.4 ± 0.1°C/s). If trial durations were shorter than 7 s, additional trials were again presented to obtain at least 60 trials for each participant with a duration comparable to the main experiment. Cooling rate was 8°C/s. During this condition, mean maximum temperature was 37.2 ± 2.7°C, mean duration of temperature increase was 15.1 ± 5.9 s, and mean latency between button presses was 22.4 ± 7.5 s. The Buttonpress condition (Fig. 1B, bottom) exactly matched the Buttonpress condition of the main experiment. The mean latency between button presses in the Buttonpress condition was 22.9 ± 11.8 s. A comparison of the peak temperatures of the first and second halves of the condition did not indicate habituation or sensitization effects (37.6°C vs. 37.5°C, P = 0.11, paired t-test).
As in the Warmth & Buttonpress and Buttonpress conditions no speeded responses to sensory stimuli were performed, reaction times were not assessed.
Electroencephalography
Recordings and preprocessing.
During both experiments, EEG data were recorded with an electrode cap (Easycap, Herrsching, Germany), BrainAmp MR plus amplifiers (Brain Products, Munich, Germany), and BrainVision Recorder software (Brain Products). The electrode montage included 64 electrodes consisting of all 10-20 system electrodes and the additional electrodes Fpz, FCz, CPz, POz, Oz, Iz, AF3/4, F5/6, FC1/2/3/4/5/6, FT7/8/9/10, C1/2/5/6, CP1/2/3/4/5/6, TP7/8/9/10, P5/6, and PO1/2/9/10. Two additional electrodes were placed below the outer canthus of each eye. The EEG was referenced to the FCz electrode, grounded at AFz, sampled at 1,000 Hz, and high-pass filtered at 0.015 Hz. The impedance was kept below 20 kΩ. The raw EEG data were preprocessed with BrainVision Analyzer software (Brain Products). Off-line analysis included downsampling to 512 Hz, digital high-pass filtering at 0.5 Hz, correction for eye movements and muscle artifacts with independent component analysis (Jung et al. 2000), and rereferencing to the average reference. Data from all conditions were segmented into trials of −7 to 5 s with respect to the button press. Trials with artifacts exceeding ±100 μV in any channel were automatically rejected, and an additional visual rejection was performed to exclude remaining artifact-contaminated trials. In the main experiment, the average number of remaining trials was 51 ± 9 for the Pain & Buttonpress condition and 54 ± 3 for the Buttonpress condition. In the control experiment, the average number of remaining trials was 51 ± 6 for the Warmth & Buttonpress condition and 53 ± 5 for the Buttonpress condition.
Analysis.
EEG data were analyzed with FieldTrip, an open-source toolbox for MATLAB (Oostenveld et al. 2011). Data were analyzed in the time and time-frequency domains. Analyses were focused on the movement preparation period before the button presses. Neural activity after the button presses was not analyzed since button presses coincided with the termination of the painful stimuli in the Pain & Buttonpress condition, which prevents an unequivocal interpretation of post-button press activity.
All statistical analyses were performed with nonparametric cluster-based permutation tests comparing the Pain & Buttonpress/Warmth & Buttonpress and Buttonpress conditions (Maris and Oostenveld 2007). First, point-by-point t-tests were calculated comparing signal amplitudes between conditions at each electrode and/or time point (see below for more details). Second, clusters of neighboring electrodes and/or time points whose t-statistic exceeded a critical threshold (P = 0.05), were selected and t-values within each cluster were summed up, resulting in cluster-level test statistics. To evaluate statistical significance, the maximum cluster-level test statistic was then compared to a reference distribution of maximum cluster t-value sums obtained by randomly interchanging data across the two conditions and recalculating the cluster-level test statistic 1,000 times. This cluster-based procedure deals with the multiple comparison problem, takes physiological plausibility into account, and is not affected by partial dependence in the data (Maris and Oostenveld 2007; van Ede and Maris 2016).
Time domain analysis.
