Choline transport across the membrane is exerted by both the high-affinity and low-affinity choline transporters. We found that choline can permeate P2 purinergic receptors, including P2X2 purinoceptors, in cholinergic neurons of the retina. Our findings show the presence of a novel choline transport pathway in cholinergic neurons. Our findings also indicate that the permeability of P2X2 purinoceptors to choline observed in the heterologous expression system may have a physiological relevance in vivo.
Keywords: cholinergic neuron, P2X purinoceptor, retina
Abstract
Choline uptake into the presynaptic terminal of cholinergic neurons is mediated by the high-affinity choline transporter and is essential for acetylcholine synthesis. In a previous study, we reported that P2X2 purinoceptors are selectively expressed in OFF-cholinergic amacrine cells of the mouse retina. Under specific conditions, P2X2 purinoceptors acquire permeability to large cations, such as N-methyl-d-glucamine, and therefore potentially could act as a noncanonical pathway for choline entry into neurons. We tested this hypothesis in OFF-cholinergic amacrine cells of the mouse retina. ATP-induced choline currents were observed in OFF-cholinergic amacrine cells, but not in ON-cholinergic amacrine cells, in mouse retinal slice preparations. High-affinity choline transporters are expressed at higher levels in ON-cholinergic amacrine cells than in OFF-cholinergic amacrine cells. In dissociated preparations of cholinergic amacrine cells, ATP-activated cation currents arose from permeation of extracellular choline. We also examined the pharmacological properties of choline currents. Pharmacologically, α,β-methylene ATP did not produce a cation current, whereas ATPγS and benzoyl-benzoyl-ATP (BzATP) activated choline currents. However, the amplitude of the choline current activated by BzATP was very small. The choline current activated by ATP was strongly inhibited by pyridoxalphosphate-6-azophenyl-2′,4′-sulfonic acid. Accordingly, P2X2 purinoceptors expressed in HEK-293T cells were permeable to choline and similarly functioned as a choline uptake pathway. Our physiological and pharmacological findings support the hypothesis that P2 purinoceptors, including P2X2 purinoceptors, function as a novel choline transport pathway and may provide a new regulatory mechanism for cholinergic signaling transmission at synapses in OFF-cholinergic amacrine cells of the mouse retina.
NEW & NOTEWORTHY Choline transport across the membrane is exerted by both the high-affinity and low-affinity choline transporters. We found that choline can permeate P2 purinergic receptors, including P2X2 purinoceptors, in cholinergic neurons of the retina. Our findings show the presence of a novel choline transport pathway in cholinergic neurons. Our findings also indicate that the permeability of P2X2 purinergic receptors to choline observed in the heterologous expression system may have a physiological relevance in vivo.
in cholinergic neurons, acetylcholine is synthesized enzymatically from choline and acetyl coenzyme A in presynaptic terminals (Sarter and Parikh 2005). Degradation of acetylcholine by acetylcholinesterase occurs with high efficiency in the synaptic cleft following vesicular release, and reuptake of choline is thought to be mediated exclusively by plasma membrane high-affinity choline transporters (high-affinity ChTrs) (Lockman and Allen 2002). Therefore, the activity of cholinergic neurons has been assessed by the rate of acetylcholine synthesis through high-affinity ChTrs (Sarter and Parikh 2005).
P2X purinoceptors are cation channels with the unusual property of pore dilation. That is, P2X7 purinoceptors exposed to ATP for extended periods acquire permeability to large cations such as N-methyl-d-glucamine (Burnstock 2004; Collo et al. 1997; Surprenant et al. 1996). The formation of large cation-permeant pores in P2X7 purinoceptors is thought to be an important mechanism for cytolysis. P2X2 purinoceptors also exhibit pore dilation when expressed at high densities (Fujiwara and Kubo 2004). However, it is not known whether pore dilation of P2X2 purinoceptors has physiological relevance in vivo.
In the mouse retina, P2X2 purinoceptors are strongly expressed in OFF-cholinergic amacrine cells (OFF-CAs) and to a lesser degree in ON-cholinergic amacrine cells (ON-CAs) (Kaneda et al. 2004). Choline is a large cation and therefore could potentially permeate densely expressed OFF-CA P2X2 purinoceptors that undergo pore dilation, thereby functioning as a novel pathway for choline transport. In the present study, we tested this hypothesis and additionally report that the expression level of the high-affinity ChTr in OFF-CAs was lower than that in ON-CAs. Our physiological and pharmacological findings raise the possibility that choline transported through cation channels, which is mediated by P2 purinoceptors, can be a secondary source of substrate for acetylcholine synthesis. Our data also support the hypothesis that, at least, P2X2 purinoceptors on OFF-CAs can work as a choline transport pathway.
MATERIALS AND METHODS
Animals.
The research protocol was approved by the Animal Experiments Ethical Review Committee of Nippon Medical School and the University Animal Welfare Committee of the Keio University School of Medicine. The IG-8 line of heterozygous transgenic mice (C57/BL6J) was used in the present study (Watanabe et al. 1998; Yoshida et al. 2001). The specific localization of green fluorescent protein (GFP) signals in the CAs of the retina was previously reported for this transgenic line.
Experimental solutions.
Extracellular and intracellular solutions used for patch-clamp recordings are summarized in Tables 1 and 2.
Table 1.
Composition of extracellular solutions
| Solution |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Type | NaCl | Choline Cl | Choline Acetate | KCl | MgCl2 | CaCl2 | NaHCO3 | NaH2PO4 | HEPES | Glucose | 4-AP | TEACl | CdCl2 |
| 1-1 | Na-rich Ringer | 115 | 5 | 1 | 2 | 26 | 1.1 | 10 | ||||||
| 1–2 | Choline-rich Ringer | 115 | 5 | 1 | 2 | 26 | 1.1 | 10 | ||||||
| 2-1 | Na-rich | 135 | 5 | 1 | 2 | 5 | 10 | |||||||
| 2-2 | Choline-rich | 135 | 5 | 1 | 2 | 5 | 10 | |||||||
| 2–3 | Choline-rich (Ca-free) | 135 | 5 | 1 | 0.2 | 5 | 10 | |||||||
| 3-1 | 140 Choline | 140 | 1 | 1 | 5 | 10 | 0.1 | |||||||
| 3-2 | 5 Choline | 5 | 1 | 1 | 5 | 10 | 0.1 | |||||||
| 4-1 | 137 Cl | 100 | 5 | 1 | 5 | 10 | 3 | 30 | 0.1 | |||||
| 4-2 | 37 Cl | 100 | 5 | 1 | 5 | 10 | 3 | 30 | 0.1 | |||||
| 5 | Cl-free Choline | 135 | 5 | 2 | 5 | 10 | ||||||||
Values are concentrations (in mM). 4-AP, 4-aminopyridine; TEACl, tetraethylammonium chloride. Na-rich Ringer and choline-rich Ringer solutions were bubbled with 95% O2-5% CO2 to adjust pH at 7.4. Na-rich solution was pH adjusted to 7.4 with NaOH. Choline-rich, choline-rich (Ca-free), 137 Cl, and Cl-free choline solutions were pH adjusted to 7.4 with Tris base. 140 Choline and 5 choline solutions were pH adjusted to 7.4 with Tris base, and osmolarity was adjusted to 300 mosM with sucrose.
Table 2.
