We aimed to determine whether somatosensory gating in patients with multiple sclerosis (MS) differed compared with healthy controls and whether a relationship exists between somatosensory gating and walking performance. We found reduced somatosensory gating responses in patients with MS, and these altered somatosensory gating responses were correlated with the mobility impairments. These novel findings show that somatosensory gating is impaired in patients with MS and is related to the mobility impairments seen in these patients.
Keywords: sensory gating, paired-pulse, cortical inhibition, magnetoencephalography
Abstract
When identical stimuli are presented in rapid temporal succession, neural responses to the second stimulation are often weaker than those observed for the first. This phenomenon is termed sensory gating and is believed to be an adaptive feature that helps prevent higher-order cortical centers from being flooded with unnecessary information. Recently, sensory gating in the somatosensory system has been linked to deficits in tactile discrimination. Additionally, studies have linked poor tactile discrimination with impaired walking and balance in individuals with multiple sclerosis (MS). In this study, we examine the neural basis of somatosensory gating in patients with MS and healthy controls and assess the relationship between somatosensory gating and walking performance. We used magnetoencephalography to record neural responses to paired-pulse electrical stimulation applied to the right posterior tibial nerve. All participants also walked across a digital mat, which recorded their spatiotemporal gait kinematics. Our results showed the amplitude of the response to the second stimulation was sharply reduced only in controls, resulting in a significantly reduced somatosensory gating in the patients with MS. No group differences were observed in the amplitude of the response to the first stimulation nor the latency of the neural response to either the first or second stimulation. Interestingly, the altered somatosensory gating responses were correlated with aberrant spatiotemporal gait kinematics in the patients with MS. These results suggest that inhibitory GABA circuits may be altered in patients with MS, which impacts somatosensory gating and contributes to the motor performance deficits seen in these patients.
NEW & NOTEWORTHY We aimed to determine whether somatosensory gating in patients with multiple sclerosis (MS) differed compared with healthy controls and whether a relationship exists between somatosensory gating and walking performance. We found reduced somatosensory gating responses in patients with MS, and these altered somatosensory gating responses were correlated with the mobility impairments. These novel findings show that somatosensory gating is impaired in patients with MS and is related to the mobility impairments seen in these patients.
multiple sclerosis (MS) is an inflammatory autoimmune disease of the central nervous system that results in demyelination of the axons in the brain and spinal cord. This demyelination reduces nerve conduction velocity, impairing the function of the central nervous system (White and Dressendorfer 2004). Although the symptoms vary widely between individuals, many experience mobility and balance impairments that limit their activities of daily living (Ellis and Motl 2013). Approximately 50% of patients with MS will require the use of a walking aid within 15 yr of onset of the disease (Tremlett et al. 2006). Furthermore, ~70% of patients with MS report gait dysfunction to be the most challenging aspect of the disease (LaRocca 2011).
Historically, the clinical impression was that these mobility impairments were due to weaker muscles that fatigue at a faster rate (Armstrong et al. 1983; Chen et al. 1987; Kent-Braun et al. 1997; Lambert et al. 2001; Ponichtera et al. 1992; Rice et al. 1992). Although this is a likely factor, several prior studies have shown that sensory deficits, particularly loss of tactile sensation, are also related to impaired standing balance and walking performance in individuals with MS (Citaker et al. 2011; Thoumie and Mevellec 2002). Despite this information, our understanding of the link between the sensory and motor systems is limited, and very few rehabilitation strategies have targeted the sensory impairments (Cattaneo et al. 2007; Gandolfi et al. 2015). Further interrogation of the sensory system, and its relation to motor function in patients with MS, is needed to improve our understanding of the link between these two systems and the disease.
Sensory gating is a physiological process by which the central nervous system inhibits or suppresses redundant sensory information. The paired-pulse stimulation paradigm, which results in an attenuated neural response to an identical second stimulation when presented with a sufficiently short stimulus onset asynchrony, is commonly employed to assess sensory gating. Such sensory gating is believed to serve as a protective mechanism, preventing higher-order cortical centers from being flooded with unnecessary or redundant information (Boutros and Belger 1999; Cheng et al. 2016). Historically, a number of sensory gating investigations have established that auditory gating deficits are associated with psychoses, including schizophrenia (Adler et al. 1982; Bramon et al. 2004; Cromwell et al. 2008). More recently, gating deficits have also been shown in individuals with autism spectrum disorder (Matsuzaki et al. 2014), epilepsy (Rosburg et al. 2008), and Alzheimer’s disease (Jessen et al. 2001) as well as elderly individuals (Lenz et al. 2012). Additionally, despite gating occurring during the earliest stages of perceptual processing, it has been suggested that aberrant gating responses impact later cognitive processing and the formation of memories (Cheng et al. 2016). Moreover, Lenz et al. (2012) have shown that with increasing age there is an increase in cortical excitability in humans, and numerous animal studies have linked this to a reduction in intracortical GABAergic inhibition. As this cortical hyperexcitability gradually increases, there is proportionate reduction of tactile perception. A similar mechanism has been explained in multiple sclerosis where studies have proven that there is reduction of GABAergic inhibition (Caramia et al. 2004; Conte et al. 2009; Liepert et al. 2005; Vucic et al. 2012).
