Abstract
When sudden environmental stimuli signaling threat occur in the portion of space surrounding the body (defensive peripersonal space), defensive responses are enhanced. Recently Bisio et al. (Bisio A, Garbarini F, Biggio M, Fossataro C, Ruggeri P, Bove M. J Neurosci 37: 2415–2424, 2017) showed that a marker of defensive peripersonal space, the defensive hand-blink reflex, is modulated by the motion of the eliciting threatening stimulus. These results can be parsimoniously explained by the continuous monitoring of environmental threats, resulting in an expansion of defensive peripersonal space when threatening stimuli approach.
Keywords: defense, blink reflex, peripersonal space, movement
the closer a threatening stimulus occurs to a body part, the more likely it is to cause damage, and the stronger the elicited defensive responses become. The region of space surrounding the body in which this increase in defensive response occurs is termed defensive peripersonal space (DPPS; Graziano and Cooke 2006). Its neural substrates in nonhuman primates likely consist of a parieto-premotor network. This network involves multisensory neurons in the ventral intraparietal area (VIP) and in the polysensory zone of area F4 (Cooke and Graziano 2004; Cléry et al. 2015b). In humans, the DPPS surrounding the face has been described by recording the enhancement of the defensive blink reflex elicited by electrical stimulation of the hand (hand-blink reflex; HBR) when the hand is nearer to the face compared with when it is far (Sambo et al. 2012). The DPPS has been suggested to have the shape of a bubble elongated asymmetrically along the rostrocaudal axis, extending further above eye level (Bufacchi et al. 2016).
In a recent paper, Bisio et al. (Bisio et al. 2017) delivered the stimuli to elicit the HBR while participants moved their hand. They instructed participants to make a single hand movement toward and then away from their face (or vice versa) roughly every 30 s and stimulated the hand at one of six time points during this motion. They reported that HBR magnitude was affected by this hand movement when the hand was near the face: in that position, when the hand was moving away from the face, the HBR magnitude was decreased compared with when the hand was moving toward the face (Fig. 1A, left). In contrast, HBR magnitude was not dependent on movement direction when the hand was in the other two positions farther from the face. Remarkably, they showed a similar effect when participants imagined moving their hand but did not actually perform the movement. They provided a convincing directional interpretation: the HBR magnitude in the near position can be reduced by a movement of the threat away from the body part that needs to be defended. The dependence of HBR magnitude on movement was also suggested by the results of Wallwork et al. (2016), which, however, consisted in a seemingly opposite pattern: where Bisio et al. reported an HBR decrease at the near position when the hand was moving away from the face, Wallwork et al. reported no difference between movement directions at the near position. Furthermore, Wallwork et al. reported an HBR increase at the far position when the hand was moving toward the face, while Bisio et al. reported no difference between movement conditions at that position (Fig. 1A, left). Both articles suggest that the cause for the observed effects might be the ability of the nervous system to predict the future location of the hand. However, their two explanations are opposite: in Bisio et al. the prediction of where the hand is going to be is assumed to cause a downregulation of HBR magnitude but not an upregulation, while in Wallwork et al. it is assumed to cause an upregulation of HBR magnitude, but not a downregulation.
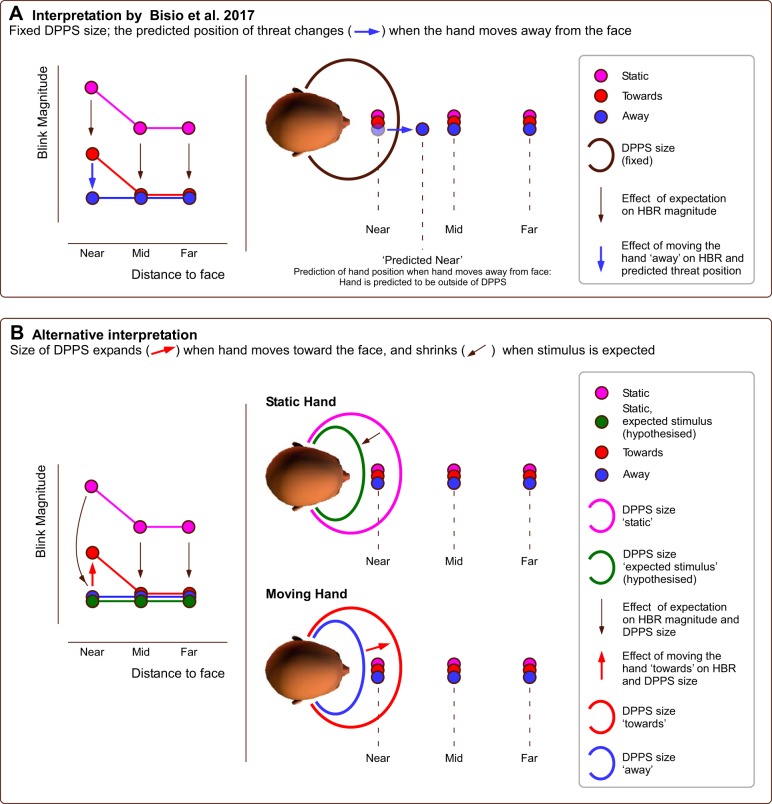
Fig. 1.