Composition of intracellular solutions
| Solution |
||||||||
|---|---|---|---|---|---|---|---|---|
| No. | Type | KCl | CsCl | CaCl2 | EGTA | HEPES | ATP-2Na | GTP-Na |
| In-1 | KCl | 135 | 0.5 | 5 | 5 | 5 | 1 | |
| In-2 | CsCl | 135 | 0.5 | 5 | 5 | |||
| In-3 | KCl(HEK) | 135 | 0.5 | 5 | 10 | 5 | 1 | |
CsCl solution was pH adjusted to 7.3 with CsOH, and osmolarity was adjusted to 281 mosM with sucrose.
Preparations for retinal slice and dissociated cells.
Details of the methods were described previously (Ishii and Kaneda 2014; Kaneda et al. 2008). In brief, both eyes were enucleated and hemisected, and the retina was isolated from the sclera. For slice preparations, detached retinas were placed on a membrane filter (pore size, 0.45 μm; Advantec Toyo, Tokyo, Japan) with the photoreceptor side up and sliced at a thickness of 150–200 μm in Na-rich Ringer solution (no. 1-1) for the slice preparation. For dissociated retinal preparations, detached retinas were incubated for 15–20 min in Na-rich solution (no. 2-1) containing 2.5 U/ml papain (Worthington Biochemical, Freehold, NJ) and its activator, l-cysteine (0.1 mg/ml), bubbled with 100% O2 at 37°C. Enzyme treatment was stopped by washing the retinas with external solution containing 0.1 mg/ml bovine serum albumin. The retinas were then triturated with a Pasteur pipette, and the cell suspension was dispensed on concanavalin-A-coated cover glasses in plastic petri dishes. After the cells had attached to the cover glasses (~30 min), the dishes were filled with Na-rich solution (no. 2-1). All experimental procedures were conducted at room temperature.
Construction of a P2X2 purinoceptor GFP expression vector.
A cDNA fragment of the P2X2 purinoceptor was amplified from a rat P2X2/pcDNA3 vector (a gift from Dr. M. Tominaga, National Institute for Physiological Sciences) by PCR using the following primers (aaaCTCGAGgccaccatggtccggcgcttggcccggggct, aaaGAATTCtaagttgggccaaacctttggggtcc); the cDNA was inserted into the XhoI/EcoRI site of GFP-N1 (Clontech).
Cell culture and transfection.
Human embryonic kidney (HEK-293T) cells (RIKEN Bio-Resource Center, Tsukuba, Japan) were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Life Technologies) containing 10% fetal bovine serum (Life Technologies), 50 U/ml penicillin, and 50 μg/ml streptomycin (Wako Pure Chemical, Wako, Japan). HEK-293T cells were maintained at 37°C in a 5% CO2-air atmosphere, and the medium was changed every other day. For electrophysiological recordings, HEK-293T cells were transfected with the DNA vector (1 μg of plasmid DNA/2 × 106 cells) with ScreenFectA (Wako Pure Chemical), according to the instructions provided by the manufacturer. The transfected cells were plated on coverslips. Cells were incubated for 1 or 2 days and used for patch-clamp recordings. For high-performance liquid chromatography (HPLC), we used an electroporation system (NEPA21; Nepa Gene, Ichikawa, Japan) to maximize the number of P2X2 purinoceptor-expressing cells. Cultured HEK-293T cells were washed with phosphate-buffered saline (PBS) and treated with Accutase (Nacalai Tesque, Kyoto, Japan) for 5 min at 37°C, according to the instructions provided by the manufacturer. Dissociated cells suspended in PBS were collected by centrifugation (1,200 g for 10 min). The concentration of cells was adjusted with Opti-MEM (1 × 106 cells/100 μl; Life Technologies), and plasmid DNA was added for transfection (5 μg plasmid DNA/1 × 106 cells). Electroporation was conducted in the cuvette (EC-002S; Nepa Gene) using poring pulses (2 pulses: initial intensity, 175 V; pulse duration, 5 ms; pulse interval, 50 ms; decay rate of intensity per pulse, 10%) and transfer pulses (5 pulses: initial intensity, 20 V; pulse duration, 50 ms; pulse interval, 50 ms; decay rate of intensity per pulse, 40%). The transfected cells were plated on 6-well plates (1 × 106 cells/well) and incubated for 2 days.
Current recordings.
For the dissociated cell preparations and the slice preparations, to maximize the chance for stable whole cell recordings from ON- or OFF-CAs, we used patch pipettes with relatively high resistance (12–18 MΩ when filled with an intrapipette solution; Ishii and Kaneda 2014; Kaneda et al. 2007, 2008). Patch pipettes with a resistance of 10–12 MΩ (when filled with an intrapipette solution) were used in the current recordings from HEK-293T cells. Current and voltage signals were recorded using a patch-clamp amplifier (Axopatch-200B; Axon Instruments, Foster City, CA) and were stored in a personal computer at 10 kHz using pCLAMP software (version 8.0; Axon Instruments) after the data were passed through a low-pass Bessel filter (<5 kHz). The holding potential was −70 mV. All experiments were conducted at room temperature. Solutions were perfused using bath applications.
Reversal potential.
Current-voltage (I-V) curves were recorded from the ramp pulses (from −100 to 30 mV; velocity, 130 mV/s). Because large cations, such as N-methyl-d-glucamine, can permeate P2X2 purinoceptors (Fujiwara and Kubo 2004), sucrose was used as a substitute for choline chloride. One molecule of choline chloride was replaced with two molecules of sucrose to adjust the osmolality, and CsCl, tetraethylammonium chloride (TEA), 4-aminopyridine (4-AP), and CdCl2 were used to block voltage-gated K+ currents and Ca2+ currents (Kaneda et al. 2007). Reversal potentials were obtained after correction for liquid junction potentials. Values of liquid junction potentials were −0.7 mV for 140 mM choline solution (140 choline, no. 3-1), −11.9 mV for 5 mM choline solution (5 choline, no. 3-2), −8.8 mV for 137 mM Cl solution (137 Cl, no. 4-1), and −4.8 mV for 37 mM Cl solution (37 Cl, no. 4-2).
Immunohistochemistry.
Details of immunohistochemistry for P2X purinoceptors were described previously (Ishii et al. 2003; Kaneda et al. 2004; Shigematsu et al. 2007). Retinas or HEK-293T cells were fixed with 4% (wt/vol) paraformaldehyde in 0.1 M phosphate buffer for 15 min at room temperature. After fixation, retinas were cryoprotected by graded dehydration in 10, 20, and 30% sucrose in 0.1 M phosphate buffer at 4°C overnight for each. The cryoprotected retinas were sectioned into 10-μm-thick sections. No cryoprotection was conducted for HEK-293T cells. Samples were reacted with a primary polyclonal antibody in 0.1 M phosphate buffer containing 0.3% Triton X-100 for 48–72 h at 4°C (Kaneda et al. 2004). The working dilution of primary polyclonal antibodies was 1:300 for rabbit anti-choline transporter (high affinity) (Chemicon) and 1:400 for P2X2 (Chemicon). The sections were then allowed to react with Alexa546-conjugated goat anti-rabbit IgG (1:500; Molecular Probes, Eugene, OR) for P2X2 or Alexa594 (1:500; Life Technologies) for choline transporter in 0.1 M phosphate buffer for 2 h at room temperature. Fluorescent images were observed under a confocal microscope (LSM-510; Carl Zeiss, Jena, Germany) at an excitation wavelength of 488 nm for GFP (bandpass 505–530 nm) and 543 nm for Alexa546 (bandpass 560–615 nm) or 561 nm for Alexa594 (bandpass 585–734 nm). The images were scanned at a slice width of 0.5–0.6 μm and digitized using a computer. Image sampling and processing were performed using the software included with the LSM5 image browser. Recording levels of each signal were adjusted in “palette mode” according to the procedure recommended by Carl Zeiss. Analysis of fluorescent signal intensity was conducted using ImageJ (Schneider et al. 2012).