The purpose of this investigation was to assess the integrity of the sensory system in patients with MS by quantifying somatosensory gating and relating this to walking performance. To this end, we applied paired-pulse electrical stimulation to the posterior tibial nerve while magnetoencephalography (MEG) was used to collect neural responses in patients with MS and a group of demographically matched controls. We also evaluated the spatiotemporal walking kinematics of these individuals to determine whether sensory gating may be related to the impaired mobility of patients with MS.
METHODS
Participants.
Eleven participants with relapsing-remitting or secondary progressive MS (age = 56.1 ± 6 yr; 9 women) and twelve healthy age- and sex-matched controls (age = 54.7 ± 7 yr; 9 women) participated in this study. The patients with MS had an average Kurtzke Expanded Disability Status Scale (EDSS) of 5.5 ± 0.8, which indicated that on average they could walk independently for ≥100 m. At the time of data collection, none of the patients had a relapse or a change in medication for ≥3 mo. All testing was done at the University of Nebraska Medical Center. The Institutional Review Board at the University of Nebraska Medical Center reviewed and approved the protocol for this investigation. Additionally, all participants provided written, informed consent before participation in this investigation.
Experimental paradigm.
The participants were seated with their eyes closed in a custom-made nonmagnetic chair with their head positioned within the MEG helmet-shaped sensor array. Unilateral electrical stimulation was applied to the right posterior tibial nerve using external cutaneous stimulators. For each participant, 120 paired-pulse trials were collected using an interstimulus interval of 500 ms and an interpair interval that randomly varied between 4.5 and 4.8 s. Each pulse comprised a 0.2-ms constant-current square-wave, with the amplitude scaled to elicit a subtle flexion of the first phalange of the foot. These stimulation parameters are known to elicit a robust somatosensory gating response (Kurz et al. 2017; Wiesman et al. 2017). Epochs were a total duration of 1.2 s, ranging from −0.2 to 1.0 s, with 0.0 s representing the onset of the first stimulation.
MEG data acquisition and coregistration.
All MEG recordings were conducted in a one-layer magnetically shielded room with active shielding engaged for advanced environmental noise compensation. During data acquisition, participants were monitored via real-time audio-video feeds from inside the shielded room. Neuromagnetic responses were acquired with a bandwidth of 0.1–330 Hz and were sampled continuously at 1 kHz using an Elekta Neuromag system (Helsinki, Finland) with 306 MEG sensors, including 204 planar gradiometers and 102 magnetometers. Each MEG data set was individually corrected for head motion during task performance and subjected to noise reduction using the signal space separation method with a temporal extension (Taulu and Simola 2006).
Four coils were affixed to the head of the participant and were used for continuous head localization during the MEG experiment. Before the experiment, the location of these coils, three fiducial points, and the scalp surface were digitized to determine their three-dimensional position (FASTRAK 3SF0002; Polhemus Navigator Sciences, Colchester, VT). Once the participant was positioned for MEG recording, an electrical current with a unique frequency label (e.g., 322 Hz) was fed to each of the four coils. This induced a measurable magnetic field and allowed each coil to be localized in reference to the sensors throughout the recording session. Since the coil locations were also known in head coordinates, all MEG measurements could be transformed into a common coordinate system. With this coordinate system (including the scalp surface points), each participant’s MEG data were coregistered with structural T1-weighted MRI data using three external landmarks (i.e., fiducials) and the digitized scalp surface points before source-space analyses. Structural MRI data were aligned parallel to the anterior and posterior commissures and transformed into the Talairach coordinate system (Talairach and Tournoux 1988) using the volumetric subspace warping method implemented in BrainVoyager QX version 2.2 (Brain Innovation).
MEG processing.
Artifact rejection was based on a fixed threshold method, supplemented with visual inspection. Artifact-free epochs were time-domain averaged with respect to stimulus onset and then digitally filtered 0.1–120 Hz. The peak response to the first stimulation was evident at the sensor level and peaked at ~80 ms after the first stimulation across all participants. Thus a 40-ms window, centered over the peak of this response, was modeled with a regional current source using the subset of sensors that covered both magnetic flux extrema (Hoechstetter et al. 2004; Scherg et al. 2002; Wilson et al. 2013). The resulting sources were all located within the leg area of the primary somatosensory cortices and had an average goodness of fit of >0.70 across the whole time window. Of note, a regional current source is similar to an equivalent current dipole except that in the current case the source has 2 orthogonal orientations (vs. 1 in the case of an equivalent current dipole). Of these 2 orientations, the dominant orientation was used for all source time-series analyses. We found the peak source amplitude of the response to the 1st stimulation (peak 1) and the 2nd stimulation (peak 2). Using these peak amplitudes, we calculated the gating ratio by dividing peak 2 by peak 1. A gating ratio that is closer to 1 indicates a reduced gating response. Additionally, we calculated the latency to the peak of each of these responses.
Mobility analysis.
All participants were instructed to walk across a 5.75-m digital mat (GAITRite, Sparta, NJ) at their preferred walking speeds. The mat digitized the locations of the feet, which were used to quantify the participant’s walking velocity, cadence, step length, and step width. Each participant completed two walking trials, averaging 7.8 ± 2.5 steps per trial, and the data from these two trials were averaged together.
Statistical analysis.