A low-pass filter of 30 Hz was applied to the segmented data. For each condition, trials were averaged time-locked to the button press. The statistical analysis was focused on a 2 s-time window preceding the button press as this period has been shown to include most movement-preparatory activity in previous time domain analyses (Brunia et al. 2012; Colebatch 2007; Shibasaki and Hallett 2006). Using the statistical approach outlined above, we tested the effect of pain on movement-preparatory brain activity by comparing the amplitude of the potentials between the Pain & Buttonpress and Buttonpress conditions. In a first step, we performed multielectrode analysis by clustering across time and electrodes. Subsequently, we restricted the statistical analysis to the electrode Cz, which has been shown to most strongly reflect the readiness potential (Brunia et al. 2012; Colebatch 2007; Shibasaki and Hallett 2006). Here, clustering was performed across time only.
Corresponding analyses were performed for the control experiment, comparing the Warmth & Buttonpress condition to the Buttonpress condition. Additionally, we assessed whether the effect of pain on motor preparation significantly differed from the effect of warmth. We computed the difference of the readiness potential between the Pain & Buttonpress and Buttonpress conditions and contrasted it against the difference between the Warmth & Buttonpress and Buttonpress conditions, both for all electrodes and at electrode Cz only.
Time-frequency domain analysis.
We performed time-frequency analysis on the segmented single trials by using a Hanning-tapered sliding window fast Fourier transform for frequencies from 1 to 100 Hz. Window length was 0.25 s, and step size was 30 ms. To obtain frequency band-specific time courses of EEG signals, we averaged power across theta (4–7 Hz), alpha (8–13 Hz), beta (14–30 Hz), and gamma (40–100 Hz) frequencies. We compared the power time courses of each frequency band in the Pain & Buttonpress and Buttonpress conditions in a 2-s time window (Cheyne 2013; Pfurtscheller and Lopes da Silva 1999; van Wijk et al. 2012) before the button press, again using the same statistical approach described above. As a first step, we implemented a multielectrode analysis testing for differences between the two conditions while clustering across time and electrodes. As previous studies showed that movement-related power changes are most prominent over the sensorimotor area contralateral to the movement (Cheyne 2013; Pfurtscheller and Lopes da Silva 1999; van Wijk et al. 2012), we restricted our analysis to an average across central electrodes contralateral to the hand performing the movement in a second step (i.e., Cz, CPz, C1, C3, CP1, CP3).
RESULTS
Time Domain Results
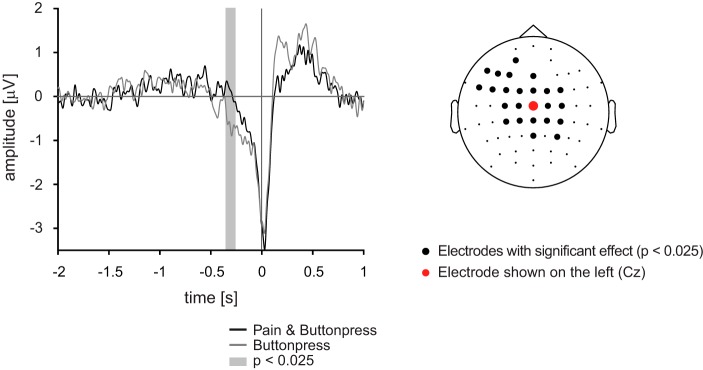
Figure 2 shows movement-related potentials for the two experimental conditions averaged across participants. Figure 2, left, shows the typical sequence of movement-related potentials at electrode Cz, where such potentials are strongest (Brunia et al. 2012; Colebatch 2007; Shibasaki and Hallett 2006). During the last 500 ms before the button press, the readiness potential was observed as an increasing negativity related to movement preparation. This was followed by the motor potential and the postmovement potential (Colebatch 2007), which were not analyzed here. The comparison of the readiness potential across conditions revealed that its amplitude was lower in the Pain & Buttonpress condition than in the Buttonpress condition. Cluster-based permutation statistics confirmed a significant difference (P < 0.025) between −0.35 s and −0.26 s before the button press (Fig. 2, left). The topography in Fig. 2, right, shows the location of the electrode shown in Fig. 2, left, and the location of electrodes where significant differences between Pain & Buttonpress and Buttonpress conditions were observed (P < 0.025) when clustering was performed across both electrodes and time. The cluster mainly covered central and frontal electrodes and was slightly lateralized to the left hemisphere, i.e., contralateral to the button press. Thus less brain activity related to motor preparation was recorded when the button press was performed during painful stimulation than when it was performed without concurrent pain.