Analysis of the intensity of immunoreactivity for P2X2 and high-affinity ChTr.
To analyze the signal intensity of P2X2, GFP and Alexa546 fluorescence signals were taken at ×40 or ×63 magnification without any adjustment for the background level. The maximum level of each fluorescence signal was adjusted to the highest level of the detector to avoid saturation of the signals. The signal intensity of the background for GFP (BGFP) and Alexa546 (B546) was calculated from the region where no retinal tissue existed. The signal intensity of GFP (SGFP) and Alexa546 (S546) in the dendritic region of CAs was defined as the region where GFP signals were positive. The ratio of the signal intensity between GFP and Alexa546 (Alexa/GFP) was calculated using the equation Alexa/GFP = (S546 − B546)/(SGFP − BGFP). In this calculation, we assumed that both ON- and OFF-CAs expressed GFP at the same level and that the difference in the signal intensity of GFP represents the signal integration along the z-axis. The selective expression of GFP signals in the ON- and OFF-CAs has been reported previously (Kaneda et al. 2004; Yoshida et al. 2001). The signal intensity of high-affinity ChTr was analyzed similarly using GFP and Alexa594 fluorescence signals.
High-performance liquid chromatography.
HEK-293T cells were washed with PBS and treated in choline-rich solution (no. 2-2) with or without ATP for 30 s at room temperature. After being washed with PBS three times, cells were dissociated in PBS by pipetting. The number of cells was counted for each well. The cells were then collected by centrifugation (1,200 g for 10 min) for each well. The pellet was treated with 1 ml PCA solution containing 0.1 M perchloric acid, 0.1 mM EDTA, 100 μM isopropyl homocholine (IPHC), and 0.1 mM eserine. The samples were centrifuged at 20,000 g for 15 min at 0°C. After adjustment of the pH to ~6.0 using 1 M KHCO3, the supernatant was filtered (UFC3LGC00 Ultrafree-MC, 10,000 MW cutoff; Millipore, Billerica, MA) by centrifugation (14,000 g for 20 min at room temperature). The sample was injected into the HPLC system (HTEC-500; Eicom, Kyoto, Japan) equipped with a guard column (CH-Gel), a separation column (AC-Gel), and an immobilized enzyme column (AC-Enzymepak II) in series. The composition of the mobile phase was 50 mM KHCO3, 300 mg/l sodium 1-decanesulfonate, and 50 mg/l EDTA. The flow rate of the mobile phase was 0.15 ml/min. Peak data were recorded and analyzed on a computer. In our system, the detection limit of the intracellular choline level was 10−10 M. The choline concentration for each sample was estimated using the internal standard (IPHC).
Statistics.
Statistical significance was assayed with the Wilcoxon rank-sum test (see Fig. 1C), Student’s unpaired t-test (see Fig. 2, D and E), Tukey’s test (see Fig. 4D), and Student’s paired t-test (Figs. 3C and 5B). Details of the results for individual statistical analysis are described in the figure legends.
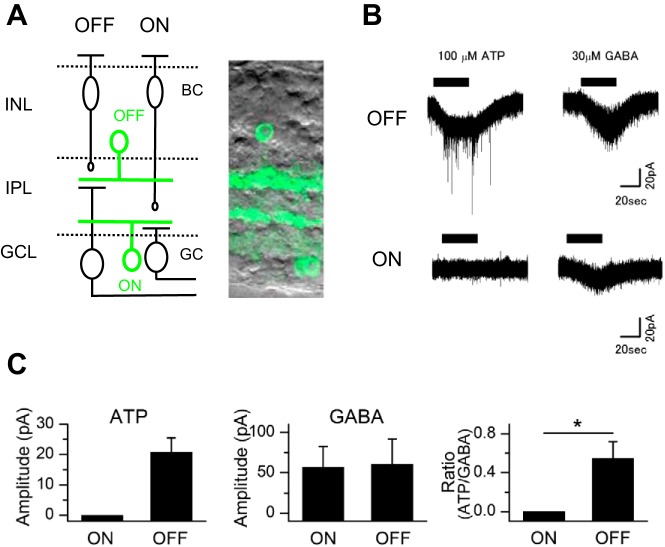
Fig. 1.
Recordings of ATP-induced choline currents. A: schematic drawings (left) of the mouse retina and photomicrographs (right) showing GFP-positive cholinergic amacrine cells (CAs) in slice preparations. The ON and OFF pathways are indicated at the top of the schema. ON- and OFF-CAs are labeled as ON (green) and OFF (green). Somas of OFF-CAs are located in the inner nuclear layer, whereas somas of ON-CAs are located in the ganglion cell layer. BC, bipolar cell; GC, ganglion cell; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer. B: responses to ATP and GABA in OFF- and ON-CAs. Currents were recorded in choline-rich Ringer solution (no. 1-2). C: average of the peak amplitude response to ATP or GABA and the relative peak amplitude of ATP to that of GABA (ATP/GABA) in ON- or OFF-CAs. The peak amplitude of ATP was normalized by the peak amplitude of GABA recorded in the same cell. Values are means ± SE; n = 4 cells from 4 mice for both ON- and OFF-CAs. *P < 0.05. The intracellular solution was KCl (no. In-1).
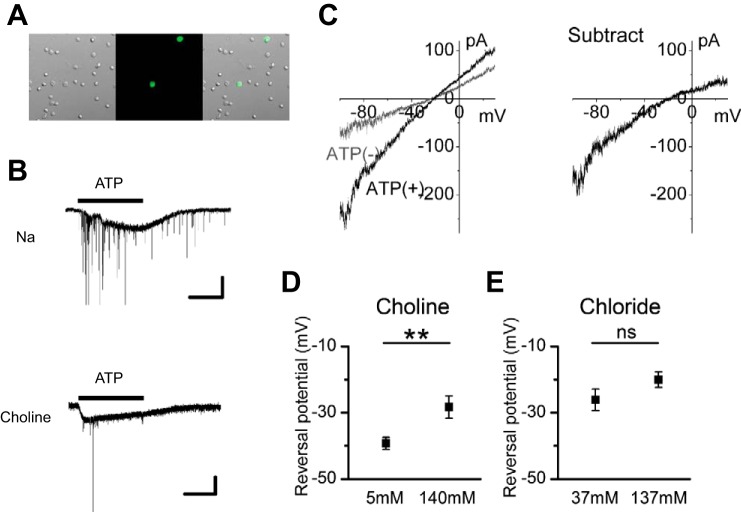
Fig. 2.
The ionic mechanism of the ATP-induced inward current. A: photomicrographs showing GFP-positive CAs in dissociated retinal preparations. Left, a differential interference image; middle, a fluorescence image; right, a merged image. B: responses to 100 μM ATP in Na-rich solution (no. 2-1; top) and in choline-rich solution (no. 2-2; bottom). The large, slow inward current is the P2 purinoceptor (including P2X2 purinoceptor)-mediated cation current. Inhibitory postsynaptic currents superimposed on the P2 purinoceptor-mediated cation current are a result of activation of P2 purinoceptors on the presynaptic terminals of CAs (Kaneda et al. 2008). The timing of ATP application is indicated by the horizontal bar above the current trace. Experiments were conducted in dissociated cell preparations. C: 2 I-V curves obtained by ramp pulse before [ATP(−); gray line] and during [ATP(+); black line] application of 100 μM ATP (left) and subtracted I-V curves (right). The extracellular solution is 140 choline (no. 3-1). D: shift of the reversal potential of the ATP-induced inward currents by choline substitution. Values are means ± SE; n = 23 cells from 6 mice for 140 choline solution (no. 3-1); n = 15 cells from 4 mice for 5 choline solution (no. 3-2). E: shift of the reversal potential of the ATP-induced inward currents by extracellular Cl− substitution. Values are means ± SE; n = 12 cells from 8 mice for 137 Cl solution (no. 4-1); n = 8 cells from 4 mice for 37 Cl solution (no. 4-2). **P < 0.01; ns, not significant (unpaired t-test). Intracellular solutions were KCl (no. In-1) in B and E and CsCl (no. In-2) in C and D.
