We used the smooth eye movement pursuit system to link between patterns of preparatory activity in the frontal eye fields and movement during a target selection task. The dominant pattern was a ramping signal that did not discriminate between selection conditions and was linked, on trial-by-trial basis, to movement latency. A weaker pattern was composed of a constant signal that discriminated between selection conditions but was only weakly linked to the movement parameters.
Keywords: FEF, latency, preparatory activity, smooth pursuit, target selection
Abstract
We investigated the composition of preparatory activity of frontal eye field (FEF) neurons in monkeys performing a pursuit target selection task. In response to the orthogonal motion of a large and a small reward target, monkeys initiated pursuit biased toward the direction of large reward target motion. FEF neurons exhibited robust preparatory activity preceding movement initiation in this task. Preparatory activity consisted of two components, ramping activity that was constant across target selection conditions, and a flat offset in firing rates that signaled the target selection condition. Ramping activity accounted for 50% of the variance in the preparatory activity and was linked most strongly, on a trial-by-trial basis, to pursuit eye movement latency rather than to its direction or gain. The offset in firing rates that discriminated target selection conditions accounted for 25% of the variance in the preparatory activity and was commensurate with a winner-take-all representation, signaling the direction of large reward target motion rather than a representation that matched the parameters of the upcoming movement. These offer new insights into the role that the frontal eye fields play in target selection and pursuit control. They show that preparatory activity in the FEF signals more strongly when to move rather than where or how to move and suggest that structures outside the FEF augment its contributions to the target selection process.
NEW & NOTEWORTHY We used the smooth eye movement pursuit system to link between patterns of preparatory activity in the frontal eye fields and movement during a target selection task. The dominant pattern was a ramping signal that did not discriminate between selection conditions and was linked, on trial-by-trial basis, to movement latency. A weaker pattern was composed of a constant signal that discriminated between selection conditions but was only weakly linked to the movement parameters.
preparatory activity, neural activity that precedes movement initiation, is believed to be an implementation of the behavioral processes of motor planning and preparation (Evarts et al. 1984; Riehle and Requin 1993). Prior to the execution movements, preparatory activity in frontal cortical areas is known to be predictive of subsequently executed movements (Churchland et al. 2010; Cisek and Kalaska 2002; Hanes and Schall 1996). Just as multiple variables can be prespecified for a given motor plan (Rosenbaum 1980), preparatory activity can be decomposed into separable components of activity that are predictive of different aspects of future behavior (Kaufman et al. 2016; Schall 2002). The approach of dissecting preparatory activity into its constituent components and linking them to future motor parameters remains a primary goal of the study of preparatory processes in any model motor system.
Our goal is to extend this type of analysis to the study of preparatory processes that influence smooth pursuit eye movements. These are smooth rotations of the eye that allow moving targets to remain on the fovea (Lisberger 2010). Prior studies have repeatedly shown that motor preparation influences the pursuit system at a behavioral level (Barnes 2008). There have also been a few studies that have also demonstrated preparatory activity in frontal cortical structures responsible for smooth eye movement control (Fukushima et al. 2011; Mahaffy and Krauzlis 2011b; Shichinohe et al. 2009). To date, however, these studies have not probed preparatory activity to the extent that prior work in other systems such as the saccade or arm movement systems has. For example, it is currently unknown whether preparatory activity in FEF can be decomposed into multiple components. Moreover, the degree to which preparatory activity in FEF is predictive of the metrics of smooth pursuit has not been rigorously quantified.
We sought to address these gaps using a recently developed target selection paradigm (Joshua and Lisberger 2012) that provides a natural context to study the different components of preparatory activity. Target selection occurs in the pursuit system when a human or monkey is faced with multiple targets to pursue and has to choose one target over others, to track it (Case and Ferrera 2007; Garbutt and Lisberger 2006). In our paradigm, an animal preselects one of two targets to track as they prepare for the forthcoming movement. In many ways, our paradigm is akin to the visual search paradigms that have been used to partition preparatory activity related to motor preparation and selection in the saccade system (Sato et al. 2001; Thompson et al. 1996). As monkeys performed this task, we recorded activity in the frontal eye fields (FEF), a region of the frontal cortex that has a well-established role in pursuit planning and control (Ilg and Thier 2008; Tanaka and Lisberger 2002a). FEF forms a critical node in the pursuit circuit (Gottlieb et al. 1994), with prior studies indicating buildup activity of pursuit neurons in FEF preceding pursuit initiation that seems related to the process of motor preparation both outside (Fukushima et al. 2011; Tanaka and Fukushima 1998) and during the context of target selection (Mahaffy and Krauzlis 2011b).
Using this paradigm, we were able to elicit robust preparatory activity in FEF neurons preceding movement onset. Furthermore, we could dissect this preparatory activity into separable components that signaled the selection of pursuit direction and pursuit latency. Specifically, we found that preparatory activity preceding pursuit initiation, in our target selection task, was dominated by buildup or ramping neural activity that terminated at the time of target motion onset. This ramping signal was linked, on a trial-by-trial basis to the latency of the subsequently executed pursuit eye movement. To a smaller degree, activity was also indicative of which target the animal selected to pursue. This was manifested as an offset in the ramping response relative to baseline and seemed more related to the selection of target direction than the actual kinematics of the future movement, as demonstrated by a set of behavioral controls. The size of this pursuit direction signal was small by comparison with the ramping signal. Therefore, we suspect that the target selection process is likely augmented by the contribution of structures outside FEF.
MATERIALS AND METHODS
We collected neural and behavior data from two male rhesus macaque monkeys (Macaca mulatta). All procedures were approved in advance by the Institutional Animal Care and Use Committee at Duke University, where the experiments were performed. Procedures were in strict compliance with the National Institutes of Health “Guide for the Care and Use of Laboratory Animals”. We implanted head holders to allow us to restrain each monkey’s head, and a coil of wire on one eye to measure eye position using the magnetic search coil technique (Judge et al. 1980). After the monkeys had recovered from surgery, we trained the monkeys to track spots of light that moved across a video monitor placed in front of them. In a later surgery, we placed a recording cylinder stereotaxically over the frontal eye fields. The center of cylinder was placed above the skull at 24 mm anterior and 20 mm lateral to the stereotaxic zero.
Up to five quartz-insulated tungsten electrodes were lowered into the caudal parts of the FEF to record spikes using a Mini-Matrix System (Thomas Recording). Signals were high-pass filtered with a cutoff frequency of 150 Hz and digitized at a sampling rate of 40 kHz (Plexon MAP). For the detailed data analysis, we sorted spikes offline (Plexon). For sorting, we used a principal component analysis and corrected manually for errors. We paid special care to the isolation of spikes from single neurons, and we included neurons for further analysis only when they formed distinct clusters in principal component analysis space. Sorted spikes were converted to time stamps with a time resolution of 1 ms and were inspected again visually to look for instability and obvious sorting errors.
Experimental design.
While lowering the electrodes, we looked for neurons that responded during pursuit eye movements. To test neurons for pursuit responses, we used a target (white circle, 0.5° diameter) that moved in one of eight directions. The targets stepped in one direction and moved in 20°/s in the other direction (step ramp). Typically, neurons in the parts of the FEF that are closer to the brain surface did not respond to our search stimuli, and we had to lower our electrodes deeper into the arcuate sulcus, where we found neurons that responded to pursuit.
After we characterized pursuit tuning, we subjected the monkeys to the main experiment protocol in which we introduced target selection and single target trials, while manipulating the reward size. Each trial started with a bright white target that appeared in the center of the screen. After 500 ms of presentation, in which the monkey was required to acquire fixation, colored targets appeared 3° eccentric to the fixation target. The monkeys were required to continue to fixate the white target in the center of the screen; gaze shifts resulted in aborting the trial.
In target selection trials, two colored cues appeared eccentric to the fixation target along orthogonal directions. The color of the target signaled the size of the reward that the monkey would receive if it tracked the target. For monkey Y, we used yellow to signal a large reward (0.1–0.2 ml) and green to signal a small reward (0.05 ml); in monkey X, we reversed these associations. After a random delay of 800–1,200 ms, the two colored targets stepped to a location 4–5° eccentric to the center of the screen and started to move toward the center of the screen at 30°/s, prompting the initiation of smooth pursuit. During the initiation of movement, we suspended the requirement to keep the eye close to the target, so as not to restrict behavior. Typically, monkeys initiated a pursuit eye movement that was biased to the large reward target, followed by a saccade that brought the eye toward one of the targets. Online, we would detect this saccade by applying a threshold on eye speed of 80°/s and blank the target that was furthest from the eye after the saccade. At the end of the trial, the target stopped, and if the eye were within a 2 × 2 degree window around the target, the monkey received a juice reward.
Single target trials were interleaved with selection trials. The single target trials had the same temporal structure and the same association between reward size and color. In these trials, we aimed to mimic the behavior observed during target selection trials. In single target trials, the target motion and target presentation before movement were rotated 0, 10, 20, 30, 45, 60, 70, 80, and 90° relative to the axis of the selection targets. For analysis, we selected the trials that were closest (L1 norm) to the average behavior observed during target selection trials; we defined these trials as “mimic behavior” trials. We constructed another set of trials in which the color cue appeared in the same location of the cue that signaled for large reward in the selection trials (i.e., 0 and 90° trials), but the behavior was different than the selection, as it was less biased. We refer to these trials as “mimic cue” trials. In several behavior sessions, the direction bias of the monkey during selection was very small, and therefore, there was very small difference between mimic behavior and mimic cue trials. This does not invalidate any of our results but led to small-power in the analysis in these sessions. Removing these sessions from our analysis did not change our conclusions. The target speed in the single target trials was optimized to mimic the eye movement speed during selection and was set to 20°/s and 15°/s for monkeys Y and X. Interleaved were additional single target trials (~15%), in which the target moved in the cardinal directions at 30°/s. However, during the preparatory period of these trials, an animal did not have an indication as to the future speed of the target. We confirmed that using these 30°/s trials for the analysis did not alter any of our conclusions.
We aimed to keep trial number similar between mimic behavior and mimic cue trials to retain a similar amount of noise in our estimates of the neural activity in these conditions. Our procedure for selecting mimic behavior trials could lead to pooling trials with different target motion conditions. We confirmed that this did not have a large impact on our results by restricting analysis to mimic behavior trials from a single target motion condition that resulted in an average behavior that was closest to the behavior observed during our target selection trials. Although this yielded a slightly larger difference in behavior between the target selection and mimic behavior trials, it did not alter any of our conclusions.