Schematic of hand movement effects on hand-blink reflex (HBR) magnitude. A: in the interpretation put forward by Bisio et al. (2017) the size of the defensive peripersonal space (DPPS) is fixed. Left: sketch of HBR magnitude across different conditions. Right: assumed DPPS (black line) and predicted positions of the threat (colored circles). In this interpretation, when 1) the threat is near the face and 2) it moves away from the face, the position of the threat is predicted to shift outside the DPPS (blue arrow in right subpanel), with a consequent decrease in HBR magnitude at the near position (blue arrow in left subpanel). In both conditions where the hand moves, the movement of the hand is linked to the stimulus onset, therefore causing an overall HBR magnitude decrease. Importantly, Bisio et al. assume this “expectation effect” to be equal at all hand positions (black arrows in left subpanel). B: an alternative interpretation is that the DPPS size is malleable. Left: sketch of HBR magnitudes across different conditions, also including hypothetical true baseline magnitude when the eliciting shock is expected. Right: implied size of DPPS (colored lines) and predicted positions of the threat (colored circles). In this alternative interpretation, when the threat moves toward the face, the DPPS expands (red arrow in right subpanel), causing an increase in HBR magnitude at the near position (red arrow in left subpanel). In this interpretation, the expectation effect results in both an overall HBR magnitude decrease and in a DPPS shrinkage (black arrows in both subpanels). While both interpretations are plausible, only the alternative interpretation fits with prior empirical observations (e.g., Wallwork et al. 2016).
A simple explanation could reconcile these two seemingly opposite observations, which could in fact be instances of the same physiological phenomenon. This explanation is that the DPPS size is not stationary but changes depending on the context: it increases when threatening stimuli move toward an endangered body area. Interestingly, this notion is present in the title of the article by Bisio et al. (2017) but not further elaborated on. Considering the wider body of work on peripersonal space (not only in relation to defense) provides support for this explanation. It has been shown that the size of peripersonal space is malleable within subject (Van der Stoep et al. 2016). For example, tool use reshapes action space around the tool (Longo and Lourenco 2007), walking expands peripersonal space forward (Noel et al. 2015), and gravitational cues warp the size of defensive peripersonal space (Bufacchi and Iannetti 2016). Even more interestingly, the firing rate of the cells thought to underlie the DPPS specifically is dependent not only on the stimulus position but also on its movement direction and its speed: these cells generally fire more when the stimulus 1) moves toward the body and 2) moves faster (Graziano and Cooke 2006).
Unfortunately, Bisio et al. did not discuss this possibility and instead assumed the opposite, namely that the DPPS is of fixed size (Fig. 1A, right). This reasoning led to the conclusion that “. . . these findings might be explained as a downregulation of the HBR response when planning to move far from the face, albeit the hand was inside the defensive peripersonal space.” In other words, they claimed that when the hand is moving away from the face, the HBR is downregulated to baseline levels, even though it is inside the DPPS of the face. This reasoning seems inconsistent with the definition of the DPPS as the zone within which the HBR magnitude exceeds a specific threshold.
That the DPPS is malleable is consistent with its survival advantage: the probability that a threat will hit the face is higher when the source of that threat (the stimulation on the wrist) is moving toward the face. This increased probability of hitting in turn increases the threat’s potential for harm and therefore necessitates a stronger HBR, to proportionally match the increased danger of the threat (Bufacchi et al. 2016). Under this framework, the HBR increase that Bisio et al. (2017) observed at the nearest position when the hand is moving toward the face can be interpreted as an expansion of DPPS. In other words, the DPPS expands from a size where it does not reach the nearest hand position from the face, to a size where it encompasses that nearest position (Fig. 1B, right). Similarly, the increase in HBR magnitude at the far position when the hand is moving toward the face observed by Wallwork et al. (2016) would then be an expansion of DPPS to encompass that furthest position, from a size where the DPPS only encompassed the nearest position. Note that this line of reasoning makes the assumption that in Wallwork et al. (2016) there is a ceiling effect: the HBR magnitude has a maximum value, and this value is reached at the near position. When the DDPS expands to encompass the furthest position, the HBR elicited when the hand is at that position also reaches the ceiling magnitude.