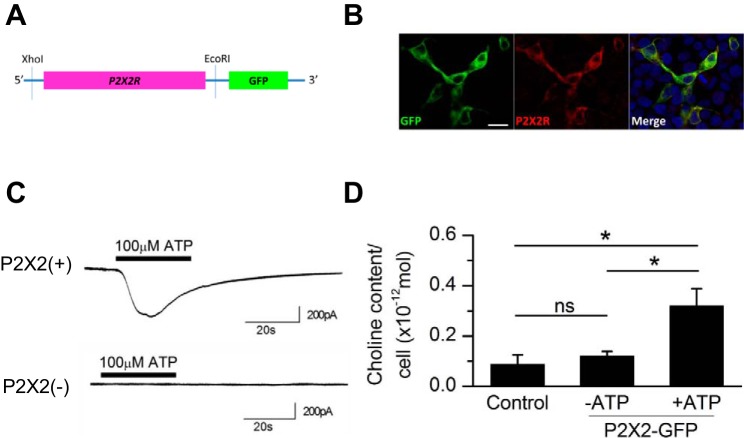
Fig. 4.
ATP-induced choline influx in transfected HEK-293T cells expressing the fusion protein of P2X2 purinoceptors and GFP. A: design of the cDNA of P2X2 purinoceptors fused with GFP. B: immunoreactivity for P2X2 purinoceptors in HEK-293T cells transfected with the P2X2R::GFP vector. Bar, 20 μm. Green, GFP; red, P2X2 purinoceptors; blue, DAPI. C: responses to ATP in GFP-positive cells [P2X2(+)] and GFP-negative cells [P2X2(−)] in choline-rich (Ca-free) solution (no. 2-3). Horizontal bars above the traces show the timing of ATP application. The intracellular solution was KCl (HEK) (no. In-3). D: effects of ATP on the cytosolic choline content in HEK-293T cells. Experiments were conducted in choline-rich solution (no. 2-2). Control cells were collected from the well without transfection; P2X2-GFP cells were collected from the well with transfection with (+ATP) or without 100 μM ATP (−ATP). Values are means ± SE; n = 4 for all 3 experimental conditions. *P < 0.05; n.s., not significant.
Fig. 3.
Physiological and pharmacological properties of the ATP-evoked choline current in the choline-rich solution (no. 2-2). A: concentration-response curve of the ATP-evoked choline current for the choline-rich solution (no. 2-2). Values are means ± SE; n = 6 from 4 mice at 10 μM, n = 7 from 4 mice at 30 μM, n = 8 from 4 mice at 50 μM, n = 9 from 6 mice at 100 μM, n = 8 from 5 mice at 500 μM, and n = 7 from 4 mice at 1 mM. The curve for the choline-rich solution (no. 2-2) was drawn using the least-square method. B: pharmacology of the ATP-evoked choline current. Values are means ± SE; n = 11 cells from 7 mice for 100 μM ATPγS, n = 10 cells from 6 mice for 100 μM MeATP, n = 9 cells from 6 mice for 20 μM PPADS + 100 μM ATP, and n = 10 cells from 7 mice for 60 μM BzATP. The extracellular solution was choline-rich (no. 2-2) or Cl−-free choline (no. 5). The difference between these two solutions is the Cl− concentration. Because P2 purinoceptor-mediated cation channels are not permeable to Cl−, both data sets were used for analysis. C: effects of extracellular Ca2+ on the ATP-evoked choline current. First, the ATP-evoked choline current in choline-rich solution (no. 2-2, [Ca2+]o = 2 mM) was recorded, and then that in choline-rich (Ca-free) solution (no. 2-3, [Ca2+]o = 0.2 mM) was recorded in the same cell. Values are means; n = 11 cells from 8 mice. The intracellular solution was KCl (no. In-1).*P < 0.05. Experiments A–C were conducted in dissociated cell preparations.
Fig. 5.

A: asymmetrical distribution of immunoreactivity for the high-affinity choline transporter (ChTr) and P2X2 purinoceptors (P2X2) between OFF- and ON-CAs. GFP signals reflect the dendrites and the soma of CAs. B: relative intensity of Alexa546/GFP in ON-CAs to Alexa546/GFP in OFF-CAs for high-affinity ChTr and P2X2. ***P < 0.001. P values were 1.4 × 10−5 for choline transporter (n = 7 mice) and 7.6 × 10−4 for P2X2 (n = 7 mice).
RESULTS
To examine whether the amplitude of the ATP-evoked current in the choline-rich Ringer solution (no. 1-2) is different between ON- and OFF-CAs, we recorded the ATP-evoked choline currents from both ON- and OFF-CAs in retinal slices from mice. ON-CAs and OFF-CAs were identified by the location of the soma (Ishii and Kaneda 2014; Kaneda et al. 2008) (Fig. 1A). Application of 100 μM ATP activated an inward current in OFF-CAs, but not in ON-CAs, in choline-rich Ringer solution (no.1-2; Fig. 1B), suggesting that only OFF-CAs expressed P2 purinoceptors at levels sufficient to form choline-permeable pores. The difference in the ATP-evoked currents between ON- and OFF-CAs was not due to the accessibility of ATP to target cells, because an application of 30 μM GABA activated inward currents in both ON- and OFF-CAs (Fig. 1C). We also measured the charge transfer during ATP application (50–70 s) for both OFF- and ON-CAs. In this calculation, the charge transfer is negative when the inward current persists, whereas the charge transfer is positive when the outward current persists. The charge transfer for OFF-CA was always negative (from −98.9 to −5.7 pC/s), which likely indicates the integrated value of the inward current. On the other hand, the charge transfer for ON-CA was negative or positive and smaller than the values of OFF-CAs (from −0.9 to 0.8 pC/s). Because the amplitude for the positive side and the negative side was almost equal in ON-CAs, it is reasonable to conclude that the calculated values of the charge transfer in ON-CAs reflect the accumulated value of the noise around the baseline. An increase in the frequency of inhibitory postsynaptic currents (IPSCs) superimposed on the ATP-evoked current in OFF-CAs is likely the result of an activation of P2 purinoceptors (presumably P2X2, P2X7, P2Y2, or P2Y14) on the presynaptic terminals of the CAs (Kaneda et al. 2008). In the present study, we focused on the choline current in the CAs and therefore did not further analyze the actions of ATP on IPSCs in the choline-rich Ringer solution (no. 1-2).
CAs express several P2 purinoceptors (Dilip et al. 2013; Kaneda et al. 2004, 2008; Shigematsu et al. 2007; Ward et al. 2008), and therefore the actions of ATP are not solely mediated by P2X2 purinoceptors. To confirm the choline permeability to P2 purinoceptors and to identify the responsible P2 purinoceptors, we conducted electrophysiological recordings from dissociated retinal preparations in which anatomical information of the soma was lost; accordingly, GFP-positive cells responsive to ATP, which consist predominantly of OFF-CAs, were used for analysis (Fig. 2A).