Fig. 2.
Time domain results of the main experiment. Left: grand averages of movement-related potentials at electrode Cz. Statistical comparison between Pain & Buttonpress and Buttonpress conditions were performed during the last 2 s of motor preparation, and the gray shaded time window highlights significantly different time periods. Right: electrodes where significant differences were observed are marked by bold black dots. Statistical analyses were performed with cluster-based permutation statistics.
Time-Frequency Domain Results
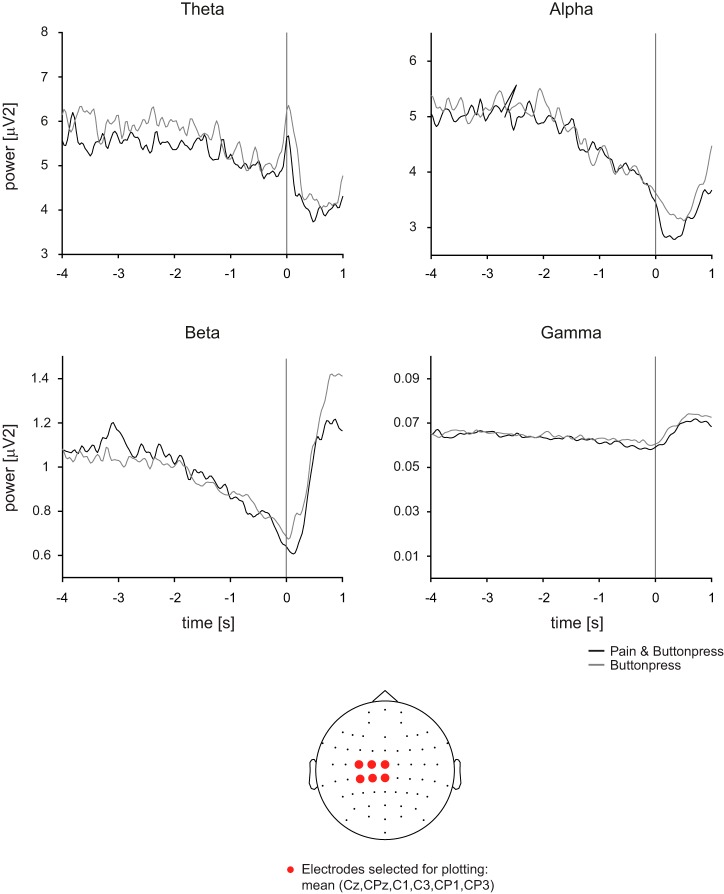
Figure 3 displays movement-related activity for the two conditions at theta (4–7 Hz), alpha (8–13 Hz), beta (14–30 Hz) and gamma (40–100 Hz) frequencies averaged across participants. Figure 3, top, shows the time courses of frequency-specific brain activity at a selection of electrodes where strongest movement-preparatory activity is observed (Cheyne 2013; Pfurtscheller and Lopes da Silva 1999; van Wijk et al. 2012), i.e., electrodes covering the sensorimotor area contralateral to the movement (Cz, CPz, C1, C3, CP1, CP3). The figure shows a decrease of power during the last 2 s before the button press, which was particularly pronounced at alpha and beta frequencies. Additional changes of neural activity were observed during and after the button press but are not further analyzed here. The topography in Fig. 3, bottom, shows the location of the electrodes shown in Fig. 3, top. Statistical comparisons performed in the last 2 s of motor preparation did not show significant differences of movement-preparatory activity between conditions in any frequency band, neither when using the contralateral electrode selection and clustering across time nor when clustering across both time and electrodes (P > 0.025).
Fig. 3.