Even if this reasoning is correct, an important issue that remains to be solved is which DPPS measure Bisio et al. (2017) considered as a baseline; Fig. 1 shows that they tested the HBR magnitude under three conditions: while the hand was moving toward the face, moving away from it, and not moving at all. They took the pattern of HBR increase in the static condition as a baseline measure: in that condition, the HBR magnitude at the near position is larger than the HBR magnitude in the other two positions. They then reasoned that, because this pattern was present in the toward condition but not in the away condition, in this latter condition the expected hand position must have been outside the DPPS. Importantly, however, in the static condition, Bisio et al. measure an HBR overall much larger than in the two movement conditions. Therefore, using the static condition as a baseline measure of DPPS size is unlikely to be correct. In fact, the difference between static and moving conditions is most likely caused by expectation; in the moving conditions, subjects spent most of their time with the hand resting on a table, then moved it toward and away from their face once per trial when prompted by the experimenter. During this movement the shock was delivered when the forearm was at a specific angle. Therefore, as Bisio et al. also point out, participants knew that they could only possibly get a shock once they started moving. This expectation effect most likely resulted in participants linking the movement of their arm to the delivery of a shock, thereby decreasing the surprise and hence the threatening value of the shock.
Therefore, in any conditions where the subject’s hand is moved, the baseline size of the DPPS might be decreased due to higher stimulus predictability. This would result in a smaller HBR magnitude difference between the near and far conditions, or even no difference at all if the baseline DPPS size were small enough. Therefore, a better baseline would have been a condition in which the participants trigger the shock, and wherein the shock occurs within ~4 s from the trigger (i.e., a similar temporal delay between when the participants began the movement and when the shock occurred in Bisio et al. (2017). If in this condition the HBR is equal in the nearest and the furthest hand positions (or at least is more similar in magnitude than when the hand is moving toward the face), this would demonstrate that, when the stimulus is expected, the DPPS is smaller than the distance between the nearest hand position and the face. In this way, when the hand is moving toward the face, the DPPS size would increase from baseline, resulting in an increase in HBR magnitude at the nearest hand position. This explanation is also supported by the observation that, when the movement of the hand is not temporally linked to the stimulus onset, baseline DPPS size (i.e., regardless of hand movement) is not substantially different from the condition in which the hand moves away from the face (Wallwork et al. 2016).
Interpreting the results of the discussed studies within the framework of an expansion of DPPS emphasizes the proposed link between the activity of cortical areas underlying the spatial modulation of defensive responses (such as VIP and F4; Cooke and Graziano 2004) and the brain stem circuits mediating the HBR (Sambo et al. 2012). Indeed, VIP neurons are highly selective to the direction and velocity of stimuli (Colby et al. 1993) and receive dense proprioceptive inputs (Lewis and Van Essen 2000), while the receptive field of many F4 neurons expands in depth when the stimulus speed increases (Fogassi et al. 1996). Such response features would be necessary to cause an expansion of DPPS in response to the movement of the forearm relative to the face. Accordingly, VIP has been proposed to subserve impact prediction (Cléry et al. 2015a). These areas receive input from the superior collicus and pulvinar (Makin et al. 2012), both of which respond to looming stimuli and are involved in time-to-collision judgements (Billington et al. 2011). The superior colliculus is also strongly involved in enacting defensive responses across species (Pereira and Moita 2016) and has multimodal response properties (Triplett et al. 2012). Interestingly, therefore, it is possible that the brain stem circuits mediating the HBR might also be influenced by midbrain areas. Regardless of the exact anatomical origin of the HBR modulation, these observations also emphasize that even simple subcortical reflexes can be modulated in sophisticated manners by other brain areas. This important notion should be kept in mind when designing and interpreting experiments measuring even the most basic behavioral responses.
In conclusion, a parsimonious explanation of the results of Bisio et al. (2017) is that the DPPS size increases when the hand carrying the threatening stimulus is moving toward the face, following the estimated increase in probability that the moving threat will harm the face.
GRANTS
This research was funded by the European Research Council (ERC). Grant acronym: PAINSTRAT.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.J.B. interpreted results of experiments, prepared figures, drafted manuscript, edited and revised manuscript, and approved final version of manuscript.