First, we confirmed that the ATP-induced current is carried by choline+. An application of 100 μM ATP induced a cation current in Na-rich solution (no. 2-1; Fig. 2B). We replaced the Na-rich solution (no. 2-1) with choline-rich solution (no. 2-2) and found that the currents were elicited by ATP application. The amplitudes of the cation currents in the choline-rich solution (no. 2-2) were less than those in Na-rich solution (no. 2-1).
Shifts in the reversal potentials of the currents in recordings with distinct extracellular choline concentrations were measured to confirm that ATP-mediated currents were carried by choline+ (Fig. 2C). Because both ON- and OFF-CAs have very large K+ conductances (Kaneda et al. 2007; Ozaita et al. 2004) but do not possess the tetrodotoxin-sensitive voltage-gated Na+ current (Kaneda et al. 2007), and pore-dilated P2X purinoceptors are permeable to TEA and other large-sized cations (P2X2: Ding and Sachs 1999; Evans et al. 1996); P2X7: Burnstock 2004; Collo et al. 1997; Surprenant et al. 1996), we designed the intracellular (no. In-2) and extracellular solutions (nos. 3-1 and 3-2) as follows. To avoid the possible passage of TEA at P2X purinoceptors, TEA was not added to the extracellular solutions to suppress K+ conductances. Instead, K+ conductances were suppressed by intracellular CsCl. Ramp pulses (from −100 to 30 mV; velocity, 130 mV/s) were applied in the presence or absence of 100 μM ATP. The I-V curve recorded in the presence of 100 μM ATP had a larger slope than the I-V curve recorded in the absence of 100 μM ATP, again suggesting that the ATP-mediated current was carried by choline+ (Fig. 2C, left). The I-V curve of the ATP-mediated current was reproduced by the subtraction of the two I-V curves (Fig. 2C, right). Reversal potentials were defined by the crossing point of the x-axis of the subtracted I-V curve. We pooled the reversal potentials, determined by the ramp pulse, and averaged them (Fig. 2D). The reversal potential (equilibrium potential) of ion X is described by the Nernst equation, as follows:
where Erev is the reversal potential of ion X, R is the gas constant, T is the absolute temperature, z is the ion valence, F is the Faraday constant, and [X]o and [X]i are the extra- and intracellular concentrations of ionized ion X. [X]o and [X]i are the product of the concentration of ion X and the activity coefficient of ion X at the individual concentration. In this equation, Erev theoretically shifts 58 mV per 10 times change of [X]o.
Therefore, the reduction of the extracellular choline+ would shift the reversal potential of choline+ to the negative potential. Indeed, switching the 140 choline solution (no. 3-1) to the 5 choline solution (no. 3-2) significantly shifted the reversal potential to the negative side (10.9 mV, P < 0.01).
To rule out the possibility that ATP responses were mediated by Cl− in the choline-rich solution (no. 2-2), we examined the effects of extracellular Cl− on the shift of the reversal potential (Fig. 2E). To block K+ conductances, we added TEA, 4-AP, and CdCl2 to the extracellular solution, and extracellular Cl− (137 Cl, no. 4-1) was substituted with equimolar acetate, an impermeable anion (37 Cl, no. 4-2). According to the Nernst equation, reduction of extracellular Cl− would shift the reversal potential of Cl− to the positive potential. As shown in Fig. 2E, however, reduction of extracellular Cl− (from 137 to 37 mM) did not significantly shift the reversal potential to the positive side (P = 0.15).
Next, we performed experiments to identify whether the pharmacological properties of choline currents driven by P2 purinoceptors are similar to those of cation currents driven by P2 purinoceptors in our previous study (Kaneda et al. 2008). We recorded the concentration response-curves of ATP-evoked choline currents in the choline-rich solution (no. 2-2). We normalized ATP-evoked choline currents recorded in the choline-rich solution (no. 2-2) to the responses evoked by application of 100 μM of ATP in the Na-rich solution (no. 2-1; Fig. 3A). The normalized ATP-evoked choline currents were pooled and averaged. The curve was fitted using the following equation:
where Imax is the maximal response, C is the concentration of ATP, n is the Hill coefficient, and EC50 is the concentration of ATP that produced a 50% response of Imax. The concentration response-curve of ATP-evoked choline currents was described by a Hill coefficient of 1.24, an EC50 of 72.9 µM, and an Imax value of 0.31.
We also examined the pharmacological properties of choline currents (Fig. 3B) according to the tables of agonists and antagonists of P2 purinoceptors (Burnstock 2004). ATPγS, an agonist of homomeric P2X1, P2X2, P2X3, P2X4, P2X5, P2X6, P2Y1, P2Y2, P2Y4, P2Y11, and P2Y12, activated choline currents, whereas α,β-methylene ATP (MeATP), a relatively selective agonist of homomeric P2X1, P2X3, P2X4, P2X5, and P2X6, did not produce a cation current. Benzoyl-benzoyl-ATP (BzATP), a relatively selective agonist of homomeric P2X1, P2X3, P2X4, P2X5, P2X7, heteromeric P2X2/3, and P2Y11, a less potent partial agonist of homomeric P2X2, and an antagonist of P2Y4, produced a small cation current. When pyridoxalphosphate-6-azophenyl-2′,4′-sulfonic acid (PPADS), an antagonist of homomeric P2X1, P2X2, P2X3, P2X5, P2X6, P2X7, P2Y1, P2Y2, and P2Y4 receptors, was added to the extracellular solution, ATP-evoked choline currents were heavily inhibited.
Because the extracellular Ca2+ concentrations affect the activity of P2X purinoceptors (Gever et al. 2006), we also compared the amplitudes of ATP-evoked choline currents with different extracellular Ca2+ concentrations in the choline-rich solution (no. 2-2; Fig. 3C). When the extracellular Ca2+ concentration was switched from 2 mM to 0.2 mM, the amplitudes of ATP-evoked choline currents increased (P < 0.05).
In the following study, we focused on whether P2X2 purinoceptors might be a candidate for choline transport in OFF-CAs, because our previous immunohistochemical findings and the current findings supported the hypothesis that P2X2 purinoceptors are one of the most likely candidates (see discussion). To confirm that P2X2 purinoceptors can form a choline permeable pore, we directly examined the permeability to choline of P2X2 purinoceptor-coupled cation channels expressed in HEK-293T cells. In the present study, we constructed a P2X2-GFP fusion protein (P2X2R::GFP) expression vector (Fig. 4A). The immunoreactivity for P2X2 purinoceptors was detected in GFP-positive cells (Fig. 4B). When 100 μM ATP was applied to GFP-negative cells in the choline-rich solution (Ca2+ free; no. 2-3), no detectable inward current was observed (n = 3), indicating that HEK-293T cells intrinsically have no ATP-activated choline-permeable channels (Fig. 4C). On the other hand, when 100 μM ATP was applied to GFP-positive cells, an inward current was detected (n = 4), confirming that P2X2 purinoceptor-coupled cation channels are permeable to choline+.
Next, we directly examined whether activation of P2X2 purinoceptors by ATP induced the increase in the intracellular choline content by using HEK-293T cells expressing P2X2 purinoceptors (Fig. 4D). When nontransfected HEK-293T cells were kept in the choline-rich solution (no. 2-2) without ATP stimulation, there was no significant increase in the cytosolic choline content (control), indicating that choline uptake by both low-affinity and high-affinity ChTrs is not detectable in our experimental system. There was also no elevation of the cytosolic choline content in P2X2 purinoceptor-expressing cells when cells were not stimulated by ATP. On the other hand, when P2X2 purinoceptor-expressing cells were treated with 100 μM ATP, the cytosolic choline content increased significantly. These findings suggest that a significant choline+ influx occurs when ATP activated P2X2 purinoceptor-coupled cation channels.