Time-frequency domain results of the main experiment. Top: grand average time courses of frequency band-specific brain activity for selected electrodes covering the sensorimotor areas contralateral to movement preparation. With cluster-based permutation statistics in a time window covering the last 2 s of motor preparation, no significant difference in movement-preparatory brain activity was observed between conditions at any frequency band. Bottom: topography shows the location of electrodes selected for analysis.
Control Experiment
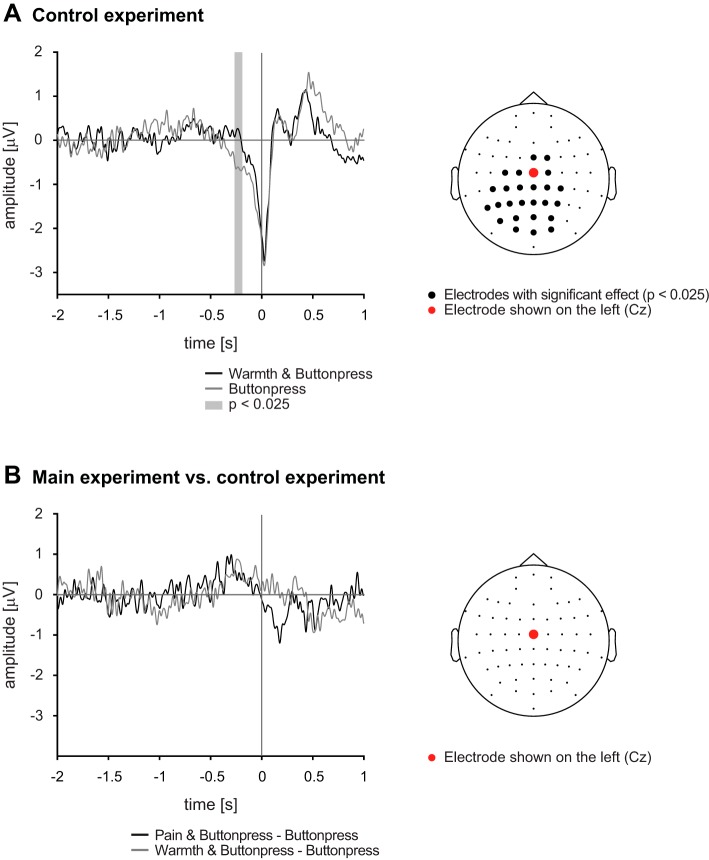
To test for the specificity of the observed difference of the readiness potential between the Pain & Buttonpress and Buttonpress conditions, we performed the same analyses for the control experiment in which nonpainful warmth stimuli were applied. Comparable to the main experiment, the amplitude of the readiness potential in the Warmth & Buttonpress condition was lower than in the Buttonpress condition at electrode Cz (Fig. 4A, left; significant cluster from −0.26 s to −0.19, P < 0.025). Figure 4A, right, shows that this difference was mainly spread over central and posterior electrodes (P < 0.025).
Fig. 4.
Time domain results of the control and the main experiment. A, left: grand average movement-related potentials at electrode Cz. Gray shaded time window indicates a significant difference between the experimental conditions of the control experiment in the last 2 s before the onset of the movement. Right: topography shows electrodes where significant differences between the Warmth & Buttonpress and Buttonpress conditions were observed. B: the difference of the readiness potentials between the experimental conditions was calculated for the main and the control experiment and contrasted against each other at Cz only (left) and across all electrodes (right). No significant differences were detected, indicating that pain and warmth affect motor preparation in a comparable way. All statistical analyses were performed with cluster-based permutation statistics during the last 2 s of motor preparation.
We finally directly compared the difference of the readiness potential between the Pain & Buttonpress and Buttonpress conditions in the main experiment with the difference of the readiness potential between the Warmth & Buttonpress and Buttonpress conditions in the control experiment. Figure 4B, left, shows the grand averages of the difference waves at electrode Cz; Fig. 4B, right, shows the topography of the comparison. No significant differences were observed, i.e., the amplitudes of the potentials differed in a similar way from the Buttonpress condition both when a painful and when a warmth stimulation was applied. Thus movement-preparatory brain activity was reduced when a button press was performed during painful as well as during nonpainful thermal stimulation, indicating a non-pain-specific effect.