To increase the power of trial-by-trial statistical tests, we presented target selection trials 4–5 times more than other trials, and as a result, these trials consisted of about 35–40% of the trials in a session. We only included neurons in our analysis that were recorded for more than five trials in all conditions and with at least 20 trials in each selection condition. For calculating trial-by-trial correlation analysis, we only used neurons that were recorded for more than 50 trials per target selection condition. We used this stricter criterion because of the large trial-by-trial variability of cortical neurons. Overall, we analyzed the activity of 198 neurons across animals (n = 119 from monkey Y, n = 79 from monkey X). Some neurons were recorded in multiple blocks that had different task configurations with respect to movement directions. This added 34 additional recording sessions (19 and 15 from monkeys Y and X, respectively) to our database. For clarity of presentation in the text, we refer to the 232 analyzed sessions as “neurons.” Keeping to only one session per each neuron did not alter any of our conclusions. Per each session, one target direction was chosen to align with the preferred direction of at least one neuron recorded using our five-channel system. Tuning could differ between neurons on different recording channels; therefore, not all neurons included in the analysis below were recorded with a target moving in their preferred direction.
Data analysis.
All analysis presented below was performed using MATLAB (MathWorks). We studied the time-varying properties of spike density functions calculated from spike times with a Gaussian window of 10 ms. For the trial-by-trial analysis, we decomposed the behavior from each trial into latency, direction, and gain using a previously published algorithm (Lee and Lisberger 2013). This algorithm constructs a template from trial-averaged pursuit velocity and calculates how much each single trial needs to be shifted in time and/or scaled in amplitude to match this template. These shift and scale parameters provide an estimate for trial-by-trial variations in latency, direction, and gain as we explain in detail below. We used the average horizontal and vertical eye velocity from 25 to 175 ms after the onset of target motion as our initial template. Using slightly longer or shorter intervals (±50 ms) did not alter any of our conclusions.
Before applying our fitting procedure, we determined the rotation matrix required to ensure that our template’s direction at the end of analysis interval averaged 45°. We then multiplied single trial velocity traces by this rotation matrix. This step simplifies the calculation of each trial’s direction and scaling factor, without altering the relevant structure of the data (Lee and Lisberger 2013). For each trial, we fit scaling factors for the horizontal and vertical velocity components and a latency shift factor that reduced the mean square error for matching the template to the traces from single trials. After calculating scaling factors and latency shift factors for all trials, we corrected each set of velocity traces using the latency factor, calculated a new template, and repeated the fitting process. In practice, across iterations, the correlation between the estimated parameters was close to 1, and our conclusions did not depend on the number of iterations. We decided to use single correction iteration because after the first iteration, the overall fit quality increased rapidly, and further iterations added very little to the quality of the fit. Given the scaling factors gv and gh, the single trial direction was defined as , and the scaling factor was defined as . The shift parameter was used as an estimate of the variation in latency on a trial-by-trial basis. We performed the trial-by-trial analysis separately for the selection conditions in which the large reward was horizontal or vertical and included both conditions in the summary of the analysis.
We used bootstrap methods to estimate the distribution of differences in neural activity between target selection and single target trials. This was done to test the null hypothesis that responses in selection trials and single trials were drawn from the same distribution. We first split up target selection trials by extracting a subset of trials that matched the number of single target trials (typically 1/5 to 1/4 of the trials). We calculated the differences between the firing rates in these target selection trials and the remaining target selection trials. This yields a distribution of firing rate differences for the entire neural population. We repeated this process 100 times and calculated the average of the resultant distributions. This procedure led to a slight difference between the number of trials in the resampled data and the actual data. We confirmed that matching the number of trials did not alter any of our conclusions.
To estimate the directional tuning of a neuron between the orthogonal directions of motion in target selection trials, we fit the average responses recorded during single target trials to a quadratic polynomial curve. We used a quadratic polynomial because it accommodates fits with extrema in the middle of the range. Moreover, the limited range of the fit (only 90°) ensures that higher-order terms are not necessary.
We used these directional tuning fits to perform simulations that could predict the size and significance of neuron-behavior correlations between firing rate and movement direction. We assumed that firing rate at each trial could be expressed as ratei = tuning (θi) + η, where tuning is the direction tuning estimated as specified above from the radial direction of movement θ, i indicates the trial number, and η is a variable that contains all rate modulations that are independent of the direction modulations. We estimated the variance of η by subtracting the variance of tuning (θi) from the firing rate variability. We then simulated a Gaussian variable with estimated variability of η such that rate (simulated)i = tuning (θi) + η (simulated). Finally, we calculated the correlation (Spearman’s rho) between the simulated rate and movement direction. This correlation (and its significance) estimates the neuron-behavior correlation with direction under the assumption that the firing rate in target selection trials contains all of the variability related to the movement direction as defined by the tuning curve. We also repeated the analysis by fitting a linear function between the movement direction and the average response in target selection conditions and found that it did not alter any of our conclusions.
RESULTS
Monkeys bias their pursuit from the initiation of movement.
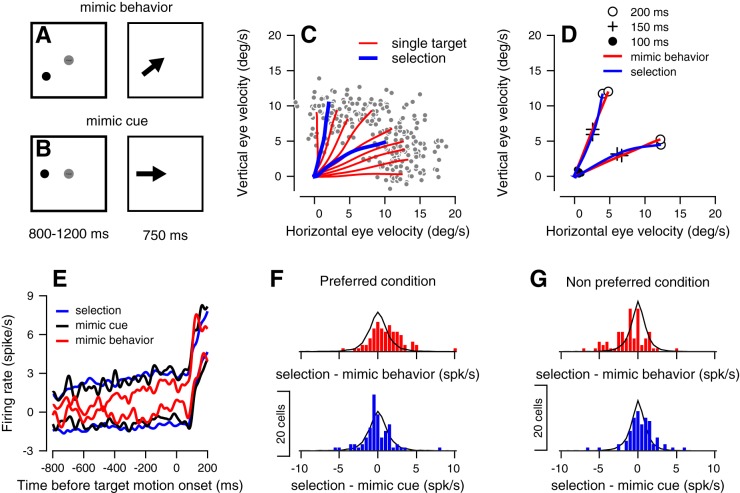
We recorded the activity of 232 neurons from the FEF, while the monkeys were engaged in a target selection task (Joshua and Lisberger 2012). In the beginning of each trial, monkeys were required to fixate the center of the screen, while two colored dots appeared peripherally. After a variable delay, both dots moved toward the center of the screen at a constant speed, at which point the monkeys could select either target to track and receive a reward. The crucial behavior manipulation involved the mapping between reward size and the color of the targets. For example, in the scheme in Fig. 1A, when the monkey selected the target that moved horizontally, it received a large amount of juice and when it tracked the target that moved along the vertical axis, it received a small amount of juice. The location of the low-reward and high-reward targets was randomly interleaved between trials. In all of the experiments, the targets were orthogonal to one another. For simplicity, we refer to these directions as vertical and horizontal directions throughout this report, although in some sessions, the targets moved along the oblique directions of the screen.
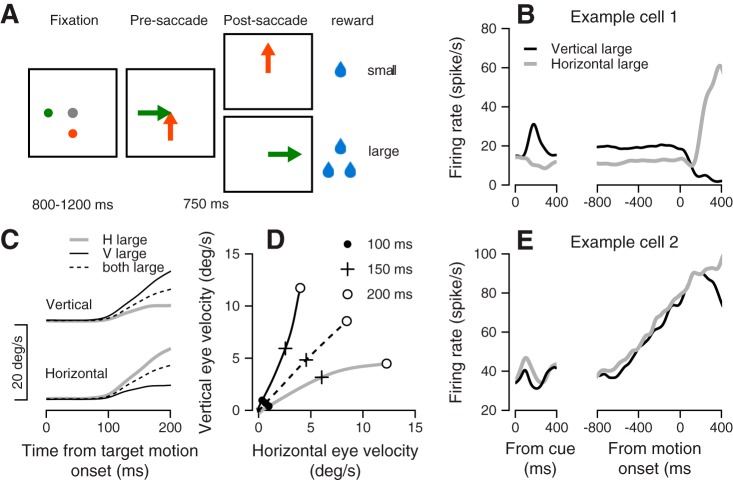
Fig. 1.
Target selection task, behavior, and example for neural responses. A: sequence of snapshots illustrates the structure of the behavioral task with two targets. B: average firing rate responses from example cell 1. C: average across all sessions of the vertical (top) and horizontal (bottom) eye velocity. D: data from C were replotted to show the trajectory of the eye velocity traces. Traces plot the average horizontal vs. vertical eye velocity in the first 200 ms after the onset of target motion. The solid circles (●), plus signs (+), and open circles (○) show the data at 100, 150 and 200 ms, respectively, after target motion onset. The gray, black, and dashed traces in C and D show the data from trials where the large reward was vertical, horizontal, or on both axes. E: average firing rate responses from example cell 2. The lines show the response in interleaved selection conditions in which the large reward was either horizontal (gray) or vertical (black). Left and right plots show activity aligned to onset of color cue and onset of target motion.
In response to target motion in target selection trials, monkeys initiated a smooth pursuit eye movement that was biased toward the target that was associated with a large reward (Fig. 1, B and C). Colored targets were presented for at least 800 ms before motion onset, giving the monkey time to prepare for the upcoming motion. Prior studies have shown that this early bias toward the high-reward target is a result of monkeys using information before target motion onset to bias their pursuit (Case and Ferrera 2007; Joshua and Lisberger 2012). Later in the trial, monkeys made corrective saccades, which in 99% of target selection trials brought their gaze toward the large-reward target. When both targets were associated with a large reward, monkeys initiated pursuit in a direction equivalent to the vector average of the two target motion directions. We were interested in studying how the preparatory activity before target motion was linked to the reward-biased behavior observed. Therefore, we focused our analysis on neural responses in the preparatory period.
Characteristics of FEF population preparatory activity preceding target motion.
Using our task, we were able to drive robust preparatory activity across many FEF neurons. The neurons presented in Fig. 1, B and E show two examples of activity patterns seen in preparatory activity during selection trials in which the larger reward target appeared either along the horizontal or vertical axis. The neuron presented in Fig. 1B encoded these different selection conditions during the preparatory period, as indicated by the difference between the black and gray lines before target motion onset. After an initial response to the appearance of the color cue, the difference in this neuron’s firing rate between the selection conditions remained close to constant. The neuron presented in Fig. 1E showed a qualitatively and quantitatively different pattern of activity preceding target motion. The neuron had a strong ramp in activity in the preparatory period, but the ramp was similar in both selection conditions, as indicated by the similarity of the black and gray traces in Fig. 1E up to motion onset.