Choline transport is thought to be mediated by high-affinity ChTrs (Lockman and Allen 2002), selectively localized at cholinergic presynaptic terminals, and choline taken up through these transporters is essential for acetylcholine (ACh) synthesis. In contrast, a low-affinity transporter system is distributed ubiquitously and provides the substrate for phospholipid synthesis (Lockman and Allen 2002). Because choline uptake through high-affinity ChTrs is the rate-limiting process for ACh synthesis (Sarter and Parikh 2005), activity of cholinergic neurons is in part determined by the expression level of high-affinity ChTrs. Therefore, choline transport through P2X2 purinoceptor-coupled cation channels in OFF-CAs could subvert the requirement for high-affinity ChTrs. To test this hypothesis, we analyzed the intensity of the immunoreactivity of high-affinity ChTrs. In the present study, we also analyzed the intensity of P2X2 purinoceptors, because the selective distribution of P2X2 purinoceptors in OFF-CA was previously reported (Kaneda et al. 2004). The intensity of immunoreactivity for P2X2 purinoceptors was greater in OFF-CAs (P < 0.001), indicating that the sensitivity of our quantitative analysis is sufficient to reproduce the previous findings (Kaneda et al. 2004). On the other hand, the immunoreactivity for high-affinity ChTrs in the dendrites of ON-CAs was stronger than the immunoreactivity for high-affinity ChTr in the dendrites of OFF-CAs (P < 0.001; Fig. 5, A and B).
DISCUSSION
In the present study, we found that P2X purinoceptors in OFF-CAs function as choline-permeable cation channels. This is the first report that the large cation permeability of P2X purinoceptors may have a physiological relevance in vivo. We also found that immunoreactivity for high-affinity ChTrs is higher in ON-CAs than in OFF-CAs. Furthermore, we examined whether P2X2 expressed at high densities in OFF-CAs (Kaneda et al. 2004) can act as a choline transport pathway. The physiological and the pharmacological properties of P2X purinoceptors in the present study supported the contribution of P2 purinoceptors, including P2X2 purinoceptors, in the formation of choline-permeable channels. In addition, we observed choline uptake through P2X2 purinoceptors in a heterologous expression system. Thus our findings suggest that choline transport through P2X purinoceptors, including P2X2 purinoceptors, in OFF-CAs acts as a novel channel-mediated choline transport pathway for ACh synthesis (Fig. 6).
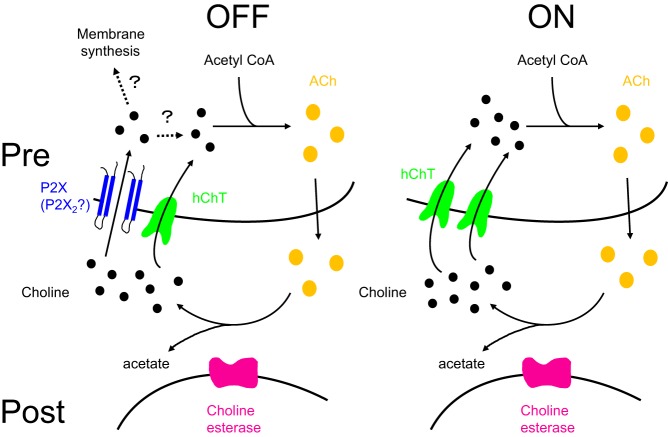
Fig. 6.
A model circuit of the choline uptake pathways in the dendrites of OFF- and ON-CAs of the mouse retina. Choline (black circles) is taken up by the high-affinity choline transporter (hChT; green) and P2X purinoceptor-coupled cation channels (blue) in OFF-CAs, and by the high-affinity choline transporter only in ON-CAs. Choline taken up by the high-affinity choline transporter is used for ACh synthesis. Pre, presynaptic terminals; Post, postsynaptic cells.
Previous immunohistochemical reports in the rodent retina show the distribution of several P2 purinoceptors in CAs (mouse: Dilip et al. 2013; Kaneda et al. 2004; Shigematsu et al. 2007; rat: Ward et al. 2008). On the basis of previous findings, P2X2, P2X3, P2X7, P2Y1, and P2Y4 purinoceptors are plausible candidates. From our pharmacological findings, we concluded that P2X3 purinoceptors are not likely candidates because MeATP did not produce any response (Burnstock 2004). At present, because previous reports of pore dilation of P2 purinoceptors have been limited in P2X subtypes (Burnstock 2004; Collo et al. 1997; Fujiwara and Kubo 2004; Surprenant et al. 1996), it is likely that P2Y1 and P2Y4 might not be candidates. To further specify the subtypes of P2 purinoceptors, we focused on the distribution of immunoreactivities for P2X2, P2X7, P2Y1, and P2Y4. Although the immunoreactivity for P2X2 purinoceptors in OFF-CA is stronger than the immunoreactivity for P2X2 purinoceptors in ON-CA (Kaneda et al. 2004), there is no significant difference in the intensity of immunoreactivities for P2X7, P2Y1, and P2Y4 between OFF-CA and ON-CA (Dilip et al. 2013; Kaneda et al. 2004; Ward et al. 2008). Therefore, it is likely that P2 purinoceptors, including P2X2 purinoceptors, on OFF-CAs acquired permeability to choline. The similar sensitivity of ATP-mediated choline currents to the extracellular Ca2+ concentration (Gever et al. 2006) also supports the contribution of P2X2 purinoceptors in the formation of choline-permeable channel pores. However, we have to be careful about the possibility that the intensity of immunoreactivities in ON-CAs and OFF-CAs might not correspond to the density of expressed purinoceptors on CAs. In addition, the presence of a small response to BzATP might also reflect the possible contribution of P2X7 purinoceptors in the formation of choline-permeable pores. Therefore, it is interesting to see if a subtle difference in the Hill coefficient (1.24) and EC50 (72.9 µM) from those of previous findings of P2 purinoceptors (Hill coefficient of 1.82, EC50 of 36.4 µM; Kaneda et al. 2008) might be explained by the presence of other P2 purinoceptors.
Permeability of P2X2 purinoceptors to large cations has been reported in heterologous expression systems (Eickhorst et al. 2002; Fujiwara and Kubo 2004, 2006; Virginio et al. 1999), but its functional significance has not yet been explored in vivo. The Km values of high-affinity ChTrs are 1.4–2.0 μM in rat brain synaptosomes (Ferguson et al. 1991; Patel et al. 1993; Yamamura and Snyder 1973), and thus the intracellular concentration of choline could be in the submicromolar range, assuming that the concentration gradient between the intra- and extracellular choline concentration acts as a driving force for the transporters. Retinal ganglion cells, the postsynaptic targets of CAs, are activated by ACh at ~10 μM (Kaneda et al. 1995; Lipton et al. 1987; Schmidt et al. 1987), and it is likely that the concentration of choline in the synaptic cleft between retinal ganglion cells and CAs following ACh degradation by acetylcholinesterase reaches micromolar levels. This estimated value is consistent with the physiological range of the concentration of choline in the plasma (5–10 μM; Lockman and Allen 2002; Sarter and Parikh 2005). Although there are no available data about the concentration of choline at the synaptic cleft and the intracellular space at present, it will be interesting to examine if the concentration gradient between the synaptic cleft and the intracellular space is sufficient to trigger choline influx through P2X2 purinoceptors at synapses of OFF-CAs in the mouse retina in future studies.