Shapiro-Wilk test of normality was used to determine whether the data were normally distributed. Those data that failed the test were subsequently logarithmically transformed for all statistical testing. Separate mixed-model (group × peak number) ANOVAs with least-significant difference post hoc were used to examine the differences between patients with MS and healthy individuals for the latency and amplitude. Additionally, separate t-tests were used to determine whether there were differences in the spatiotemporal kinematics, as well as the gating ratio, between the two groups. Spearman rho rank-order correlations were subsequently performed across all subjects between variables that differed significantly between the two groups. All statistical analyses were performed in SPSS version 23 (IBM, Armonk, NY) at a 0.05 α-level.
RESULTS
MEG analysis.
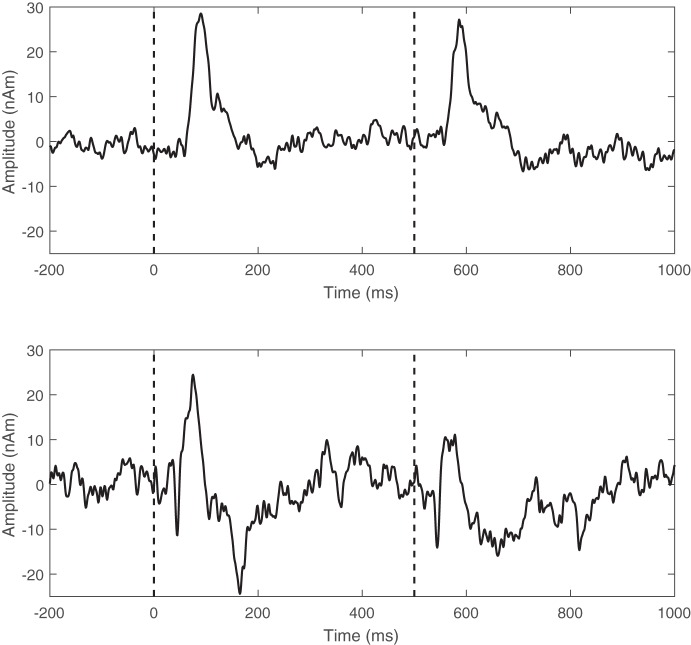
Exemplary regional source time series from an individual with MS and a healthy individual are shown in Fig. 1. Inspection of these time series clearly shows that the amplitude of the somatosensory response to the second stimulation was accentuated in the participant with MS compared with the healthy control. This response was typical of what was seen across all of the participants with MS.
Fig. 1.
Exemplary regional source time series taken from the primary somatosensory cortices for a patient with MS (top) and a healthy individual (bottom). Stimulus onset is indicated by the dashed line, which occurred at times 0.0 and 0.5 s.
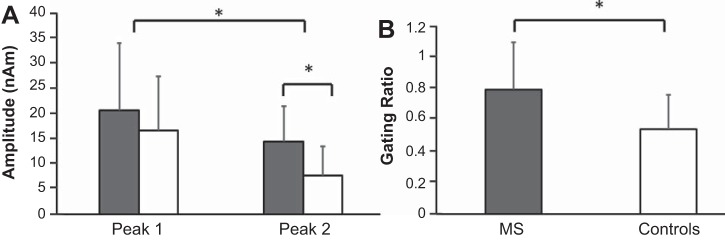
Statistical analyses revealed a significant group main effect [F(1,21) = 8.97; P = 0.007], with patients with MS having greater response amplitudes compared with healthy controls (MS = 20.02 ± 2.70 nAm, Controls = 11.09 ± 2.58 nAm). There was also a significant peak main effect [F(1,21) = 19.806; P = 0.001], indicating that the amplitude of peak 1 was stronger than the amplitude of peak 2 across both groups (peak 1 = 18.06 ± 10.93 nAm, peak 2 = 12.66 ± 9.55 nAm). There also was a significant peak × group interaction [F(1,21) = 6.32; P = 0.02], and post hoc analyses indicated that there was no significant difference between the two groups for the amplitude of peak 1 (MS = 21.97 ± 13.29 nAm, Controls = 14.46 ± 6.99 nAm; P = 0.10) but that the amplitude of peak 2 was significantly greater in patients with MS compared with healthy controls (Fig. 2A; P = 0.006). Finally, there was no significant difference between the amplitude of peak 1 and peak 2 in the patients with MS (P = 0.44), whereas the healthy controls had a significantly reduced peak 2 relative to peak 1 amplitude (Fig. 2A; P = 0.02). Thus only controls exhibited a significant gating response, and consequently the gating ratio was also significantly larger in patients with MS (P = 0.04; Fig. 2B).
Fig. 2.
A: group averages (means ± SD) for the amplitude of peak 1 and peak 2 (MS, gray; Controls, white). B: the gating ratios. *P < 0.05.
In regard to latency effects, analysis of the regional source time series revealed no significant group [F(1,21) = 1.65; P = 0.21] or peak [F(1,21) = 1.13; P = 0.30] main effect for latency. Thus there were no differences in latencies between the two groups (MS = 83.41 ± 5.29 ms, Controls = 76.29 ± 5.06 ms) or between the response to the first and second stimuli (stimulus 1 = 80.35 ± 17.90 ms, stimulus 2 = 79.04 ± 17.61 ms).
Mobility analyses.