DISCUSSION
In the present study, we investigated whether and how pain influences motor preparation in the human brain. The results show that a movement that stops an increasingly painful thermal stimulus is associated with a smaller readiness potential than a similar movement without a concomitant painful stimulus. However, a control experiment indicated that a similar reduction of the readiness potentials occurs when the movement stops an ongoing nonpainful thermal stimulus. The pain-associated reduction of the readiness potential is thus not pain specific but likely represents a modality-spanning effect.
Pain and Motor Processes in the Human Brain
Despite the crucial relevance of motor responses for the protective function of pain, interactions between pain and motor processes in the brain have rarely been investigated. Anatomical studies revealed that nociceptive pathways project directly and substantially to cortical motor areas (Dum et al. 2009). Functional imaging studies showed overlaps and interactions between pain-related and motor-related activations (Misra and Coombes 2015; Perini et al. 2013). However, because of the limited temporal resolution of functional magnetic resonance imaging, these studies could not analyze processes related to the preparation, execution, and aftereffects of movements separately. Neurophysiological recordings using EEG, magnetoencephalography, or intracranial recordings can disentangle these processes by showing their temporal sequence. A few EEG studies have addressed interactions between pain and motor processes so far. Some studies investigated the neural correlates of the anticipation of a painful stimulus that serves as a go-cue for a motor response (Babiloni et al. 2006, 2008, 2010). The results showed that the anticipation of pain yielded an increase of movement-preparatory alpha desynchronization. However, in these studies the painful stimulus served as the go-cue for the movement, so that it was not possible to unequivocally disentangle pain anticipation and movement preparation. Moreover, in these studies the motor response did not have an effect on the pain stimulus and thus no biological function. In contrast, in the present study we did not simply investigate pain anticipation and motor preparation but the effects of pain on the preparation of a biologically meaningful motor response. Another study investigated EEG activity during the anticipation of painful stimuli that could be stopped by a motor response (Piedimonte et al. 2017). The results showed that EEG responses related to movement preparation did not consistently differ between different expectations of pain. However, that study assessed the influence of different expectations of pain on motor preparatory brain activity, whereas the present study assessed the influence of pain on motor preparation. Hence, a direct comparison of both studies appears inappropriate. Another recent EEG study compared arm movements during ongoing pain, during ongoing warm stimulation, or without concurrent stimuli (Misra et al. 2017). Concomitant pain led to increases of movement-preparatory beta desynchronizations and a shortening of reaction times compared with warm stimulation or no stimulation, suggesting a facilitating effect of pain on the motor system. In contrast, the present study does not show an effect of pain on movement-preparatory desynchronizations at beta frequencies. However, the previous study used an externally cued paradigm in which the movement was always performed 2,500 ms after onset of the painful stimulus, whereas in the present study participants were free to press the button at any time. Moreover, in the previous study the painful stimulus had no functional relationship to the movement, whereas participants performed biologically relevant movements that stopped the painful stimuli in the present study. These differences between paradigms could explain the lack of effects on event-related desynchronizations in the present study compared with previous studies.
Externally Paced vs. Self-Paced Movements
We observed a significant decrease in the amplitude of the preparatory readiness potential when movements were performed to stop a painful or nonpainful thermal stimulus compared with movements without these stimuli. In principle, this finding can be explained by three different factors. First, the simple presence of a thermal stimulus or any sensory stimulus might attenuate the readiness potential by directing attention from movement preparation toward the sensory stimulus, which is known to influence the readiness potential (Birbaumer et al. 1990). Second, not the simple presence of the stimulus but the pacing of the movement by the stimulus might yield the decrease of the readiness potential. In light of previous findings, this factor could well explain the present observations. Although each individual could freely determine when to stop the stimulation, the Pain & Buttonpress and Warmth & Buttonpress conditions included responding to an external stimulus whereas movements in the Buttonpress condition were performed at an internally paced rate. A few studies investigating the difference in preparatory activity between internally generated and externally driven movements indeed reported that the amplitude of the readiness potential was significantly smaller for externally paced than for self-paced movements (Jahanshahi et al. 1995; Jankelowitz and Colebatch 2002), which would be well compatible with the present findings. Third, neither the simple presence of the stimulus nor responding to the stimulus but rather the option to stop the stimulus, i.e., the sense of agency, might cause the decrease of the readiness potential. However, the influence of the sense of agency on the readiness potential has not yet been investigated, and its contribution to the present findings therefore remains unknown. Further studies might investigate which of the three factors or which combination of factors explains the effect observed here. In particular, studies comparing the effects of pain with the effects of other sensory modalities might help to more precisely interpret the observed effects.