We assessed whether P2X2 purinoceptor-mediated choline influx could produce significant elevation of the intracellular choline concentration in HEK-293T cells. The amount of choline that entered during ATP stimulation was found to be 0.13 ± 0.11 pmol/cell by HPLC analysis. (The proportion of GFP-positive cells in the sample was assumed to be 64.6 ± 8.3%, n = 173.) If we assume the cell is a cylinder (15 μm in diameter and 30 mm in length; see Fig. 4B), the volume of a single HEK-293T cell would be 5.3 pl, so the observed choline influx would increase the intracellular choline concentration by 25 mM. The immunoreactivity for high-affinity ChTrs in ON-CAs is higher than in OFF-CAs, whereas the immunoreactivity for P2X2 purinoceptors in OFF-CAs is stronger than that in ON-CAs. The different immunoreactivities of high-affinity ChTrs between ON- and OFF-CAs indicate that there is a difference in the number of high-affinity ChTrs between ON- and OFF-CAs. Because the activity of cholinergic neurons is determined by the choline uptake by high-affinity ChTrs (Sarter and Parikh 2005), it is likely that there is a difference in the neuronal activity between ON- and OFF-CAs if choline taken up by the high-affinity ChTrs is the only source for ACh synthesis. Although the extracellular choline concentration in our experimental condition was very high (135 mM), our results raise the possibility that the ability for ACh synthesis between ON- and OFF-CAs might be balanced by additional choline transport through P2 purinoceptors, including P2X2 purinoceptors, in OFF-CAs. In previous studies, ACh has been reported to increase the firing rate of ganglion cells (Ikeda and Sheardown 1982; Schmidt et al. 1987), and the light-evoked release of ACh is regulated by P2X purinoceptors (Neal and Cunningham 1994). In the mouse retina, inhibition of P2X purinoceptors by PPADS increased the firing rate of OFF-ganglion cells and decreased that of ON-ganglion cells (Kaneda et al. 2008). Therefore, it will be interesting to determine whether significant choline transport through P2X2 purinoceptors occurs in OFF-CAs, but not in ON-CAs, in vivo in future studies. Because choline transported through low-affinity ChTrs is used as a substrate for phospholipid synthesis (Lockman and Allen 2002), it is possible that choline transported through P2 purinoceptors, including P2X2 purinoceptors, might similarly be used as a lipid precursor (Fig. 6).
Neal and Cunningham (1994) reported that glycinergic pathways are involved in the regulation of light-evoked release of ACh. In addition, our group previously reported that glycine receptors function selectively in ON-CAs (Ishii and Kaneda 2014). Our findings suggest that glycinergic signaling in ON-CAs is also involved in cholinergic regulation of ON-retinal ganglion cells. Because the functional role for ACh in the retina is not well elucidated, studies of the regulatory mechanism of cholinergic activity by P2X2 purinoceptors and glycine receptors might provide important clues for understanding the functional roles of ACh in the retina.
In the present study, we found that P2 purinoceptors, including P2X2 purinoceptors, in OFF-CAs are permeable to choline and may regulate the activity of OFF-CAs. We have reported that ON-CAs selectively receive glycinergic inputs (Ishii and Kaneda 2014). In the mouse retina, ON- and OFF-pathways act in an opposing manner in bright light conditions, whereas breakdown of the symmetry between ON- and OFF-pathways occurs in dim conditions (Pandarinath et al. 2010). In addition, recent findings show that the mode of synaptic computation is different between ON-and OFF-pathways at multiple states in retinal circuits (Eggers et al. 2007; Molnar and Werblin 2007; Murphy and Rieke 2006; Pandarinath et al. 2010; Zaghloul et al. 2003). Thus the OFF-pathway specific distribution of channel-mediated choline transport might be important for the formation of pathway-specific signal processing. Further investigation is needed to reveal whether channel-mediated choline transport promotes the pathway-specific signal processing of CAs in the mouse retina.
GRANTS
This work was supported by Japan Society for the Promotion of Science (JSPS) Grants-in-Aid for Scientific Research (C) (Nos. 18500312 and 21500373; to M. Kaneda), a Nippon Medical School Grant-in-Aid for Medical Research (to M. Kaneda), Novartis Pharma Research Grants (to M. Kaneda), JSPS Grant-in-Aid for Young Scientists (B) (KAKENHI No. 26860150 and 17K16990; to T. Ishii), Novartis Pharma Research Grants 2017 (to T. Ishii), and JSPS Grant-in-Aid for Young Scientists (B) (KAKENHI No. 26861473; to K. Homma).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.K. conceived the experiments; T.I., K.H., and M.K. designed the experiments; T.I., K.H., T.A., Y. Shigematsu, Y. Shimoda, H.I., and M.K. performed experiments; T.I., K.H., A.M., T.A., Y.K., and M.K. analyzed data; T.I., K.H., A.M., Y.K., and M.K. interpreted results of experiments; T.I., K.H., and M.K. prepared figures; T.I., K.H., A.M., Y.K., and M.K. drafted manuscript; T.I., K.H., and M.K. edited and revised manuscript; T.I., K.H., A.M., Y. Shigematsu, Y. Shimoda, H.I., Y.K., and M.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. G. T. Swanson for comments and editing of the manuscript. We also thank Dr. M. Tominaga for providing the vector for the P2X2 purinoceptor. Present address for K. Homma: Department of Ophthalmology, Keio University School of Medicine, Tokyo, Japan (e-mail: hommak@keio.jp).