Individual gait characteristics and demographics are presented for patients with MS in Table 1. The spatiotemporal walking kinematics were significantly different between the two groups for all variables. At preferred walking speeds, patients with MS had slower walking velocity (MS = 0.70 ± 0.27 m/s, Controls = 1.20 ± 0.16 m/s; P < 0.01), slower cadence (MS = 87.20 ± 16.44 steps per minute, Controls = 107.73 ± 8.73 steps per minute; P < 0.01), shorter step length (MS = 0.47 ± 0.11 m, Controls = 0.67 ± 0.07 m; P < 0.01), and wider step width (MS = 0.13 ± 0.06 m, Controls = 0.09 ± 0.03 m; P < 0.01) relative to the healthy control group.
Table 1.
Demographics of participants with MS
| Age, yr | Sex | Gating Ratio | EDSS | Walking Velocity, m/s | Cadence, Steps per Minute | Step Length, m | Step Width, m |
|---|---|---|---|---|---|---|---|
| 57 | F | 1.31 | 6 | 0.34 | 67.8 | 0.30 | 0.20 |
| 58 | M | 0.85 | 6 | 1.15 | 108.8 | 0.64 | 0.12 |
| 48 | F | 0.88 | 4.5 | 0.88 | 90.8 | 0.58 | 0.10 |
| 60 | F | 0.41 | 4.5 | 0.97 | 99.9 | 0.58 | 0.11 |
| 48 | F | 0.95 | 6.5 | 0.46 | 70.6 | 0.40 | 0.09 |
| 63 | F | 1.00 | 5 | 0.63 | 87.2 | 0.43 | 0.11 |
| 65 | M | 0.52 | 6.5 | 0.41 | 60.9 | 0.41 | 0.07 |
| 47 | F | 0.95 | 4.5 | 0.83 | 100.3 | 0.50 | 0.12 |
| 60 | F | 0.66 | 5 | 0.71 | 85.9 | 0.50 | 0.12 |
| 53 | F | 0.97 | 6 | 0.97 | 108.7 | 0.53 | 0.11 |
| 57 | F | 0.84 | 5.5 | 0.41 | 78.3 | 0.31 | 0.26 |
| 56 | 0.85 | 5.5 | 0.70 | 87.2 | 0.47 | 0.13 |
Means are presented in the last row. M, male; F, female.
There were moderate negative rank-order correlations between the amplitude of peak 2 and walking velocity (r = −0.52, P < 0.01) and step length (r = −0.53, P < 0.01). These correlations implied that individuals who walked slower and used a shorter step length tended to have a larger amplitude for peak 2. Additionally, we found a moderate positive correlation between the amplitude of peak 2 and step width (r = 0.47, P = 0.01). This correlation implies that individuals who used a wider step width tended to have a larger amplitude for peak 2.
Moderate negative rank-order correlations were also found between the gating ratio and walking velocity (r = −0.37, P = 0.04; Fig. 3A) and step length (r = −0.39, P = 0.03; Fig. 3B). These correlations suggested that individuals who had stronger somatosensory gating tended to walk faster and used a longer step length. Additionally, we found a moderate positive correlation between the gating ratio and step width (r = 0.40, P = 0.03; Fig. 3C). This correlation implied that weaker somatosensory gating was associated with a wider step width. Altogether, these correlations imply that reduced somatosensory gating may be partially related to the mobility impairments seen in patients with MS.
Fig. 3.
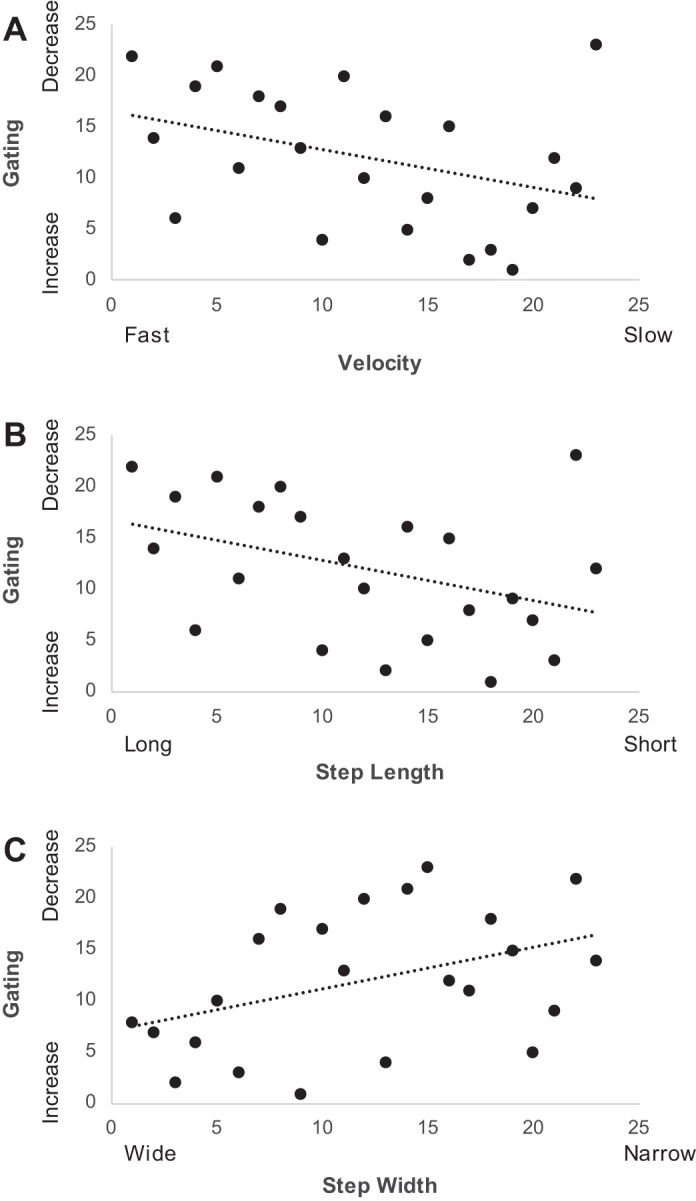
Scatter plots of the relationship between the rank of the gating ratio and the rank of walking velocity (A), the rank of step length (B), and the rank of step width (C).