The pattern of activity displayed by our two example neurons was mirrored at the population level. When we tested whether the number of spikes in the 800-ms window preceding target motion was dependent on whether the large reward target moved horizontally or vertically, we found that 30% of all recorded neurons responded differently (P < 0.01, Mann-Whitney U test) between these conditions. We refer to the selection condition that elicited the largest preparatory response as the preferred condition for the neuron in question, and the other condition as the nonpreferred condition of the neuron. Large-reward target motion and small-reward target motion always moved along orthogonal directions, but depending on the neuron in question, the direction of large-reward target motion could be along any one of eight radially spaced directions. This is because we attempted to tailor our stimuli to the movement fields of the neurons we recorded.
To study the dynamics of the population preparatory responses across selection conditions, we calculated the average responses of the neurons that passed the significance test for a difference between the preferred and nonpreferred condition. Around 100 ms after cue onset, the average population activity diverged depending on whether the neuron was recorded in the preferred and nonpreferred condition (Fig. 2A difference between the blue and red curves). In both cases, there was a cue-locked change in activity followed by a sustained rate in firing (Fig. 2B). The time taken to achieve this representation was much shorter than the length of delay epoch preceding target motion that was randomly selected to be between 800 and 1,200 ms.
Fig. 2.
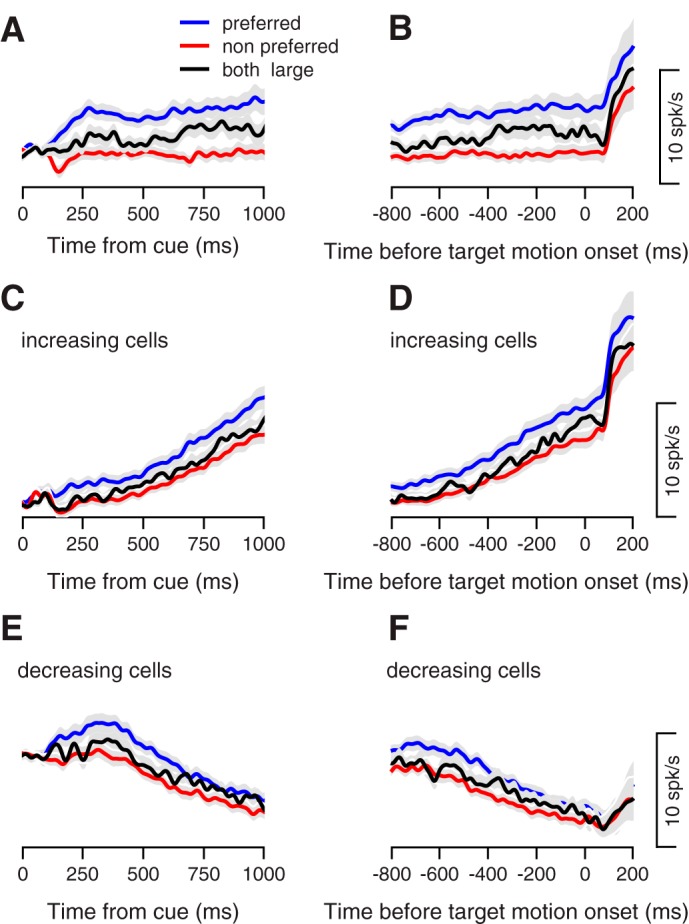
Neural responses during the planning of target selection. Average firing rate across the population during the delay period. A, C, E: traces show activity aligned to the onset of color cue. B, D, and F: traces show activity aligned to target motion onset. Blue and red traces show the neural activity in the preferred and nonpreferred selection conditions. Black traces show activity in the condition where both targets signaled a large reward. Gray shading shows the means ± SE. Negative values of the horizontal axis correspond to time before movement onset. In A and B, we only used the neurons with a significantly different response (P < 0.01, Mann-Whitney U-test) in the selection conditions (n = 72). In C–F, we only used neurons that increased (n = 82) or decreased (n = 48) their rate in the last 800 ms before target motion onset (P < 0.01, Wilcoxon signed rank test).
We next quantified the dynamics of population preparatory responses, per each selection condition, by dividing the 800-ms window preceding target motion into two bins and computing the firing rate change over time. In 55% of the neurons, we found a significant difference between the firing rates in the beginning vs. the end of the preparatory period (P < 0.01, Wilcoxon rank sum test). Of these, most neurons (64%) increased their rate across the epoch, and a minority (36%) decreased their rate. Examining increasing and decreasing cell populations separately, Fig. 2, C and E shows how these responses approximated smooth ramps of activity that began shortly after cue offset. These ramps in activity persisted up to the time of pursuit onset, after which neurons responded to target motion onset and the initiation of pursuit. Like the overall population average shown in Fig. 2, A and B, the average responses of upward and downward ramping subpopulations showed an offset in ramp activity for pursuit in the preferred and nonpreferred conditions (note difference between blue and red lines in Fig. 2, C–F). This offset in activity at the subpopulation level was the source of the offset in sustained activity that differentiated preferred and nonpreferred conditions at the population level for all neurons. One notable difference between the population of upward- and downward-ramping neurons was that before ramp initiation activity in the downward-ramping subpopulation underwent a transient increase (Fig. 2E). Finally, we examined activity in a behavioral condition, in which both targets signaled a large reward, and in which selection biases were absent (Fig. 1C, dashed line). At both the population and ramping subpopulation level, firing rates wound up in between the preferred and nonpreferred conditions, (Fig. 2, A–F, black lines).
Figure 2 makes clear that at a population average level, aligning to either cue or target motion onset leads to very similar conclusions about preparatory activity. Next, we tested whether this population response was the result of a cluster of neurons that responded to either cue onset or with sustained activity across the preparatory period. We found that a neuron’s firing rate 100–200 ms after cue appearance was correlated with its rate in the 100-ms preceding target motion (Pearson correlation = 0.43). We did not see strong evidence of clustering when these firing rates were plotted against one another (not shown), suggesting a continuum in the response to cue onset vs. sustained activity. Finally, across the population, responses after cue onset were smaller than responses at the end of the preparatory epoch (3.5 spike/s vs. 5.2 spike/s absolute deviation from baseline, P < 0.01, Wilcoxon rank sum test), a second confirmation that neurons robustly modulate their firing rate across the preparatory epoch.
From these analyses, we conclude that following cue onset, subpopulations of neurons exhibit activity that ramps upward or downward up to the time of pursuit onset. When pursuit is in the preferred condition of each neuron, there is an additional positive offset in activity that is maintained throughout the preparatory epoch. This offset in activity, which discriminates target directions, seems to emerge early in the preparatory epoch and well before pursuit onset. We suspect that the difference between conditions appeared early in neural activity because the brightly colored targets used in our task could be easily detected and distinguished from one another, reducing the time needed to select a given target. Had the stimuli been more ambiguous, it may have taken a longer time for neural activity (and the animal) to select one target over the other. While this has been shown in the saccadic eye movement system in the context of visual search (Thompson et al. 1996) or perceptual decisions (Kim and Shadlen 1999), future work is needed to test this hypothesis in the context of this pursuit selection task.
In the subsequent analysis, we decompose the patterns of activity that we found in the preparatory activity in this FEF population into separable components and link them to behavior in our task using trial-by-trial analysis. We then combine this analysis with control experiments that allow for deeper interpretation of the preparatory signals that we observe.
Parcellating components of preparatory activity in FEF.
The above analysis of average firing rates across the preparatory epoch suggests that there are two separate components of activity preceding pursuit onset, one component is a positive offset of activity that differs on the basis of the direction of the large reward target, the other is a ramp of activity that is agnostic to future pursuit direction. To test whether activity could be partitioned in this way, we deconstructed neural activity using two basis functions (Fig. 2). The first basis function was a ramp in activity over the preparatory epoch that was the same in both target selection conditions (Fig. 3A, blue line). The second basis function was a flat pattern of activity that encoded preferred pursuit direction via a positive constant offset (Fig. 3A, red line). For simplicity, we refer to this second basis function as a selection function, but we stress that the underlying process of selection may be completed shortly after cue onset (as discussed above). The orthogonality of these basis functions allowed us to factorize the response of every neuron that we recorded into a combination of these two patterns of activity. For each neuron, we concatenated the responses to both target-selection conditions, which resulted in a vector whose length was twice the preparatory epoch (1,600 ms). We then projected the activity of each neuron in our recorded population onto both basis functions. This procedure quantifies how much variability in preparatory activity these basis functions account for.
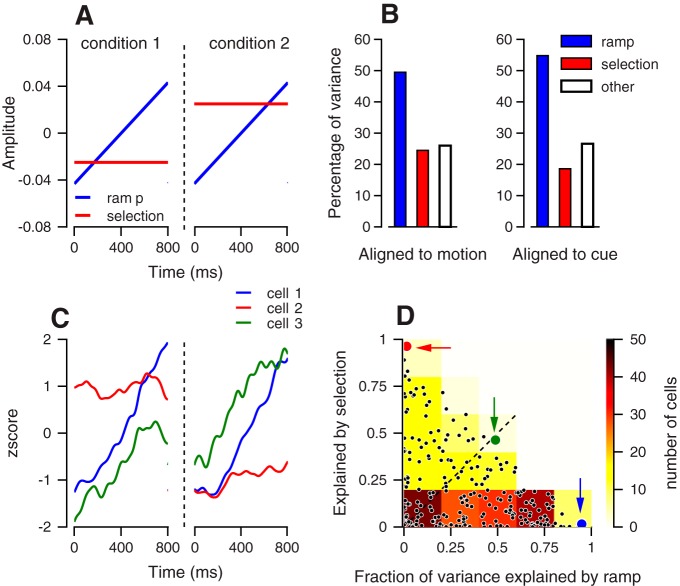
Fig. 3.
Decomposing neural activity into selection and ramping. A: basis functions for extracting selection-related and ramping activity. The response from the selection conditions was concatenated, and the dashed vertical line differentiates selection conditions. B: percentage of the variance explained by projection on the ramping (blue), selection basis functions (red), and the residual variance that cannot be explained by ramping or selection base function (white). Data were aligned to target motion onset (left) or to color cue onset (right). When aligned to cue, we excluded the time of the transient visual response (0–350 ms) and analyzed activity in the following 800 ms C: z-scores of the responses of three neurons in the last 800 ms before target motion onset. Different colors correspond to the different neurons, and the dashed vertical line differentiates selection conditions. D: fraction of the variance explained by the projection of the neuron activity on the ramp (horizontal) and selection (vertical) basis functions. Each dot shows the data from a single neuron. The red, green and blue dots correspond to the neurons in C. The background colors show the distribution of the dots binned in squares of 0.2 × 0.2, where the number of neurons in the squares determines their color. Data from all of the neurons in the data set (n = 232) were used for constructing the figure.