Limitations
Several limitations apply to the present study. First, we performed the different conditions in a fixed order. This was done to adjust the timing of the stimulations and movements of the Pain and Buttonpress conditions to that of the Pain & Buttonpress condition. However, the fixed order implies the risk of an order effect. However, in line with repetition effects in sensory (Grill-Spector et al. 2006) and motor (Hamilton and Grafton 2009) systems, repeated finger movements would be expected to be associated with a decrease of related brain activity. In contrast, the present findings could only be explained by an increase of movement-preparatory brain activity over time with repeated performance. Second, we did not directly assess muscle activity and/or movement kinematics. Thus we cannot determine whether the observed difference between movement-preparatory brain activity with and without concomitant stimulation is associated with a difference in preparatory muscle activity and/or a difference in movement characteristics. Third, the lack of a pain-specific effect of pain on motor preparation in our study does not rule out the existence of such effects. They may manifest in other EEG features such as connectivity and/or at other locations such as subcortical regions that are not well captured by EEG.
Conclusions
The present study shows that pain reduces movement-preparatory activity in the brain in a paradigm with a high ecological validity. This effect is, however, not pain specific but seems to represent a modality-spanning phenomenon that might reflect the basic difference between stimulus-related and self-paced movements. Pain-specific interactions between pain and motor preparation thus remain to be demonstrated. A better understanding of these interactions promises novel insights into how the protective function of pain is implemented in the brain and how these processes might deviate in chronic pain.
GRANTS
The study was supported by the Deutsche Forschungsgemeinschaft (Grants PL 321/11-1 and RTG 1373 to M. Ploner).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M. Postorino, E.S.M., M.M.N., L.T., and M. Ploner conceived and designed research; M. Postorino, E.S.M., M.M.N., and L.T. performed experiments; M. Postorino and E.S.M. analyzed data; M. Postorino, E.S.M., M.M.N., L.T., and M. Ploner interpreted results of experiments; M. Postorino prepared figures; M. Postorino, E.S.M., and M. Ploner drafted manuscript; M. Postorino, E.S.M., M.M.N., L.T., and M. Ploner edited and revised manuscript; M. Postorino, E.S.M., M.M.N., L.T., and M. Ploner approved final version of manuscript.