DISCUSSION
This study examined somatosensory gating in patients with MS using a paired-pulse electrical stimulation paradigm applied to the posterior tibial nerve. Our results demonstrated that patients with MS had a significantly larger somatosensory gating ratio compared with a demographically matched group of healthy controls. In addition, we found differences in the spatiotemporal walking kinematics of patients with MS compared with healthy controls, which have been well-documented in the MS literature (Arpin et al. 2016; Benedetti et al. 1999; Kelleher et al. 2010). Our results extend these observations by suggesting that sensory gating deficits are related to the poor mobility seen in persons with MS.
Our most important findings were related to the amplitude of the neural responses and the gating ratio. Essentially, we observed no differences in the amplitude of peak 1 between the two groups, although patients with MS had a greater peak 2 amplitude compared with the healthy controls. This pattern of differences resulted in a larger somatosensory gating ratio for the patients with MS compared with the healthy controls. Since sensory gating is thought to represent the filtering of unnecessary information (Boutros and Belger 1999; Cheng et al. 2016), our results may suggest that patients with MS are unable to filter out unnecessary stimuli to process relevant sensory information properly. If this is the case, deficits in sensory processing may contribute to the motor impairments seen in patients with MS, as sensory feedback is necessary to make corrections to the movement trajectory (Körding et al. 2004; Shadmehr 2004; Wolpert 2007).
Currently, the exact mechanisms behind sensory gating are not fully understood; however, evidence suggests that γ-aminobutyric acid (GABA) neurotransmitters modulate somatosensory gating (Huttunen et al. 2008). Damage to inhibitory interneurons and dysregulation of GABA neurotransmitters have been reported in a histological study of individuals with progressive MS (Dutta et al. 2006). Therefore, the reduced somatosensory gating observed here may indicate that the activity of inhibitory intracortical circuits is altered in patients with MS. Prior transcranial magnetic stimulation studies have supported this idea by showing that patients with MS have reduced intracortical inhibition (Caramia et al. 2004; Conte et al. 2009; Liepert et al. 2005; Vucic et al. 2012). Furthermore, this notion is supported by other transcranial magnetic stimulation studies that have shown that impaired intracortical inhibition is related to EDSS scores (Conte et al. 2009; Vucic et al. 2012) in patients who are in the relapsing phase (Caramia et al. 2004). Alternatively, animal studies have identified the hippocampus and thalamus as possible mediators of auditory sensory gating (Freedman et al. 1996; Krause et al. 2003). However, these mechanisms have not yet been established during somatosensory gating. Prior work has, however, shown structural and functional changes in the hippocampus and thalamus of patients with MS (Kern et al. 2015; Roosendaal et al. 2010), which may contribute to the somatosensory gating deficits we report. Future work is needed to explore further these somatosensory gating deficits in patients with MS and elucidate the exact mechanisms behind sensory gating.
Negative correlations were also found between the amplitude of peak 2 and walking velocity as well as between the amplitude of peak 2 and step length. This indicates that patients with aberrant sensory gating tended to walk slower and selected a shorter step length. Additionally, we found a positive correlation between the amplitude of peak 2 and the step width, indicating that patients with an uncharacteristic sensory gating response also took wider steps, presumably to increase their base of support. Taken together, these results may suggest that reduced intracortical inhibition is partially related to the altered walking performance of individuals with MS, which is supported by prior work showing that lower GABA concentrations in the sensorimotor cortex are related to reduced motor performance in patients with secondary progressive MS (Cawley et al. 2015). In addition, several other studies have shown that the sensory deficits, particularly loss of tactile sensation and proprioception, are related to impaired standing balance and walking performance in patients with MS (Citaker et al. 2011; Thoumie and Mevellec 2002). Together, this evidence suggests that the motor impairments present in patients with MS are partially related to the neural computations associated with processing sensory information, and this processing may be dysfunctional due to alterations in the GABA system.
It is also important to note that gait is a complex process, requiring the coordinated contraction of numerous muscles as well as the integration of multiple sensory systems (e.g., somatosensory, visual, and vestibular). Thus, although altered sensory gating may be one mechanism that contributes to the gait deficits seen in patients with MS, other factors such as lesion burden and location likely also play a role. Similarly, although alterations in the GABA system appear to impact the function of patients with MS, other mechanisms such as potassium channel dysfunction may impact motor function. Evidence of this can be seen through the use of dalfampridine (4-aminopyridine), a potassium channel blocker that is believed to improve the motor function of patients with MS by increasing nerve conduction through demyelinated axons (Filli et al. 2017; Judge and Bever 2006). Future work is needed to increase our understanding of the disease and the mechanisms responsible for gait dysfunction in patients with MS.