The result of this analysis shows that over 75% of the variability in the recorded population can be accounted for via these two basis functions. Furthermore, the ramping basis function that was agnostic to pursuit direction accounted for twice the amount of population variance as the selection function (Fig. 3B). When we aligned activity to cue onset and repeated our analysis, there was a slight increase in the variability accounted by the ramping function and a slight decrease in the variability accounted by the selection function (Fig. 3B, right), indicating this finding was robust to alignment changes across the preparatory epoch.
We also quantified the degree to which each basis function accounted for activity at a single neuron level by projecting preparatory activity onto each function on a neuron-by-neuron basis. Figure 3C shows the average activity of three neurons during the preparatory epoch, and Fig. 3D shows these neurons in a plot that compares how much of the variability in preparatory activity was captured by the ramping function vs. the selection-related function. The neuron plotted in blue in Fig. 3, C and D is an example of a neuron that was dominated by a ramping component of activity. The neuron plotted in red in Fig. 3, C and D is an example of a neuron that was dominated by a selection component of activity, but did not ramp. The neuron plotted in green in Fig. 3, C and D is an example of a neuron with a mixed representation in which about half its response was captured by the selection function and half its response was captured by the ramping function. Single neurons maintained the population level bias toward responses that were related to a ramping function vs. the selection function. As seen in Fig. 3D, the majority of the neurons were biased toward the ramping axis. For 63% of the neurons, the ramping basis function accounted for more of the variability in preparatory activity than the selection function.
The decomposition method that we used above suggests that preparatory activity in FEF during our target selection task is actually a superposition of independent components of activity. Overall, this preparatory activity is low-dimensional and dominated by ramping activity, with an additional offset component. This confirms the intuition gleaned from examining average activity. However, thus far, we do not know what aspects of behavior these two components of activity reflect. For example, many neurons in our population seem to exhibit ramping activity that does not signal the target selection condition. A reasonable hypothesis is that this activity reflects the preparation of future motor production, akin to what has been suggested for movement neurons in FEF in the context of visual search (Bichot et al. 2001; Purcell et al. 2010). We test this prediction explicitly below.
Quantifying behavioral variation in the pursuit system during target selection.
If preparatory activity in the frontal eye fields plays a role in motor production, it should vary as a function of future movement parameters. Our approach to examine the link between preparatory activity and behavior is to calculate the trial-by-trial correlations between preparatory activity and concomitant variations in pursuit initiation metrics. We first sought to independently quantify the motor parameters that exhibited significant variation on a trial-to-trial basis during pursuit initiation. For each trial, we used a previously published algorithm to extract the latency, direction, and gain of pursuit during movement initiation (Lee and Lisberger 2013; and see methods). For single trials, these three components could explain 97% of the variance in the behavior, akin to the findings of prior studies (Osborne et al. 2005). To illustrate the results of the analysis, we plot eye speed during selection trials in one example session separated according to differences in latency, direction, and gain. Trials in shades of blue were collected in the preferred condition of the neuron recorded during this session, and trials in shades of red were collected in the nonpreferred condition. We maintain this convention throughout Fig. 4. In Fig. 4A, we plot eye speed on the fifth of trials with the earliest response time (dark-shaded lines) and latest response time (light shaded lines). The clear temporal offset between these dark- and light-shaded lines demonstrates the difference in movement latency between early and late trials, respectively. Figure 4B shows the difference between trials with the greatest bias toward the larger reward target (dark shaded lines) and the trials that were furthest away from this target (light-shaded lines). Finally, Fig. 4C shows the trials with the largest and smallest pursuit gain (again, using dark- vs. light-shaded lines). We note that in contrast to data in Fig. 4, A and B, we have aligned trials in Fig. 4C to movement onset to prevent masking of gain differences by latency differences.
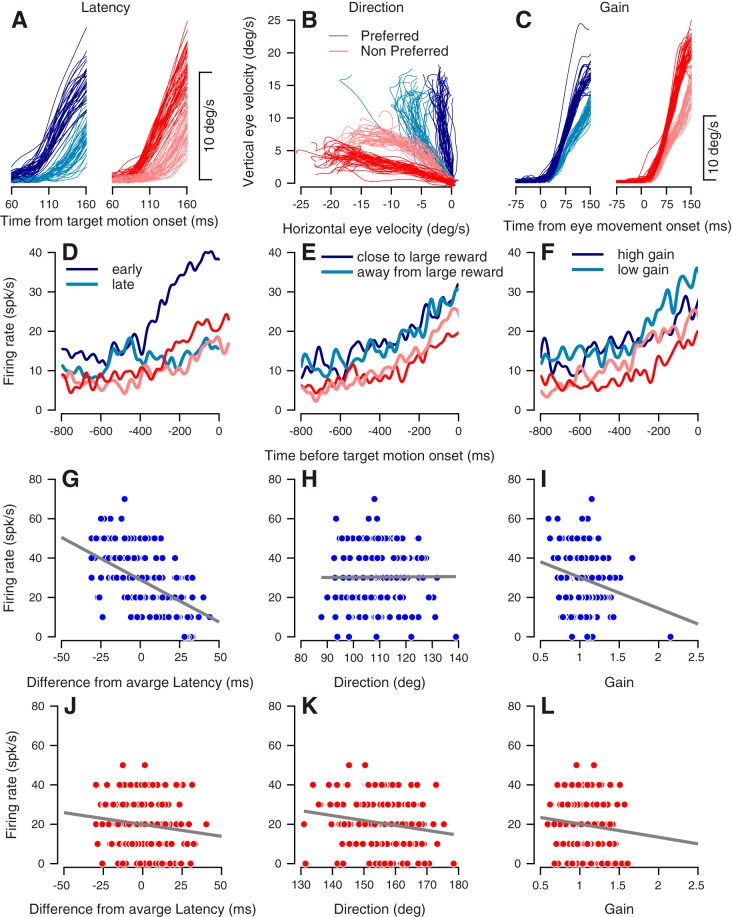
Fig. 4.
Example of trial-by-trial correlation of activity with behavior. A: eye speed as a function of time from target motion onset. Each thin line shows data from a single trial. Trials in the preferred condition are in shades of blue, and trials in the nonpreferred condition are in shades of red. Traces show one-fifth of the data from the trials with the earliest (dark-shaded lines) and latest (light-shaded lines) latency. B: horizontal vs. vertical eye velocity in the first 200 ms after target motion onset. Traces show one-fifth of the data from the trials in which behavior was the least (dark-shaded lines) and the most (light-shaded lines) biased toward the target that signaled the large reward. C: eye speed as a function of time from eye movement onset. Traces show one-fifth of the data in which the gain of the movement was maximal (dark-shaded lines) and minimal (light-shaded lines). D–F: example of one neuron’s firing rate in the last 800 ms before target motion onset. Traces in D–F show the average rate in the trials with corresponding colors in A–C. Firing rate correlations with movement latency (G), movement direction (H), and gain (I) in the preferred condition. Each dot represents a single trial; the vertical values show the firing rate at target motion onset. The gray lines show the results of the linear regression between the behavior and the rate. Firing rate correlations with movement latency (J), direction (K), and gain (L) in the nonpreferred condition of this neuron. Data in A–L are taken from a single recording session.
We found a weak correlation between eye movement latency and direction (Spearman rho = 0.06). Here, we used a convention whereby biases toward the large reward were considered as a decrease in direction, so that positive correlations between latency and direction would indicate that when the monkey moved later, the movement direction would be biased away from the large reward target. The lack of such direction-latency correlations reveals a key difference between the pursuit and saccadic eye movement systems during selection tasks. In the saccade system, there is a significant correlation between direction and latency in similar paradigms (Chou et al. 1999; Ottes et al. 1985).
We found that movement latency and gain were positively correlated (Spearman rho = 0.36), indicating that when the monkeys started to move later, they also moved faster. The level of these correlations is consistent with prior studies of the pursuit system (Osborne et al. 2005) and might result from the longer time available to integrate visual motion in trials with longer latency. Gain and direction were negatively correlated (Spearman rho = −0.15), indicating a slight tendency to move faster when pursuit was more biased toward the large reward target. This correlation contrasts with the nonsignificant correlations between direction and gain in single target step ramp task (Osborne et al. 2005), indicating that these correlations likely result from the nature of the task that we have used. Ultimately, the low values of correlations between most of the behavioral variables in our task allowed us to correlate firing rates on a trial-by-trial basis each set of behavioral variables independently. This is the method we adopt in the next section.
Trial-by-trial analysis demonstrates the strongest correlations exist between preparatory activity and pursuit latency.
We next correlated preparatory activity with behavioral metrics of pursuit during our target selection task. As an example, Fig. 4D shows one example upward ramping neuron with the average firing rate separated according to trials with early and late response latencies in the preferred condition of this neuron (dark and light blue lines, corresponding to the trials in 4A). The ramp upward in early response trials was much steeper than the ramp upward in late response trials. The difference in ramp slope between early and late trials was reduced in the nonpreferred condition (dark and light red lines, corresponding to trials in Fig. 4A). In both the preferred and nonpreferred choice conditions, this neuron’s ramping activity was weakly affected by directional bias toward the large reward target (Fig. 4E) and by movement gain (Fig. 4F).
To quantify the relationship between neural activity and behavior across all trials for a given neuron, we calculated the correlation between the instantaneous firing rate on a single trial and trial-by-trial variations in the three examined movement parameters (latency, direction, and gain). The instantaneous firing rate in a single trial was calculated by counting spikes in window of 100 ms and converting the count to spike/s units. For this example neuron and in the preferred condition, we found a strong negative correlation between the instantaneous firing rates in the 100-ms window preceding target motion onset and latency (Fig. 4G, Spearman’s rho = −0.58, P < 0.01), no correlation between the direction and firing rate (Fig. 4H, rho = 0.04, P = 0.56), and a weak correlation between gain and firing rate (Fig. 4I, rho = −0.18, P < 0.05). In the nonpreferred condition, we found a weak negative correlation between instantaneous rate in the 100-ms window preceding target motion onset and latency (Fig. 4J, rho = −0.17, P < 0.05), a weak negative correlation between direction and firing rate (Fig. 4K, rho = −0.17, P < 0.05), and no significant correlation between gain and firing rate (Fig. 4L, rho = −0.11, P = 0.11).