REFERENCES
- Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 9: 463–484, 2005. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Brancucci A, Del Percio C, Capotosto P, Arendt-Nielsen L, Chen AC, Rossini PM. Anticipatory electroencephalography alpha rhythm predicts subjective perception of pain intensity. J Pain 7: 709–717, 2006. doi: 10.1016/j.jpain.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Capotosto P, Brancucci A, Del Percio C, Petrini L, Buttiglione M, Cibelli G, Romani GL, Rossini PM, Arendt-Nielsen L. Cortical alpha rhythms are related to the anticipation of sensorimotor interaction between painful stimuli and movements: a high-resolution EEG study. J Pain 9: 902–911, 2008. doi: 10.1016/j.jpain.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Capotosto P, Del Percio C, Babiloni F, Petrini L, Buttiglione M, Cibelli G, Marusiak J, Romani GL, Arendt-Nielsen L, Rossini PM. Sensorimotor interaction between somatosensory painful stimuli and motor sequences affects both anticipatory alpha rhythms and behavior as a function of the event side. Brain Res Bull 81: 398–405, 2010. doi: 10.1016/j.brainresbull.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Bank PJ, Peper CE, Marinus J, Beek PJ, van Hilten JJ. Motor consequences of experimentally induced limb pain: a systematic review. Eur J Pain 17: 145–157, 2013. doi: 10.1002/j.1532-2149.2012.00186.x. [DOI] [PubMed] [Google Scholar]
- Birbaumer N, Elbert T, Canavan AG, Rockstroh B. Slow potentials of the cerebral cortex and behavior. Physiol Rev 70: 1–41, 1990. [DOI] [PubMed] [Google Scholar]
- Brunia CH, van Boxtel GJ, Böcker KB. Negative slow waves as indices of anticipation: the bereitschaftspotential, the contingent negative variation, and the stimulus-preceding negativity. In: The Oxford Handbook of Event-Related Potential Components, edited by Luck SJ, Kappenman ES. Oxford, UK: Oxford Univ. Press, 2012, p. 189–207. [Google Scholar]
- Cheyne DO. MEG studies of sensorimotor rhythms: a review. Exp Neurol 245: 27–39, 2013. doi: 10.1016/j.expneurol.2012.08.030. [DOI] [PubMed] [Google Scholar]
- Colebatch JG. Bereitschaftspotential and movement-related potentials: origin, significance, and application in disorders of human movement. Mov Disord 22: 601–610, 2007. doi: 10.1002/mds.21323. [DOI] [PubMed] [Google Scholar]
- Dum RP, Levinthal DJ, Strick PL. The spinothalamic system targets motor and sensory areas in the cerebral cortex of monkeys. J Neurosci 29: 14223–14235, 2009. doi: 10.1523/JNEUROSCI.3398-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer S, Luppino G, Rozzi S. Motor cortex. In: The Human Nervous System, edited by Mai JK, Paxinos G. Amsterdam: Elsevier, 2012, p. 1012–1035. doi: 10.1016/B978-0-12-374236-0.10027-6. [DOI] [Google Scholar]
- Grill-Spector K, Henson R, Martin A. Repetition and the brain: neural models of stimulus-specific effects. Trends Cogn Sci 10: 14–23, 2006. doi: 10.1016/j.tics.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Hagander LG, Midani HA, Kuskowski MA, Parry GJ. Quantitative sensory testing: effect of site and skin temperature on thermal thresholds. Clin Neurophysiol 111: 17–22, 2000. doi: 10.1016/S1388-2457(99)00192-3. [DOI] [PubMed] [Google Scholar]
- Hamilton AF, Grafton ST. Repetition suppression for performed hand gestures revealed by fMRI. Hum Brain Mapp 30: 2898–2906, 2009. doi: 10.1002/hbm.20717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges PW, Smeets RJ. Interaction between pain, movement, and physical activity: short-term benefits, long-term consequences, and targets for treatment. Clin J Pain 31: 97–107, 2015. doi: 10.1097/AJP.0000000000000098. [DOI] [PubMed] [Google Scholar]
- Hodges PW, Tucker K. Moving differently in pain: a new theory to explain the adaptation to pain. Pain 152, Suppl: S90–S98, 2011. doi: 10.1016/j.pain.2010.10.020. [DOI] [PubMed] [Google Scholar]
- Jahanshahi M, Jenkins IH, Brown RG, Marsden CD, Passingham RE, Brooks DJ. Self-initiated versus externally triggered movements: I. An investigation using measurement of regional cerebral blood flow with PET and movement-related potentials in normal and Parkinson’s disease subjects. Brain 118: 913–933, 1995. doi: 10.1093/brain/118.4.913. [DOI] [PubMed] [Google Scholar]