Conclusion.
Our results indicated that patients with MS have a reduced somatosensory gating response. This suggests that MS may be associated with dysfunctional inhibitory intracortical circuitry. Additionally, the altered spatiotemporal gait kinematics seen in the patients with MS were related to the somatosensory gating ratio. This suggests that the motor performance impairments seen in patients with MS are related to sensory processing deficits. We suggest that future investigations and clinical treatment protocols aimed at improving motor performance in these patients should place greater emphasis on improving sensory processing deficits.
GRANTS
Funding for this investigation was partially provided by the University of Nebraska Foundation and the National Science Foundation (Grant 1539067).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.J.A., T.W.W., and M.J.K. conceived and designed research; D.J.A. and J.E.G. performed experiments; D.J.A. analyzed data; D.J.A., T.W.W., and M.J.K. interpreted results of experiments; D.J.A. prepared figures; D.J.A. drafted manuscript; D.J.A., T.W.W., and M.J.K. edited and revised manuscript; D.J.A., J.E.G., T.W.W., and M.J.K. approved final version of manuscript.
REFERENCES
- Adler LE, Pachtman E, Franks RD, Pecevich M, Waldo MC, Freedman R. Neurophysiological evidence for a defect in neuronal mechanisms involved in sensory gating in schizophrenia. Biol Psychiatry 17: 639–654, 1982. [PubMed] [Google Scholar]
- Armstrong LE, Winant DM, Swasey PR, Seidle ME, Carter AL, Gehlsen G. Using isokinetic dynamometry to test ambulatory patients with multiple sclerosis. Phys Ther 63: 1274–1279, 1983. doi: 10.1093/ptj/63.8.1274. [DOI] [PubMed] [Google Scholar]
- Arpin DJ, Davies BL, Kurz MJ. Multiple sclerosis influences the precision of the ankle plantarflexon muscular force production. Gait Posture 45: 170–174, 2016. doi: 10.1016/j.gaitpost.2016.02.001. [DOI] [PubMed] [Google Scholar]
- Benedetti MG, Piperno R, Simoncini L, Bonato P, Tonini A, Giannini S. Gait abnormalities in minimally impaired multiple sclerosis patients. Mult Scler 5: 363–368, 1999. doi: 10.1177/135245859900500510. [DOI] [PubMed] [Google Scholar]
- Boutros NN, Belger A. Midlatency evoked potentials attenuation and augmentation reflect different aspects of sensory gating. Biol Psychiatry 45: 917–922, 1999. doi: 10.1016/S0006-3223(98)00253-4. [DOI] [PubMed] [Google Scholar]
- Bramon E, Rabe-Hesketh S, Sham P, Murray RM, Frangou S. Meta-analysis of the P300 and P50 waveforms in schizophrenia. Schizophr Res 70: 315–329, 2004. doi: 10.1016/j.schres.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Caramia MD, Palmieri MG, Desiato MT, Boffa L, Galizia P, Rossini PM, Centonze D, Bernardi G. Brain excitability changes in the relapsing and remitting phases of multiple sclerosis: a study with transcranial magnetic stimulation. Clin Neurophysiol 115: 956–965, 2004. doi: 10.1016/j.clinph.2003.11.024. [DOI] [PubMed] [Google Scholar]
- Cattaneo D, Jonsdottir J, Zocchi M, Regola A. Effects of balance exercises on people with multiple sclerosis: a pilot study. Clin Rehabil 21: 771–781, 2007. doi: 10.1177/0269215507077602. [DOI] [PubMed] [Google Scholar]
- Cawley N, Solanky BS, Muhlert N, Tur C, Edden RA, Wheeler-Kingshott CA, Miller DH, Thompson AJ, Ciccarelli O. Reduced gamma-aminobutyric acid concentration is associated with physical disability in progressive multiple sclerosis. Brain 138: 2584–2595, 2015. doi: 10.1093/brain/awv209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WY, Pierson FM, Burnett CN. Force-time measurements of knee muscle functions of subjects with multiple sclerosis. Phys Ther 67: 934–940, 1987. doi: 10.1093/ptj/67.6.934. [DOI] [PubMed] [Google Scholar]
- Cheng CH, Chan PY, Niddam DM, Tsai SY, Hsu SC, Liu CY. Sensory gating, inhibition control and gamma oscillations in the human somatosensory cortex. Sci Rep 6: 20437, 2016. doi: 10.1038/srep20437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citaker S, Gunduz AG, Guclu MB, Nazliel B, Irkec C, Kaya D. Relationship between foot sensation and standing balance in patients with multiple sclerosis. Gait Posture 34: 275–278, 2011. doi: 10.1016/j.gaitpost.2011.05.015. [DOI] [PubMed] [Google Scholar]
- Conte A, Lenzi D, Frasca V, Gilio F, Giacomelli E, Gabriele M, Marini Bettolo C, Iacovelli E, Pantano P, Pozzilli C, Inghilleri M. Intracortical excitability in patients with relapsing-remitting and secondary progressive multiple sclerosis. J Neurol 256: 933–938, 2009. doi: 10.1007/s00415-009-5047-0. [DOI] [PubMed] [Google Scholar]