REFERENCES
- Burnstock G. Introduction: P2 receptors. Curr Top Med Chem 4: 793–803, 2004. doi: 10.2174/1568026043451014. [DOI] [PubMed] [Google Scholar]
- Collo G, Neidhart S, Kawashima E, Kosco-Vilbois M, North RA, Buell G. Tissue distribution of the P2X7 receptor. Neuropharmacology 36: 1277–1283, 1997. doi: 10.1016/S0028-3908(97)00140-8. [DOI] [PubMed] [Google Scholar]
- Dilip R, Ishii T, Imada H, Wada-Kiyama Y, Kiyama R, Miyachi E, Kaneda M. Distribution and development of P2Y1-purinoceptors in the mouse retina. J Mol Histol 44: 639–644, 2013. doi: 10.1007/s10735-013-9525-4. [DOI] [PubMed] [Google Scholar]
- Ding S, Sachs F. Single channel properties of P2X2 purinoceptors. J Gen Physiol 113: 695–720, 1999. doi: 10.1085/jgp.113.5.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, McCall MA, Lukasiewicz PD. Presynaptic inhibition differentially shapes transmission in distinct circuits in the mouse retina. J Physiol 582: 569–582, 2007. doi: 10.1113/jphysiol.2007.131763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhorst AN, Berson A, Cockayne D, Lester HA, Khakh BS. Control of P2X2 channel permeability by the cytosolic domain. J Gen Physiol 120: 119–131, 2002. doi: 10.1085/jgp.20028535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RJ, Lewis C, Virginio C, Lundstrom K, Buell G, Surprenant A, North RA. Ionic permeability of, and divalent cation effects on, two ATP-gated cation channels (P2X receptors) expressed in mammalian cells. J Physiol 497: 413–422, 1996. doi: 10.1113/jphysiol.1996.sp021777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SS, Diksic M, Collier B. Stereospecificity of high- and low-affinity transport of choline analogues into rat cortical synaptosomes. J Neurochem 57: 915–921, 1991. doi: 10.1111/j.1471-4159.1991.tb08238.x. [DOI] [PubMed] [Google Scholar]
- Fujiwara Y, Kubo Y. Density-dependent changes of the pore properties of the P2X2 receptor channel. J Physiol 558: 31–43, 2004. doi: 10.1113/jphysiol.2004.064568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara Y, Kubo Y. Regulation of the desensitization and ion selectivity of ATP-gated P2X2 channels by phosphoinositides. J Physiol 576: 135–149, 2006. doi: 10.1113/jphysiol.2006.115246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gever JR, Cockayne DA, Dillon MP, Burnstock G, Ford AP. Pharmacology of P2X channels. Pflugers Arch 452: 513–537, 2006. doi: 10.1007/s00424-006-0070-9. [DOI] [PubMed] [Google Scholar]
- Ikeda H, Sheardown MJ. Acetylcholine may be an excitatory transmitter mediating visual excitation of ‘transient’ cells with the periphery effect in the cat retina: iontophoretic studies in vivo. Neuroscience 7: 1299–1308, 1982. doi: 10.1016/0306-4522(82)91135-6. [DOI] [PubMed] [Google Scholar]
- Ishii K, Kaneda M, Li H, Rockland KS, Hashikawa T. Neuron-specific distribution of P2X7 purinergic receptors in the monkey retina. J Comp Neurol 459: 267–277, 2003. doi: 10.1002/cne.10608. [DOI] [PubMed] [Google Scholar]
- Ishii T, Kaneda M. ON-pathway-dominant glycinergic regulation of cholinergic amacrine cells in the mouse retina. J Physiol 592: 4235–4245, 2014. doi: 10.1113/jphysiol.2014.271148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda M, Hashimoto M, Kaneko A. Neuronal nicotinic acetylcholine receptors of ganglion cells in the cat retina. Jpn J Physiol 45: 491–508, 1995. doi: 10.2170/jjphysiol.45.491. [DOI] [PubMed] [Google Scholar]
- Kaneda M, Ishii K, Morishima Y, Akagi T, Yamazaki Y, Nakanishi S, Hashikawa T. OFF-cholinergic-pathway-selective localization of P2X2 purinoceptors in the mouse retina. J Comp Neurol 476: 103–111, 2004. doi: 10.1002/cne.20208. [DOI] [PubMed] [Google Scholar]
- Kaneda M, Ishii T, Hosoya T. Pathway-dependent modulation by P2-purinoceptors in the mouse retina. Eur J Neurosci 28: 128–136, 2008. doi: 10.1111/j.1460-9568.2008.06317.x. [DOI] [PubMed] [Google Scholar]
- Kaneda M, Ito K, Morishima Y, Shigematsu Y, Shimoda Y. Characterization of voltage-gated ionic channels in cholinergic amacrine cells in the mouse retina. J Neurophysiol 97: 4225–4234, 2007. doi: 10.1152/jn.01022.2006. [DOI] [PubMed] [Google Scholar]
- Lipton SA, Aizenman E, Loring RH. Neural nicotinic acetylcholine responses in solitary mammalian retinal ganglion cells. Pflugers Arch 410: 37–43, 1987. doi: 10.1007/BF00581893. [DOI] [PubMed] [Google Scholar]
- Lockman PR, Allen DD. The transport of choline. Drug Dev Ind Pharm 28: 749–771, 2002. doi: 10.1081/DDC-120005622. [DOI] [PubMed] [Google Scholar]
- Molnar A, Werblin F. Inhibitory feedback shapes bipolar cell responses in the rabbit retina. J Neurophysiol 98: 3423–3435, 2007. doi: 10.1152/jn.00838.2007. [DOI] [PubMed] [Google Scholar]
- Murphy GJ, Rieke F. Network variability limits stimulus-evoked spike timing precision in retinal ganglion cells. Neuron 52: 511–524, 2006. doi: 10.1016/j.neuron.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal M, Cunningham J. Modulation by endogenous ATP of the light-evoked release of ACh from retinal cholinergic neurones. Br J Pharmacol 113: 1085–1087, 1994. doi: 10.1111/j.1476-5381.1994.tb17106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaita A, Petit-Jacques J, Völgyi B, Ho CS, Joho RH, Bloomfield SA, Rudy B. A unique role for Kv3 voltage-gated potassium channels in starburst amacrine cell signaling in mouse retina. J Neurosci 24: 7335–7343, 2004. doi: 10.1523/JNEUROSCI.1275-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandarinath C, Victor JD, Nirenberg S. Symmetry breakdown in the ON and OFF pathways of the retina at night: functional implications. J Neurosci 30: 10006–10014, 2010. doi: 10.1523/JNEUROSCI.5616-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel PJ, Messer WS Jr, Hudson RA. Inhibition and inactivation of presynaptic cholinergic markers using redox-reactive choline analogs. J Med Chem 36: 1893–1901, 1993. doi: 10.1021/jm00065a012. [DOI] [PubMed] [Google Scholar]
- Sarter M, Parikh V. Choline transporters, cholinergic transmission and cognition. Nat Rev Neurosci 6: 48–56, 2005. doi: 10.1038/nrn1588. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Humphrey MF, Wässle H. Action and localization of acetylcholine in the cat retina. J Neurophysiol 58: 997–1015, 1987. [DOI] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9: 671–675, 2012. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigematsu Y, Shimoda Y, Kaneda M. Distribution of immunoreactivity for P2X3, P2X5, and P2X6-purinoceptors in mouse retina. J Mol Histol 38: 369–371, 2007. doi: 10.1007/s10735-007-9107-4. [DOI] [PubMed] [Google Scholar]
- Surprenant A, Rassendren F, Kawashima E, North RA, Buell G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7). Science 272: 735–738, 1996. doi: 10.1126/science.272.5262.735. [DOI] [PubMed] [Google Scholar]
- Virginio C, MacKenzie A, Rassendren FA, North RA, Surprenant A. Pore dilation of neuronal P2X receptor channels. Nat Neurosci 2: 315–321, 1999. doi: 10.1038/7225. [DOI] [PubMed] [Google Scholar]
- Ward MM, Puthussery T, Fletcher EL. Localization and possible function of P2Y4 receptors in the rodent retina. Neuroscience 155: 1262–1274, 2008. doi: 10.1016/j.neuroscience.2008.06.035. [DOI] [PubMed] [Google Scholar]
- Watanabe D, Inokawa H, Hashimoto K, Suzuki N, Kano M, Shigemoto R, Hirano T, Toyama K, Kaneko S, Yokoi M, Moriyoshi K, Suzuki M, Kobayashi K, Nagatsu T, Kreitman RJ, Pastan I, Nakanishi S. Ablation of cerebellar Golgi cells disrupts synaptic integration involving GABA inhibition and NMDA receptor activation in motor coordination. Cell 95: 17–27, 1998. doi: 10.1016/S0092-8674(00)81779-1. [DOI] [PubMed] [Google Scholar]
- Yamamura HI, Snyder SH. High affinity transport of choline into synaptosomes of rat brain. J Neurochem 21: 1355–1374, 1973. doi: 10.1111/j.1471-4159.1973.tb06022.x. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Watanabe D, Ishikane H, Tachibana M, Pastan I, Nakanishi S. A key role of starburst amacrine cells in originating retinal directional selectivity and optokinetic eye movement. Neuron 30: 771–780, 2001. doi: 10.1016/S0896-6273(01)00316-6. [DOI] [PubMed] [Google Scholar]
- Zaghloul KA, Boahen K, Demb JB. Different circuits for ON and OFF retinal ganglion cells cause different contrast sensitivities. J Neurosci 23: 2645–2654, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]