We next extended this neuron-behavior correlation analysis to our population of recorded neurons. Specifically, we correlated variations in instantaneous firing rate, assessed using a sliding 100-ms window, with variations in latency, gain, and direction during pursuit initiation (with variables assessed separately for each selection condition). In Fig. 5A, we illustrate, across time, the fraction of significant correlations found between the preparatory activity and individual motor parameters. The largest fraction of significant correlations (P < 0.05, Spearman’s rho) was found with latency (Fig. 5A, blue line). This fraction reached a maximum just before target motion onset. The fraction of significant correlations between preparatory activity and gain was smaller (Fig. 5A, black lines) and correlations between activity and direction were the smallest (Fig. 5A, red lines).
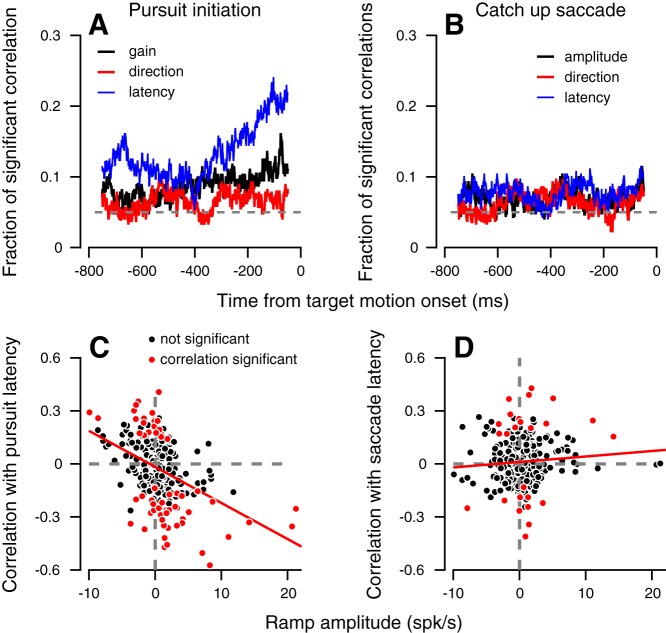
Fig. 5.
Summary of neuron behavior correlations and their relationship to ramps. Fraction of neurons with significant neuron-behavior correlations with pursuit (A) and saccade (B) parameters. The dashed line shows the chance level (0.05) used for the analysis. Correlation and their significance were calculated for trials in which the monkey had to choose between two targets. Correlations were calculated separately for the different selection conditions. Neuron behavior correlation with latency of pursuit (C) and catch-up saccades (D) as a function of the size of the ramp in activity. Each dot shows the data for a single neuron in a single selection condition. Positive and negative values on the horizontal axis correspond to ramping up and down. Significant and insignificant correlations are plotted in red and black, respectively. Data are presented only for conditions that passed the inclusion criteria for neuron-behavior correlation analysis (more than 50 trials per selection condition, n = 277 conditions).
The small fraction of significant firing rate-direction correlations might seem surprising given the directional selectivity that neurons clearly exhibited during target selection trials. We hypothesized that the small number of significant correlations, we observed were a result of the small magnitude of this direction signal. Even in the cells that significantly signaled the selection condition, there was, on average, only a four spikes/s difference between conditions (illustrated in Fig. 2A). This signal might easily be masked by noise in the firing rate and lead to small trial-by-trial correlations. On the basis of the size of the average difference in firing rate between preferred and nonpreferred selection conditions and the observed variability of firing rates, we ran simulations (see methods for details), which showed that even if all the variability in movement direction were shared between neurons in the FEF, only 8% of neurons would show significant firing rate-direction correlations. This is consistent with our hypothesis that the low-amplitude direction signal in FEF is too small to support significant trial-by-trial correlations. Further analyses, which are beyond the scope of this paper, could take into account the correlation between neurons (Schoppik et al. 2008) to get better estimates as to how informative preparatory activity in FEF is about movement direction on a trial-by-trial basis. Additionally, it is possible that neuron-behavior correlations between firing rate and direction may be task dependent and could increase when an animal pursues a single target or has to choose between two equally rewarded targets. We did not collect enough trials in these target conditions to perform our neuron-behavior correlation analysis with the requisite statistical power, but future work will examine this possibility.
Ramp amplitude is linked to movement latency and not movement direction or gain.
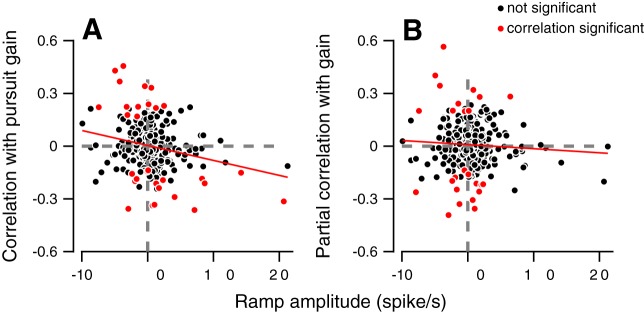
We hypothesized that the component of preparatory activity that was likely to be driving these trial-by-trial correlations with latency was selection-independent ramping activity. This hypothesis was motivated by prior studies that have shown the magnitude or slope of ramping activity in FEF to be linked with saccade response latency (Hanes and Schall 1996). To test this explicitly, for each neuron, we calculated the correlation between a neuron’s ramping amplitude in a given selection condition and its firing rate-latency correlation in the 100-ms window preceding the onset of target motion (when we observe the most sizable firing rate latency correlations). We quantified ramping amplitude by dividing the 800-ms window preceding target motion into two equally sized bins and computing the firing rate change over time. We found an overall negative correlation (Fig. 5C, Spearman’s rho = −0.40) between ramp amplitude and the neuron-behavior correlation with latency. Neurons that ramped up tended to have negative correlations with response latency (e.g., Fig. 4G), with higher firing rates leading to earlier eye movements. The neurons that ramped down tended to have positive correlation with response latency, with lower firing rates in these neurons leading to earlier eye movements. The magnitude of ramping activity was not significantly correlated with firing rate-direction correlations (P > 0.1). This is expected given the chance correlations found between firing rates and pursuit direction throughout the preparatory epoch (Fig. 5A, red lines).
In contrast to movement direction, we did find evidence that ramp amplitude was correlated with firing rate-gain correlations, however. An example of this can be observed in Fig. 4, F and I, where the magnitude of a neuron’s ramping response was negatively correlated with pursuit gain. This example was representative of the structure of our recorded population, as there was a negative correlation between ramping amplitude and neuron-behavior correlations with gain (Fig. 6A, Spearman’s rho = −0.19, P < 0.01). Moreover, this relationship was similar to the one observed with latency, with upward ramping neurons having negative correlations between firing rate and gain, and downward ramping neurons having positive correlations between firing rate and gain. A caveat to this finding is that a correlation exists between latency and gain behaviorally, as documented above. This raises the question of whether the correlation of firing rate with gain is a result of a correlation between latency and gain.
Fig. 6.
Neuron-behavior correlation with pursuit gain depends on pursuit latency. Values on the vertical axis show the neuron-behavior correlation with pursuit gain (A) and partial neuron-behavior correlation with gain when controlled for pursuit latency (B). Positive and negative values on the horizontal axis correspond to ramping up and down. Neuron-behavior correlations were calculated from the firing rate in the last 100 ms before target motion onset. Significant and insignificant correlations are plotted in red and black, respectively. Each dot shows the data for a single neuron in a single selection condition. Red line shows the results of the linear regression between the ramp amplitude and the neuron-behavior correlation with gain.
To test this, we calculated the partial correlation between firing rate in the 100 ms preceding target motion onset and pursuit gain, while controlling for pursuit latency. We found doing so abolished the correlation between ramp amplitude and firing rate-gain correlations (Fig. 6B, Spearman’s rho = −0.02, P = 0.72). Even neurons that had large ramp amplitudes were no longer correlated with gain when we controlled for the latency (compare red and black dots in the fourth quadrant of Fig. 6, A and B). By contrast, when we calculated the partial correlation between firing rate and latency while controlling for gain, we found ramp amplitude was still strongly correlated with these firing-rate latency partial correlations (Spearman’s rho = 0.37, P < 0.001). These findings suggest that the ramp activity that we found was most strongly linked to pursuit latency on a trial-by-trial basis, rather than direction or gain.
Controls concerning the sources of neuron-behavior correlations.
The core finding presented above is that FEF neurons are correlated on a trial-by-trial basis with pursuit latency over other pursuit metrics. However, it is possible that some variability in firing rates before target onset might be accounted for by the random temporal jitter between cue and target motion onset (Fig. 1A, 800–1,200 ms). This can occur if, after the color cue appears, each neuron ramps deterministically to movement initiation. This would cause the peak of ramping activity to be larger on longer trials, which could, in turn, be correlated with faster behavioral responses. We tested this possibility by calculating the partial correlation between movement latency and preparatory epoch firing rate, while controlling for the time from cue onset to target motion onset. If task event structure completely determines the firing rate-latency correlation, we would expect neurons not to be significantly correlated with latency when we control for task timing. Alternatively, if firing rate-latency correlations were driven by variability in an internal process unrelated to task structure, we would expect the same number of neurons to correlate with behavior. Intermediate values would indicate that both factors are important. We found that calculating partial correlation coefficients decreased the percentage of significant correlations with response latency (16% vs. 22%, at 0–100 ms before target motion onset). Nevertheless, this fraction remained larger than the fraction of significant correlations with either gain or direction, and suggests that both cue timing and internally generated variability contribute to firing rate-latency correlations.
We also considered whether the ramping structure and variations in preparatory activity might also be related to nonpursuit sources. The largest nonpursuit component of behavior in the task is catch-up saccades that bring the eye close to the large reward target shortly after pursuit initiation. We wanted to test whether preparatory activity might reflect prespecification of the parameters of these corrective saccades. To do so, we measured the correlation between firing rates preceding target motion and catch-up saccade parameters (Fig. 5, B and D). In the 800 ms preceding target motion, a small percentage of neurons exhibited significant (Spearman rho, P < 0.05) correlations with saccade latency (8%), amplitude (8%), or direction (5%). Ultimately, fewer neurons had significant correlations with saccade parameters than with the latency of pursuit (χ2-test, P < 0.01). In addition, ramp amplitude during the preparatory epoch did not correlate significantly with the latency of these saccades (Fig. 5D, Spearman’s rho = 0.08, P = 0.15).
In summary, the preparatory firing rates of the FEF neurons in our recording sample were most strongly linked to variations in pursuit latency over other pursuit metrics or saccade motor parameters. These correlations were driven by variations in cue timing and variations in an internal process unrelated to task structure and were linked to ramping patterns of activity preceding motion onset.
The selection component of preparatory activity encodes a winner-take-all competition between large and small reward targets.