- Cromwell HC, Mears RP, Wan L, Boutros NN. Sensory gating: a translational effort from basic to clinical science. Clin EEG Neurosci 39: 69–72, 2008. doi: 10.1177/155005940803900209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta R, McDonough J, Yin X, Peterson J, Chang A, Torres T, Gudz T, Macklin WB, Lewis DA, Fox RJ, Rudick R, Mirnics K, Trapp BD. Mitochondrial dysfunction as a cause of axonal degeneration in multiple sclerosis patients. Ann Neurol 59: 478–489, 2006. doi: 10.1002/ana.20736. [DOI] [PubMed] [Google Scholar]
- Ellis T, Motl RW. Physical activity behavior change in persons with neurologic disorders: overview and examples from Parkinson disease and multiple sclerosis. J Neurol Phys Ther 37: 85–90, 2013. doi: 10.1097/NPT.0b013e31829157c0. [DOI] [PubMed] [Google Scholar]
- Filli L, Zörner B, Kapitza S, Reuter K, Lörincz L, Weller D, Sutter T, Killeen T, Gruber P, Petersen JA, Weller M, Linnebank M. Monitoring long-term efficacy of fampridine in gait-impaired patients with multiple sclerosis. Neurology 88: 832–841, 2017. doi: 10.1212/WNL.0000000000003656. [DOI] [PubMed] [Google Scholar]
- Freedman R, Adler LE, Myles-Worsley M, Nagamoto HT, Miller C, Kisley M, McRae K, Cawthra E, Waldo M. Inhibitory gating of an evoked response to repeated auditory stimuli in schizophrenic and normal subjects. Human recordings, computer simulation, and an animal model. Arch Gen Psychiatry 53: 1114–1121, 1996. doi: 10.1001/archpsyc.1996.01830120052009. [DOI] [PubMed] [Google Scholar]
- Gandolfi M, Munari D, Geroin C, Gajofatto A, Benedetti MD, Midiri A, Carla F, Picelli A, Waldner A, Smania N. Sensory integration balance training in patients with multiple sclerosis: a randomized, controlled trial. Mult Scler 21: 1453–1462, 2015. doi: 10.1177/1352458514562438. [DOI] [PubMed] [Google Scholar]
- Hoechstetter K, Bornfleth H, Weckesser D, Ille N, Berg P, Scherg M. BESA source coherence: a new method to study cortical oscillatory coupling. Brain Topogr 16: 233–238, 2004. doi: 10.1023/B:BRAT.0000032857.55223.5d. [DOI] [PubMed] [Google Scholar]
- Huttunen J, Pekkonen E, Kivisaari R, Autti T, Kähkönen S. Modulation of somatosensory evoked fields from SI and SII by acute GABAA-agonism and paired-pulse stimulation. Neuroimage 40: 427–434, 2008. doi: 10.1016/j.neuroimage.2007.12.024. [DOI] [PubMed] [Google Scholar]
- Jessen F, Kucharski C, Fries T, Papassotiropoulos A, Hoenig K, Maier W, Heun R. Sensory gating deficit expressed by a disturbed suppression of the P50 event-related potential in patients with Alzheimer’s disease. Am J Psychiatry 158: 1319–1321, 2001. doi: 10.1176/appi.ajp.158.8.1319. [DOI] [PubMed] [Google Scholar]
- Judge SI, Bever CT Jr. Potassium channel blockers in multiple sclerosis: neuronal Kv channels and effects of symptomatic treatment. Pharmacol Ther 111: 224–259, 2006. doi: 10.1016/j.pharmthera.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Kelleher KJ, Spence W, Solomonidis S, Apatsidis D. The characterisation of gait patterns of people with multiple sclerosis. Disabil Rehabil 32: 1242–1250, 2010. doi: 10.3109/09638280903464497. [DOI] [PubMed] [Google Scholar]
- Kent-Braun JA, Ng AV, Castro M, Weiner MW, Gelinas D, Dudley GA, Miller RG. Strength, skeletal muscle composition, and enzyme activity in multiple sclerosis. J Appl Physiol (1985) 83: 1998–2004, 1997. [DOI] [PubMed] [Google Scholar]
- Kern KC, Gold SM, Lee B, Montag M, Horsfall J, O’Connor MF, Sicotte NL. Thalamic-hippocampal-prefrontal disruption in relapsing-remitting multiple sclerosis. Neuroimage Clin 8: 440–447, 2015. doi: 10.1016/j.nicl.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Körding KP, Ku SP, Wolpert DM. Bayesian integration in force estimation. J Neurophysiol 92: 3161–3165, 2004. doi: 10.1152/jn.00275.2004. [DOI] [PubMed] [Google Scholar]
- Krause M, Hoffmann WE, Hajós M. Auditory sensory gating in hippocampus and reticular thalamic neurons in anesthetized rats. Biol Psychiatry 53: 244–253, 2003. doi: 10.1016/S0006-3223(02)01463-4. [DOI] [PubMed] [Google Scholar]
- Kurz MJ, Wiesman AI, Coolidge NM, Wilson TW. Children with cerebral palsy hyper-gate somatosensory stimulations of the foot. Cereb Cortex. First published June 7, 2017; doi: 10.1093/cercor/bhx144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert CP, Archer RL, Evans WJ. Muscle strength and fatigue during isokinetic exercise in individuals with multiple sclerosis. Med Sci Sports Exerc 33: 1613–1619, 2001. doi: 10.1097/00005768-200110000-00001. [DOI] [PubMed] [Google Scholar]