REFERENCES
- Billington J, Wilkie RM, Field DT, Wann JP. Neural processing of imminent collision in humans. Proc Biol Sci 278: 1476–1481, 2011. doi: 10.1098/rspb.2010.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisio A, Garbarini F, Biggio M, Fossataro C, Ruggeri P, Bove M. Dynamic shaping of the defensive peripersonal space through predictive motor mechanisms: when the “near” becomes “far”. J Neurosci 37: 2415–2424, 2017. doi: 10.1523/JNEUROSCI.0371-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bufacchi RJ, Iannetti GD. Gravitational cues modulate the shape of defensive peripersonal space. Curr Biol 26: R1133–R1134, 2016. doi: 10.1016/j.cub.2016.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bufacchi RJ, Liang M, Griffin LD, Iannetti GD. A geometric model of defensive peripersonal space. J Neurophysiol 115: 218–225, 2016. doi: 10.1152/jn.00691.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cléry J, Guipponi O, Odouard S, Wardak C, Ben Hamed S. Impact prediction by looming visual stimuli enhances tactile detection. J Neurosci 35: 4179–4189, 2015a. doi: 10.1523/JNEUROSCI.3031-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cléry J, Guipponi O, Wardak C, Ben Hamed S. Neuronal bases of peripersonal and extrapersonal spaces, their plasticity and their dynamics: knowns and unknowns. Neuropsychologia 70: 313–326, 2015b. doi: 10.1016/j.neuropsychologia.2014.10.022. [DOI] [PubMed] [Google Scholar]
- Colby CL, Duhamel JR, Goldberg ME. Ventral intraparietal area of the macaque: anatomic location and visual response properties. J Neurophysiol 69: 902–914, 1993. [DOI] [PubMed] [Google Scholar]
- Cooke DF, Graziano MS. Super-flinchers and nerves of steel: defensive movements altered by chemical manipulation of a cortical motor area. Neuron 43: 585–593, 2004. doi: 10.1016/j.neuron.2004.07.029. [DOI] [PubMed] [Google Scholar]
- Fogassi L, Gallese V, Fadiga L, Luppino G, Matelli M, Rizzolatti G. Coding of peripersonal space in inferior premotor cortex (area F4). J Neurophysiol 76: 141–157, 1996. [DOI] [PubMed] [Google Scholar]
- Graziano MS, Cooke DF. Parieto-frontal interactions, personal space, and defensive behavior. Neuropsychologia 44: 845–859, 2006. doi: 10.1016/j.neuropsychologia.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Lewis JW, Van Essen DC. Corticocortical connections of visual, sensorimotor, and multimodal processing areas in the parietal lobe of the macaque monkey. J Comp Neurol 428: 112–137, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- Longo MR, Lourenco SF. Space perception and body morphology: extent of near space scales with arm length. Exp Brain Res 177: 285–290, 2007. doi: 10.1007/s00221-007-0855-x. [DOI] [PubMed] [Google Scholar]
- Makin TR, Holmes NP, Brozzoli C, Farnè A. Keeping the world at hand: rapid visuomotor processing for hand-object interactions. Exp Brain Res 219: 421–428, 2012. doi: 10.1007/s00221-012-3089-5. [DOI] [PubMed] [Google Scholar]
- Noel J-P, Grivaz P, Marmaroli P, Lissek H, Blanke O, Serino A. Full body action remapping of peripersonal space: the case of walking. Neuropsychologia 70: 375–384, 2015. doi: 10.1016/j.neuropsychologia.2014.08.030. [DOI] [PubMed] [Google Scholar]
- Pereira AG, Moita MA. Is there anybody out there? Neural circuits of threat detection in vertebrates. Curr Opin Neurobiol 41: 179–187, 2016. doi: 10.1016/j.conb.2016.09.011. [DOI] [PubMed] [Google Scholar]
- Sambo CF, Forster B, Williams SC, Iannetti GD. To blink or not to blink: fine cognitive tuning of the defensive peripersonal space. J Neurosci 32: 12921–12927, 2012. doi: 10.1523/JNEUROSCI.0607-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triplett JW, Phan A, Yamada J, Feldheim DA. Alignment of multimodal sensory input in the superior colliculus through a gradient-matching mechanism. J Neurosci 32: 5264–5271, 2012. doi: 10.1523/JNEUROSCI.0240-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Stoep N, Serino A, Farnè A, Di Luca M, Spence C. Depth: the forgotten dimension in multisensory research. Multisens Res 29: 493–524, 2016. doi: 10.1163/22134808-00002525. [DOI] [Google Scholar]
- Wallwork SB, Talbot K, Camfferman D, Moseley GL, Iannetti GD. The blink reflex magnitude is continuously adjusted according to both current and predicted stimulus position with respect to the face. Cortex 81: 168–175, 2016. doi: 10.1016/j.cortex.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]