The second component of activity that we found in the basis function analysis discussed above was an offset in preparatory firing rates that differed on the basis of the direction of large-reward target motion. Left open is the question of what is represented in this selection component. We considered two hypothetical representations. The first is that the firing rate of each neuron is the result of a competition between two separate target cue signals with the larger-amplitude target signal ultimately “winning” control over the behavior. In this case, we would expect that preparatory activity during selection trials would be close to the pattern of activity associated with pursuit toward a single large-reward target, as the large-reward target wins control over behavior in target selection trials. We refer to this pattern of differences between selection and single large-reward target trials as a winner-take-all representation (Tanaka and Lisberger 2002b). An alternative hypothesis is that the offset of activity represents the parameters of the forthcoming movement, what we term a movement trajectory hypothesis. In this case, we would expect the preparatory activity to be dictated by the unique trajectory of the movement that results from the simultaneous motion of the large- and small-reward targets (Fig. 1C).
To test this, we compared the activity of neurons in target-selection trials (henceforth, referred to as selection trials) to trials where the monkeys were presented with a single target of high reward value. The critical manipulation of these single target trials was that their location before target motion cued either 1) for a movement that was intermediate between the directions of the targets in our selection task (Fig. 7A) or 2) for a movement in the same direction as the targets in our selection task (Fig. 7B).
Fig. 7.
Dissociating a winner-take-all from a movement trajectory representation. A and B: sequence of snapshots illustrates the structure of the behavioral task with a single target. C and D: traces plot the horizontal vs. vertical eye velocity in selection (blue) and single target trials (red) from one session (C) and from the average across all the recording sessions (D). In C, gray dots show the eye velocity from single trials at 200 ms after target motion. In D, only the single target trials that were most similar to the selection trials are presented (mimic behavior trials). The solid circles (●), plus signs (+), and open circles (○) show the data at 100, 150, and 200 ms after target motion onset. E: average firing rate from 800 ms before target motion onset to 200 ms after target motion onset in the selection (blue), single large reward target (black), and mimic trials (red). The upper and lower traces show the average in the preferred and nonpreferred conditions. Histograms show the distribution of differences between the activity in the selection and mimic behavior (red) or mimic cue (blue) trials in the preferred (F) and nonpreferred condition (G). The solid black lines show the corresponding distributions of the resampling method. For each neuron, the response was averaged across the 800 ms before target motion onset. In E–G, we only used the neurons with significantly different responses (P < 0.01, Mann-Whitney U-test) between selection conditions (n = 72).
We constructed two sets of single target trials. The first was a set of trials that we refer to as mimic behavior trials. To construct this group of trials, we had monkeys pursue a set of high-reward targets that moved in directions that were between the motions used in the selection trials (Fig. 7C). We then selected the subset of trials that were closest to the behavior that we observed during our selection task. We illustrate the outcome of this trial selection procedure in Fig. 7D. The blue lines in this figure illustrate pursuit in selection trials, while the red lines indicate pursuit averaged over the subset of mimic behavior trials that were determined to be the closest pursuit in selection trials.
In the second set of trials, which we term mimic cue trials, a color cue appeared in the same location of the cue that signaled for large reward in the selection trials. Given the lack of a low-reward target in these trials, the behavior was different from that observed in selection trials, in that there was no bias away from the direction of large-reward target motion. Indeed, in these mimic cue trials, bias away from the cardinal direction along which the target moved was small in comparison to the much larger bias observed in selection trials (2 vs. 20°, P < 0.01, Wilcoxon rank sum test).
Comparing the preparatory activity of neurons during selection trials and our control trials suggests that the offset in activity that we observed above was most consistent with a winner-take-all representation. Figure 7E plots the preparatory neural responses during selection trials and control trials. The average population response in mimic behavior trials was different from the response observed during target selection trials (Fig. 7E, note the separation between the red and blue lines). Remember that behavior in selection trials and mimic behavior trials are nearly matched (Fig. 7D). This suggests that the difference between preparatory activity in the preferred and nonpreferred condition is not accounted for by the behavior that follows target motion, but is driven by different factors. By contrast, the average preparatory neural response during mimic cue trials (black lines) was very similar to the average neural response during target selection trials (blue lines). The closer proximity of mimic cue trials to selection trials seems consistent with the idea that preparatory activity is responding to the cue specifying future target motion regardless of task context, in line with the winner-take-all hypothesis.
To see whether these differences extended to the single neuron level and test for statistical significance, we calculated the difference between preparatory firing rates in selection trials and preparatory firing rates in either mimic behavior or mimic cue trials. For each neuron, we measured the average firing rate in the 800-ms window preceding target motion in the different conditions. The top histogram in Fig. 7F quantifies the firing rate difference between selection trials (in the preferred condition of each neuron) and corresponding mimic behavior trials. The firing rate in selection trials in the preferred selection condition was significantly larger than the firing rate in the mimic behavior trials (Fig. 7F, red histogram, P < 0.05 Wilcoxon rank sum test). When we looked at the firing rate difference between selection trials in the nonpreferred condition of each neuron and corresponding mimic behavior trials, we found the opposite trend; the firing rate was larger in the mimic behavior trials (Fig. 7G, red histogram, P < 0.05). By contrast, the firing rate difference between selection and mimic cue trials did not reach significance in either preferred or nonpreferred conditions (Fig. 7, F and G, blue histograms P > 0.1). Moreover, the difference in activity between the mimic behavior and selection trials was significantly larger than the difference in activity between mimic cue and selection trials (P < 0.05, Mann-Whitney U-test, on the absolute differences). From this, we conclude that the activity patterns that we observed at the population level are consistent with what we find at the single neuron level, and once again commensurate with a winner-take-all hypothesis. There are some potential caveats, however, which we discuss below.
Although in most sessions, pursuit in selection trials was substantially biased away from the cardinal directions, in some sessions, this difference was smaller. For example, the blue traces in Fig. 7C indicate that in this session, pursuit in vertical selection trials was less biased away from the nearest cardinal direction than pursuit in horizontal selection trials. There could be situations, therefore, where mimic cue trials are similar to mimic behavior trials. This would make it difficult to tease apart whether a given neural representation is consistent with the movement trajectory and winner take all hypothesis, as they make very similar predictions. To see whether this was the case, we restricted our analysis to sessions where the bias during selection trials was significantly large (more than 20°) and where there would be a large difference in behavior during mimic cue, and mimic behavior trials. Doing so did not alter any of our conclusions.
A second potential caveat is that some bias could have arisen in the above analysis from the criteria used to define each neuron’s preferred and nonpreferred condition. To address this, we took advantage of the fact that target selection trials were presented four or five times more than other trials (minimum of 24 trials, median 70). The large number of these trials allowed us to directly control for the bias, while maintaining enough statistical power to detect significance. For each neuron and each selection condition, we split the trials into two sets. We used half of the data to test whether the neuron responded differently depending on the direction of the large-reward target and the other half to calculate the firing-rate differences between target selection trials and both types of mimic trials. Applying this method did not alter any of our conclusions, suggesting our criteria for determining the preferred conditions of each neuron was not a source of bias.
In addition, the large number of selection trials also allowed us to apply resampling methods to estimate the distribution of the rate differences between conditions under the null hypothesis that there is no difference between conditions (Fig. 7, G and F, black lines, and see methods for more details). We found only small differences between this resampled distribution and the empirical distribution of the firing rate differences between selection trials and mimic cue trials. This similarity indicates that any differences between selection trials and mimic cue trials likely arise from noise in our firing rate estimates.
Firing rate modulations during the preparatory period are weakly predictive of modulations during movement.
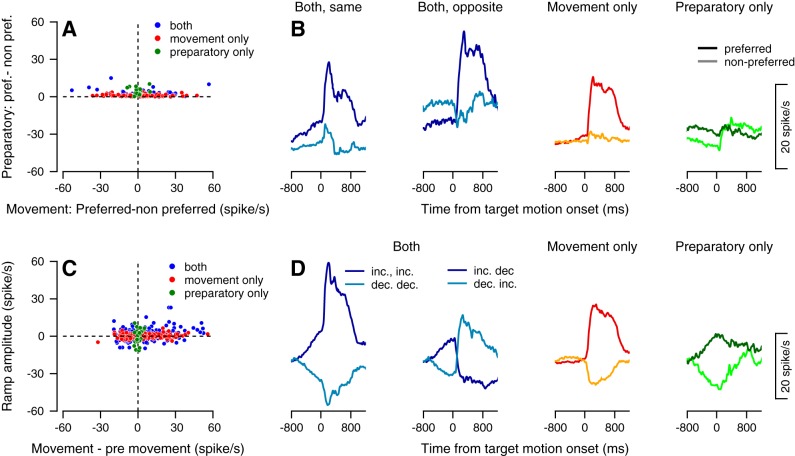
Thus far, our examination of selection related signals has been restricted to their presence in preparatory activity. We next quantified the degree to which the structure of this preparatory activity was shared by perimovement activity that followed both target motion onset and pursuit initiation (during what we term the movement epoch). We first examined to what degree the offset in preparatory activity that discriminated preferred and nonpreferred selection conditions carried over into the movement epoch. For most neurons, the difference in activity between selection conditions was usually much larger during the first 200 ms of pursuit than during the preparatory epoch. This is evidenced by the tendency of individual neurons to fall along the horizontal axis of Fig. 8A. Eleven percent of the neurons exhibited significant differences between selection conditions during only the preparatory epoch (green dots), 19% exhibited significant differences during only the movement epoch (red dots), and 42% exhibited differences in both epochs (Fig. 8A and subpopulations of neurons in Fig. 8B). Moreover, even when neurons discriminated target selection conditions during both the preparatory and movement epochs, the sign of this signal might be the same, or might be different (62% vs. 38%, see Fig. 8B left vs. right panels with blue curves, respectively). This suggests that the preferred condition during the preparatory epoch was often not predictive of the preferred condition during movement (exemplified by the neuron depicted in Fig. 1B).
Fig. 8.