- Jankelowitz SK, Colebatch JG. Movement-related potentials associated with self-paced, cued and imagined arm movements. Exp Brain Res 147: 98–107, 2002. doi: 10.1007/s00221-002-1220-8. [DOI] [PubMed] [Google Scholar]
- Jung TP, Makeig S, Humphries C, Lee TW, McKeown MJ, Iragui V, Sejnowski TJ. Removing electroencephalographic artifacts by blind source separation. Psychophysiology 37: 163–178, 2000. doi: 10.1111/1469-8986.3720163. [DOI] [PubMed] [Google Scholar]
- Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods 164: 177–190, 2007. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Melzack R, Casey KL. Sensory, motivational, and central control determinants of pain: a new conceptual model in pain. In: The Skin Senses, edited by Kenshalo DR. Springfield, IL: Thomas, 1968, p. 423–439. [Google Scholar]
- Misra G, Coombes SA. Neuroimaging evidence of motor control and pain processing in the human midcingulate cortex. Cereb Cortex 25: 1906–1919, 2015. doi: 10.1093/cercor/bhu001. [DOI] [PubMed] [Google Scholar]
- Misra G, Ofori E, Chung JW, Coombes SA. Pain-related suppression of beta oscillations facilitates voluntary movement. Cereb Cortex 27: 2592–2606, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison I, Perini I, Dunham J. Facets and mechanisms of adaptive pain behavior: predictive regulation and action. Front Hum Neurosci 7: 755, 2013. doi: 10.3389/fnhum.2013.00755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mylius V, Borckardt JJ, Lefaucheur JP. Noninvasive cortical modulation of experimental pain. Pain 153: 1350–1363, 2012. doi: 10.1016/j.pain.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Naugle KM, Fillingim RB, Riley JL III. A meta-analytic review of the hypoalgesic effects of exercise. J Pain 13: 1139–1150, 2012. doi: 10.1016/j.jpain.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen JP, Nizard J, Keravel Y, Lefaucheur JP. Invasive brain stimulation for the treatment of neuropathic pain. Nat Rev Neurol 7: 699–709, 2011. doi: 10.1038/nrneurol.2011.138. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113, 1971. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen JM. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci 2011: 156869, 2011. doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perini I, Bergstrand S, Morrison I. Where pain meets action in the human brain. J Neurosci 33: 15930–15939, 2013. doi: 10.1523/JNEUROSCI.3135-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol 110: 1842–1857, 1999. doi: 10.1016/S1388-2457(99)00141-8. [DOI] [PubMed] [Google Scholar]
- Piedimonte A, Guerra G, Vighetti S, Carlino E. Measuring expectation of pain: contingent negative variation in placebo and nocebo effects. Eur J Pain 21: 874–885, 2017. doi: 10.1002/ejp.990. [DOI] [PubMed] [Google Scholar]
- Shibasaki H, Hallett M. What is the Bereitschaftspotential? Clin Neurophysiol 117: 2341–2356, 2006. doi: 10.1016/j.clinph.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Sullivan MJ. Toward a biopsychomotor conceptualization of pain: implications for research and intervention. Clin J Pain 24: 281–290, 2008. doi: 10.1097/AJP.0b013e318164bb15. [DOI] [PubMed] [Google Scholar]
- Tabor A, Keogh E, Eccleston C. Embodied pain-negotiating the boundaries of possible action. Pain 158: 1007–1011, 2017. doi: 10.1097/j.pain.0000000000000875. [DOI] [PubMed] [Google Scholar]
- van Ede F, Maris E. Physiological plausibility can increase reproducibility in cognitive neuroscience. Trends Cogn Sci 20: 567–569, 2016. doi: 10.1016/j.tics.2016.05.006. [DOI] [PubMed] [Google Scholar]
- van Wijk BC, Beek PJ, Daffertshofer A. Neural synchrony within the motor system: what have we learned so far? Front Hum Neurosci 6: 252, 2012. doi: 10.3389/fnhum.2012.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Sikes WR. Cingulate nociceptive circuitry and roles in pain processing: the cingulate premotor pain model. In: Cingulate Neurobiology and Disease, edited by Vogt BA. New York: Oxford Univ. Press, 2009, p. 311–338. [Google Scholar]
- Wiech K, Tracey I. Pain, decisions, and actions: a motivational perspective. Front Neurosci 7: 46, 2013. doi: 10.3389/fnins.2013.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]