- LaRocca NG. Impact of walking impairment in multiple sclerosis: perspectives of patients and care partners. Patient 4: 189–201, 2011. doi: 10.2165/11591150-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Lenz M, Tegenthoff M, Kohlhaas K, Stude P, Höffken O, Gatica Tossi MA, Kalisch T, Kowalewski R, Dinse HR. Increased excitability of somatosensory cortex in aged humans is associated with impaired tactile acuity. J Neurosci 32: 1811–1816, 2012. doi: 10.1523/JNEUROSCI.2722-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liepert J, Mingers D, Heesen C, Bäumer T, Weiller C. Motor cortex excitability and fatigue in multiple sclerosis: a transcranial magnetic stimulation study. Mult Scler 11: 316–321, 2005. doi: 10.1191/1352458505ms1163oa. [DOI] [PubMed] [Google Scholar]
- Matsuzaki J, Kagitani-Shimono K, Sugata H, Hirata M, Hanaie R, Nagatani F, Tachibana M, Tominaga K, Mohri I, Taniike M. Progressively increased M50 responses to repeated sounds in autism spectrum disorder with auditory hypersensitivity: a magnetoencephalographic study. PLoS One 9: e102599, 2014. doi: 10.1371/journal.pone.0102599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponichtera JA, Rodgers MM, Glaser RM, Mathews TA, Camaione DN. Concentric and eccentric isokinetic lower extremity strength in persons with multiple sclerosis. J Orthop Sports Phys Ther 16: 114–122, 1992. doi: 10.2519/jospt.1992.16.3.114. [DOI] [PubMed] [Google Scholar]
- Rice CL, Vollmer TL, Bigland-Ritchie B. Neuromuscular responses of patients with multiple sclerosis. Muscle Nerve 15: 1123–1132, 1992. doi: 10.1002/mus.880151011. [DOI] [PubMed] [Google Scholar]
- Roosendaal SD, Hulst HE, Vrenken H, Feenstra HE, Castelijns JA, Pouwels PJ, Barkhof F, Geurts JJ. Structural and functional hippocampal changes in multiple sclerosis patients with intact memory function. Radiology 255: 595–604, 2010. doi: 10.1148/radiol.10091433. [DOI] [PubMed] [Google Scholar]
- Rosburg T, Trautner P, Ludowig E, Helmstaedter C, Bien CG, Elger CE, Boutros NN. Sensory gating in epilepsy – effects of the lateralization of hippocampal sclerosis. Clin Neurophysiol 119: 1310–1319, 2008. doi: 10.1016/j.clinph.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Scherg M, Ille N, Bornfleth H, Berg P. Advanced tools for digital EEG review: virtual source montages, whole-head mapping, correlation, and phase analysis. J Clin Neurophysiol 19: 91–112, 2002. doi: 10.1097/00004691-200203000-00001. [DOI] [PubMed] [Google Scholar]
- Shadmehr R. Generalization as a behavioral window to the neural mechanisms of learning internal models. Hum Mov Sci 23: 543–568, 2004. doi: 10.1016/j.humov.2004.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach G, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. New York: Thieme, 1988. [Google Scholar]
- Taulu S, Simola J. Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys Med Biol 51: 1759–1768, 2006. doi: 10.1088/0031-9155/51/7/008. [DOI] [PubMed] [Google Scholar]
- Thoumie P, Mevellec E. Relation between walking speed and muscle strength is affected by somatosensory loss in multiple sclerosis. J Neurol Neurosurg Psychiatry 73: 313–315, 2002. doi: 10.1136/jnnp.73.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremlett H, Paty D, Devonshire V. Disability progression in multiple sclerosis is slower than previously reported. Neurology 66: 172–177, 2006. doi: 10.1212/01.wnl.0000194259.90286.fe. [DOI] [PubMed] [Google Scholar]
- Vucic S, Burke T, Lenton K, Ramanathan S, Gomes L, Yannikas C, Kiernan MC. Cortical dysfunction underlies disability in multiple sclerosis. Mult Scler 18: 425–432, 2012. doi: 10.1177/1352458511424308. [DOI] [PubMed] [Google Scholar]
- White LJ, Dressendorfer RH. Exercise and multiple sclerosis. Sports Med 34: 1077–1100, 2004. doi: 10.2165/00007256-200434150-00005. [DOI] [PubMed] [Google Scholar]
- Wiesman AI, Heinrichs-Graham E, Coolidge NM, Gehringer JE, Kurz MJ, Wilson TW. Oscillatory dynamics and functional connectivity during gating of primary somatosensory responses. J Physiol 595: 1365–1375, 2017. doi: 10.1113/JP273192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Franzen JD, Heinrichs-Graham E, White ML, Knott NL, Wetzel MW. Broadband neurophysiological abnormalities in the medial prefrontal region of the default-mode network in adults with ADHD. Hum Brain Mapp 34: 566–574, 2013. doi: 10.1002/hbm.21459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert DM. Probabilistic models in human sensorimotor control. Hum Mov Sci 26: 511–524, 2007. doi: 10.1016/j.humov.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]