Linking preparatory activity to movement-related activity. A: Each dot shows the difference between the responses of a neuron in its preferred and nonpreferred condition during the preparatory epoch spanning the 800 ms before motion onset (vertical axis) and during a movement epoch spanning 100–300 ms after motion onset (horizontal axis). Vertical values are only positive since the preferred condition was defined as the direction with larger response in the preparatory epoch. Red, green, and blue dots correspond to neurons that signaled selection only after motion onset (n = 99/232), only in the preparatory period (n = 26) and both in the perpetrator period and after motion onset (n = 45). B: peristimulus time histograms of subpopulations of neurons divided according to the epochs in which they significantly differentiated selection conditions. Neurons that responded in both epochs were further divided into neurons that had the same direction of modulation (leftmost panel of 8B; n = 28) and opposite modulation (second from the left panel of 8B; n = 17) in the preparatory and movement epochs. C: each dot shows the modulation in movement epoch activity (relative to end of the preparatory epoch activity) vs. the amplitude of ramping activity during the preparatory epoch (horizontal vs. vertical axes, respectively). Each dot shows a single selection condition of a single neuron; therefore, each neuron in our population contributes two points to this figure. Positive and negative values on the horizontal axis correspond to increases and decreases in rate after motion onset. Positive and negative values on the vertical axis correspond to neurons that ramp up and down, respectively. Red, green, and blue dots correspond to neurons that modulated their activity only after motion onset (105 increasing and 79 decreasing out of 464 conditions), only before motion onset (16 increasing and 15 decreasing) and during both the preparatory and movement epochs (n = 141). D: peristimulus time histograms of subpopulations of neurons divided according to the epochs in which they exhibited significant modulation in activity and the sign of the responses. We further split neurons that responded in both epochs into subpopulation responses that spanned all combinations of the sign of responses (increasing or decreasing, the four curves in the two leftmost panels in Fig. 8D). In this manner, 60 neurons increased their activity in both epochs, and 31 neurons decreased their activity in both epochs. Twenty-eight neurons had an increase in activity during the preparatory epoch and a decrease in activity during the movement epoch. Finally, 22 neurons showed a decrease in activity during the preparatory epoch and an increase in activity during the movement epoch.
Much the same could be said about the degree to which preparatory ramping activity predicted activity in the movement epoch. Across our recording population, both the sign (upward or downward) and magnitude of ramping activity could differ significantly from the magnitude of pursuit-driven activity (Fig. 8C). In the panels of Fig. 8D, we have plotted peristimulus time histograms of subpopulations of neurons from Fig. 8C divided according to the epoch in which they exhibited significant modulations in activity. It is clear that the slope of a given neuron’s preparatory ramping activity did not predict activity during the movement epoch. We note that this apparent dissociation between the preferred condition of preparatory and perimovement activity quantified in Fig. 8 is similar to what has been previously reported in the premotor and motor cortices during arm reaching tasks (Churchland et al. 2010). These prior studies have suggested that these differences may be reconciled by considering preparatory activity as an initial state within a dynamical system that evolves according to fixed rules. These rules can, in turn, be revealed via larger-scale interrogation and analysis of population dynamics. Future work will need to examine whether these findings from the motor cortices generalizes to pursuit neurons within the frontal eye field.
DISCUSSION
We analyzed preparatory activity in the frontal eye fields during pursuit target selection. Our goals were to see the degree to which preparatory activity could be elicited by pursuit target selection and to understand the composition of these preparatory signals commensurate with the approach of prior studies of preparatory activity in the arm movement and saccadic eye movement system. To this end, we report robust preparatory activity frontal eye fields preceding pursuit target selection. Within our population, preparatory activity could be decomposed into a ramping signal, linked to response latency via trial-by-trial analysis, and post cue firing rate offset that encoded the direction of a large reward target motion in a winner-take-all fashion. Apart from providing a quantitative account of FEF’s role in pursuit preparation, we think our results are important for additional reasons. First, they provide novel insights into the sources of variation in the pursuit system. Second, they suggest a mechanism by which a weighted vector average might be implemented in the pursuit system, downstream of FEF and motion representations in area MT. Finally, they provide important insights into the function of the frontal eye fields in pursuit target selection, complementing what is known about the structure from studies of saccade target selection. We discuss these matters more in depth below.
Central source of variability in pursuit.
A considerable amount of the variability in pursuit initiation can be accounted for by errors in the sensory estimation of the target motion (Hohl et al. 2013; Osborne et al. 2005). Studies across the pursuit circuit have confirmed that variability in pursuit initiation arises upstream to the final motor pathways (Joshua and Lisberger 2014; Medina and Lisberger 2007). The view that has emerged from these studies is that sensory errors propagate through a feedforward network to drive motor variability.
Our results indicate a need to rethink the way the pursuit system is structured. In selection trials, monkeys used the information about future target motion timing and direction to plan movements. In these trials, we found that trial-by-trial variations in firing rate during the preparatory epoch preceding target motion were correlated with trial-by-trial variations with latency. Hence, the behavioral variability that we observe cannot be only a result of feedforward processing of target motion, but likely is driven by central sources as well. These central sources of variation do not impact all motor variables equally. For example, we did not find strong correlations between preparatory activity and variations in either pursuit direction or gain. We hypothesize that the observed behavioral variation in gain and direction might be driven by errors in sensory estimation, motor noise during movement execution, or even variation in the preparatory activity of other areas of the pursuit circuit.
We note our findings are not exclusive to the pursuit eye movement system. For example, in the saccade system, the buildup and peak of premovement activity correlate with response timing (Hanes and Schall 1996; Segraves and Park 1993). In the arm movement system, variations in preparatory neural activity are predictive of variations in upcoming reach kinematics (Churchland et al. 2006), indicating that some variability in arm movements also arises before sensory feedback. Our findings mirror these prior findings in the arm movement system. We think the reason we are able to observe central sources of variability is that we have placed the pursuit system into a situation where we can observe the influence of both processes simultaneously. Prior studies on the variability of pursuit eye movements focused only on tasks that minimized the need to plan upcoming movements. Without something to plan for, however, it is unlikely that one will observe such central sources of variability in any motor system. We think these results speak to a generalizable principle underlying motor planning across effectors, whereby whenever planning mechanisms are engaged, the structure of preparatory activity is changed in a manner that leads it to covary with future movement parameters.
Implementing a weighted vector average.
Prior studies have shown that when pursuit is driven by multiple visual motion signals, in the absence of other instructive signal, the resulting pursuit eye movement follows the vector average of the constituent motion signals (Lisberger and Ferrera 1997). In our task, however, it is clear that additional processes intervene and bias the initial pursuit trajectory toward the larger rewarded target (Fig. 1 and see Joshua and Lisberger (2012) for further details). We interpret the presence of winner-take-all representations that we observe (Fig. 7) as an instantiation of a motor plan to follow the large reward target while ignoring the small reward target.
We suggest that behavioral bias results from an interaction between visual drive and winner-take-all signals resulting in a weighted vector average. The fact that visual drive from area MT is only slightly modulated during action selection (Ferrera 2015) leads us to hypothesize that the neural implementation of weighted-vector averaging results from the combination of weakly selection-modulated visual signals from area MT with stronger selection-modulated signals from FEF. We note that certain regions of the pontine nuclei or cerebellum that are jointly targeted by these regions seem appropriately positioned to fulfill this role (Voogd et al. 2012).
Finally, we have interpreted the winner-take-all activity as consistent with a target selection signal, but we note that the short period of time (less than 250 ms) needed to reach this plateau in activity is also consistent with the idea that it is the initial rise in activity that encodes the target selection process. This interpretation is consistent with prior studies of visual search, which show that saccade target selection occurs relatively quickly after cue onset (Thompson et al. 1996). It is possible, therefore, that different processes might drive the sustained portion of the response that persists throughout the preparatory epoch, including attention to the colored cues or valuation of the large reward target. Future work is needed to disentangle these possibilities and determine which processes comprise the winner-take-all signal and how they underlie the selection process.
The neural mechanism of target selection by the frontal eye fields.
Studies have repeatedly shown that the FEF activity is linked to the target selection process (Schall 2002). Moreover, inactivation of FEF has been shown to disrupt target selection (Keller et al. 2008; Schiller and Tehovnik 2003). However, these studies have been mostly restricted to the study of target selection in the context of saccade control. In the pursuit system, the neural mechanisms underlying target selection have rarely been studied. In one study of FEF during a pursuit selection task, the initial response of neurons was a stereotypical ramp in activity, with most neurons discriminating selection conditions only around movement onset (Mahaffy and Krauzlis 2011b). Moreover, both inactivation and stimulation of the FEF chiefly affected the parameters of pursuit movement but not selection between targets in this task (Mahaffy and Krauzlis 2011a). In these studies, the direction of the selected target was revealed only at the time of the target motion onset, limiting the ability of the monkey to plan the direction of the movement. By contrast, different studies have shown that when monkeys are cued for target selection, subpopulations of neurons clearly encode future movement direction (Fukushima et al. 2011; Shichinohe et al. 2009). However, in these experiments, there was no attempt to link the preparatory signal to the initiation of pursuit, or to compare the signal to forced choice conditions.
Our results suggest that while preparatory activity in FEF encodes target selection conditions, it more consistently encodes the selection-independent parameter of movement latency through ramping activity. This fact, combined with the small size of selection-related signals we did observe, suggests that the frontal eye fields serve a secondary role in the target selection process, augmented by structures whose primary role is to drive the target selection process. A plausible candidate for such computations is the basal ganglia. The basal ganglia are embedded in the frontal pursuit pathways (Cui et al. 2003; Krauzlis 2004), and neurons in the basal ganglia respond during pursuit (Basso et al. 2005; Yoshida and Tanaka 2009). Moreover, a rich history of work demonstrates how basal ganglia circuits can interact with cortical structures to drive action (Mink 1996; Redgrave et al. 1999). If the basal ganglia drive the selection process during pursuit planning, we would expect to find that premovement selection signals in the basal ganglia, in contrast to FEF, would be both larger in amplitude and more strongly correlated with future movement direction in our task.
Finally, we note the similarity that our findings bear to recent work in the arm-movement system that suggest that the majority of response variability in the motor cortices is unrelated to target direction or speed (Kaufman et al. 2016). In this work, the authors suggest that motor cortical activity instead comprises a large condition-invariant component that we believe is similar to the ramping component of neural activity discussed above. This hints toward a second generalizable principle of motor planning across effectors, in addition to the central source of pursuit variability discussed above.
GRANTS
M. Joshua received support from the Human Frontiers Science Program and the National Institute for Psychobiology in Israel. This study also received funding from the Howard Hughes Medical Institute (to R. T. Raghavan and M. Joshua).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.J. conceived and designed research; R.T.R. and M.J. analyzed data; R.T.R. and M.J. interpreted results of experiments; R.T.R. and M.J. prepared figures; R.T.R. and M.J. drafted manuscript; R.T.R. and M.J. edited and revised manuscript; R.T.R. and M.J. approved final version of manuscript; M.J. performed experiments.
ACKNOWLEDGMENTS
We thank Stephen Lisberger for his valuable input and support in the early stages of this project.
REFERENCES
- Barnes GR. Cognitive processes involved in smooth pursuit eye movements. Brain Cogn 68: 309–326, 2008. doi: 10.1016/j.bandc.2008.08.020. [DOI] [PubMed] [Google Scholar]
- Basso MA, Pokorny JJ, Liu P. Activity of substantia nigra pars reticulata neurons during smooth pursuit eye movements in monkeys. Eur J Neurosci 22: 448–464, 2005. doi: 10.1111/j.1460-9568.2005.04215.x. [DOI] [PubMed] [Google Scholar]
- Bichot NP, Chenchal Rao S, Schall JD. Continuous processing in macaque frontal cortex during visual search. Neuropsychologia 39: 972–982, 2001. doi: 10.1016/S0028-3932(01)00022-7. [DOI] [PubMed] [Google Scholar]
- Case GR, Ferrera VP. Coordination of smooth pursuit and saccade target selection in monkeys. J Neurophysiol 98: 2206–2214, 2007. doi: 10.1152/jn.00021.2007. [DOI] [PubMed] [Google Scholar]
- Chou IH, Sommer MA, Schiller PH. Express averaging saccades in monkeys. Vision Res 39: 4200–4216, 1999. doi: 10.1016/S0042-6989(99)00133-9. [DOI] [PubMed] [Google Scholar]
- Churchland MM, Afshar A, Shenoy KV. A central source of movement variability. Neuron 52: 1085–1096, 2006. doi: 10.1016/j.neuron.2006.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland MM, Cunningham JP, Kaufman MT, Ryu SI, Shenoy KV. Cortical preparatory activity: representation of movement or first cog in a dynamical machine? Neuron 68: 387–400, 2010. doi: 10.1016/j.neuron.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek P, Kalaska JF. Simultaneous encoding of multiple potential reach directions in dorsal premotor cortex. J Neurophysiol 87: 1149–1154, 2002. doi: 10.1152/jn.00443.2001. [DOI] [PubMed] [Google Scholar]
- Cui DM, Yan YJ, Lynch JC. Pursuit subregion of the frontal eye field projects to the caudate nucleus in monkeys. J Neurophysiol 89: 2678–2684, 2003. doi: 10.1152/jn.00501.2002. [DOI] [PubMed] [Google Scholar]
- Evarts EV, Shinoda Y, Wise SP. Neurophysiological Approaches to Higher Brain Functions. New York: Wiley, 1984. [Google Scholar]
- Ferrera VP. Smooth pursuit preparation modulates neuronal responses in visual areas MT and MST. J Neurophysiol 114: 638–649, 2015. doi: 10.1152/jn.00636.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima J, Akao T, Shichinohe N, Kurkin S, Kaneko CR, Fukushima K. Neuronal activity in the caudal frontal eye fields of monkeys during memory-based smooth pursuit eye movements: comparison with the supplementary eye fields. Cereb Cortex 21: 1910–1924, 2011. doi: 10.1093/cercor/bhq261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbutt S, Lisberger SG. Directional cuing of target choice in human smooth pursuit eye movements. J Neurosci 26: 12479–12486, 2006. doi: 10.1523/JNEUROSCI.4071-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb JP, MacAvoy MG, Bruce CJ. Neural responses related to smooth-pursuit eye movements and their correspondence with electrically elicited smooth eye movements in the primate frontal eye field. J Neurophysiol 72: 1634–1653, 1994. [DOI] [PubMed] [Google Scholar]
- Hanes DP, Schall JD. Neural control of voluntary movement initiation. Science 274: 427–430, 1996. doi: 10.1126/science.274.5286.427. [DOI] [PubMed] [Google Scholar]
- Hohl SS, Chaisanguanthum KS, Lisberger SG. Sensory population decoding for visually guided movements. Neuron 79: 167–179, 2013. doi: 10.1016/j.neuron.2013.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilg UJ, Thier P. The neural basis of smooth pursuit eye movements in the rhesus monkey brain. Brain Cogn 68: 229–240, 2008. doi: 10.1016/j.bandc.2008.08.014. [DOI] [PubMed] [Google Scholar]
- Joshua M, Lisberger SG. Reward action in the initiation of smooth pursuit eye movements. J Neurosci 32: 2856–2867, 2012. doi: 10.1523/JNEUROSCI.4676-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshua M, Lisberger SG. A framework for using signal, noise, and variation to determine whether the brain controls movement synergies or single muscles. J Neurophysiol 111: 733–745, 2014. doi: 10.1152/jn.00510.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge SJ, Richmond BJ, Chu FC. Implantation of magnetic search coils for measurement of eye position—an improved method. Vision Res 20: 535–538, 1980. [DOI] [PubMed] [Google Scholar]
- Kaufman MT, Seely JS, Sussillo D, Ryu SI, Shenoy KV, Churchland MM. The largest response component in the motor cortex reflects movement timing but not movement type. eNeuro 3: 3, 2016. doi: 10.1523/ENEURO.0085-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller EL, Lee KM, Park SW, Hill JA. Effect of inactivation of the cortical frontal eye field on saccades generated in a choice response paradigm. J Neurophysiol 100: 2726–2737, 2008. doi: 10.1152/jn.90673.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JN, Shadlen MN. Neural correlates of a decision in the dorsolateral prefrontal cortex of the macaque. Nat Neurosci 2: 176–185, 1999. doi: 10.1038/5739. [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ. Recasting the smooth pursuit eye movement system. J Neurophysiol 91: 591–603, 2004. doi: 10.1152/jn.00801.2003. [DOI] [PubMed] [Google Scholar]
- Lee J, Lisberger SG. Gamma synchrony predicts neuron-neuron correlations and correlations with motor behavior in extrastriate visual area MT. J Neurosci 33: 19677–19688, 2013. doi: 10.1523/JNEUROSCI.3478-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisberger SG. Visual guidance of smooth-pursuit eye movements: sensation, action, and what happens in between. Neuron 66: 477–491, 2010. doi: 10.1016/j.neuron.2010.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisberger SG, Ferrera VP. Vector averaging for smooth pursuit eye movements initiated by two moving targets in monkeys. J Neurosci 17: 7490–7502, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahaffy S, Krauzlis RJ. Inactivation and stimulation of the frontal pursuit area change pursuit metrics without affecting pursuit target selection. J Neurophysiol 106: 347–360, 2011a. doi: 10.1152/jn.00669.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahaffy S, Krauzlis RJ. Neural activity in the frontal pursuit area does not underlie pursuit target selection. Vision Res 51: 853–866, 2011b. doi: 10.1016/j.visres.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina JF, Lisberger SG. Variation, signal, and noise in cerebellar sensory-motor processing for smooth-pursuit eye movements. J Neurosci 27: 6832–6842, 2007. doi: 10.1523/JNEUROSCI.1323-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol 50: 381–425, 1996. doi: 10.1016/S0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- Osborne LC, Lisberger SG, Bialek W. A sensory source for motor variation. Nature 437: 412–416, 2005. doi: 10.1038/nature03961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottes FP, Van Gisbergen JA, Eggermont JJ. Latency dependence of colour-based target vs nontarget discrimination by the saccadic system. Vision Res 25: 849–862, 1985. doi: 10.1016/0042-6989(85)90193-2. [DOI] [PubMed] [Google Scholar]
- Purcell BA, Heitz RP, Cohen JY, Schall JD, Logan GD, Palmeri TJ. Neurally constrained modeling of perceptual decision making. Psychol Rev 117: 1113–1143, 2010. doi: 10.1037/a0020311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redgrave P, Prescott TJ, Gurney K. The basal ganglia: a vertebrate solution to the selection problem? Neuroscience 89: 1009–1023, 1999. doi: 10.1016/S0306-4522(98)00319-4. [DOI] [PubMed] [Google Scholar]
- Riehle A, Requin J. The predictive value for performance speed of preparatory changes in neuronal activity of the monkey motor and premotor cortex. Behav Brain Res 53: 35–49, 1993. doi: 10.1016/S0166-4328(05)80264-5. [DOI] [PubMed] [Google Scholar]
- Rosenbaum DA. Human movement initiation: specification of arm, direction, and extent. J Exp Psychol Gen 109: 444–474, 1980. doi: 10.1037/0096-3445.109.4.444. [DOI] [PubMed] [Google Scholar]
- Sato T, Murthy A, Thompson KG, Schall JD. Search efficiency but not response interference affects visual selection in frontal eye field. Neuron 30: 583–591, 2001. doi: 10.1016/S0896-6273(01)00304-X. [DOI] [PubMed] [Google Scholar]
- Schall JD. The neural selection and control of saccades by the frontal eye field. Philos Trans R Soc Lond B Biol Sci 357: 1073–1082, 2002. doi: 10.1098/rstb.2002.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller PH, Tehovnik EJ. Cortical inhibitory circuits in eye-movement generation. Eur J Neurosci 18: 3127–3133, 2003. doi: 10.1111/j.1460-9568.2003.03036.x. [DOI] [PubMed] [Google Scholar]
- Schoppik D, Nagel KI, Lisberger SG. Cortical mechanisms of smooth eye movements revealed by dynamic covariations of neural and behavioral responses. Neuron 58: 248–260, 2008. doi: 10.1016/j.neuron.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segraves MA, Park K. The relationship of monkey frontal eye field activity to saccade dynamics. J Neurophysiol 69: 1880–1889, 1993. [DOI] [PubMed] [Google Scholar]
- Shichinohe N, Akao T, Kurkin S, Fukushima J, Kaneko CR, Fukushima K. Memory and decision making in the frontal cortex during visual motion processing for smooth pursuit eye movements. Neuron 62: 717–732, 2009. doi: 10.1016/j.neuron.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Fukushima K. Neuronal responses related to smooth pursuit eye movements in the periarcuate cortical area of monkeys. J Neurophysiol 80: 28–47, 1998. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Lisberger SG. Role of arcuate frontal cortex of monkeys in smooth pursuit eye movements. I. Basic response properties to retinal image motion and position. J Neurophysiol 87: 2684–2699, 2002a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Lisberger SG. Role of arcuate frontal cortex of monkeys in smooth pursuit eye movements. II. Relation to vector averaging pursuit. J Neurophysiol 87: 2700–2714, 2002b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson KG, Hanes DP, Bichot NP, Schall JD. Perceptual and motor processing stages identified in the activity of macaque frontal eye field neurons during visual search. J Neurophysiol 76: 4040–4055, 1996. [DOI] [PubMed] [Google Scholar]
- Voogd J, Schraa-Tam CK, van der Geest JN, De Zeeuw CI. Visuomotor cerebellum in human and nonhuman primates. Cerebellum 11: 392–410, 2012. doi: 10.1007/s12311-010-0204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida A, Tanaka M. Neuronal activity in the primate globus pallidus during smooth pursuit eye movements. Neuroreport 20: 121–125, 2009. doi: 10.1097/WNR.0b013e32831af055. [DOI] [PubMed] [Google Scholar]