Abstract
Introduction
Chronic venous ulcers (CVU) commonly have poorly controlled pain.
Report
Thirty patients older than 65 years of age with painful CVU were reviewed. At the initial visit, cleaning without sevoflurane was performed. Cleaning visits with sevoflurane every 2 days for 1 month were scheduled. The results of subsequent treatment with sevoflurane at the first, second, seventh, and twelfth cleanings were analysed. Pain was measured using a visual analog scale (VAS), quality of life by the Charing Cross Venous Leg Ulcer Questionnaire, and functional capacity by the Barthel Index.
Discussion
Initial VAS was 8.8 ± 1.3 points and at the twelfth cleaning VAS was 0.8 ± 1 points (p = .001). Latency time ranged between 2 and 7 m and duration ranged between 8 and 18 h. It improved quality of life (83 ± 14 points before treatment vs. 50 ± 14 at the twelfth cleaning) and functional capacity (82 ± 13.3 before treatment vs. 91 ± 11.6 points at the twelfth cleaning) (p = .001). The safety profile was favourable with mild and self limited local cutaneous adverse effects, including pruritus, erythema, and heat. No systemic toxicity was detected. Topical sevoflurane may be a therapeutic alternative for painful CVU with a fast, intense, and long-lasting analgesic effect.
Keywords: Analgesic treatment, Pain, Satisfaction, Sevoflurane, Ulcers
Introduction
Pain in chronic venous ulcers (CVUs) commonly increases with usual cleaning, surgical debridement, and dressing changes.1, 2, 3 Approximately, 85% of CVUs have pain with a median visual analog scale (VAS) score of 4.6.1 CVU usually causes diminished physical activity and quality of life.4, 5 Adequate analgesic control is essential. In spite of several systemic analgesic treatments pain is usually poorly controlled.1, 2, 3, 4, 5
Sevoflurane is an inhaled halogenated anaesthetic agent with an adequate safety profile, used for induction and maintenance of general anaesthesia.6 There have been a few case reports of its efficacy as a topical anaesthetic in leg ulcers.7, 8, 9, 10, 11, 12, 13, 14, 15, 16
Material and methods
A retrospective pilot study of a series of 30 patients over 65 years of age with painful CVU is presented. VAS was ≥ 4 points. Written informed consent was obtained from all patients for the off label use of topical sevoflurane. The drug and study protocol were approved by a pharmacy committee and the hospital ethics committee, respectively. Hospitalised patients, patients with ulceration of any other cause, patients with generalised arteriosclerosis, patients with cognitive impairment, or patients with pain of another cause were excluded.
During an initial visit, VAS score, Charing Cross Venous Leg Ulcer Questionnaire (CCVUQ),17 and Barthel Index18 were registered, corresponding to the previous cleanings without sevoflurane. During the initial visit, a cleaning without sevoflurane was performed. Cleaning visits with sevoflurane every 2 days for a period of 1 month were scheduled. VAS, CCVUQ, and Barthel Index results of the previous and initial cleanings without sevoflurane and the subsequent treatments with sevoflurane at the first, second, seventh, and twelfth cleanings were compared.
Treatment consisted of irrigating the ulcer with 1 mL liquid sevoflurane per cm2 wound size without covering the healthy skin edges; sterile gauze was used to protect the perilesional skin. Next the wound was quickly covered with a sterile hydrophilic braided cotton pad, soaked in physiological saline. Later, usual routine cleaning was performed.
Results
Analgesic effect
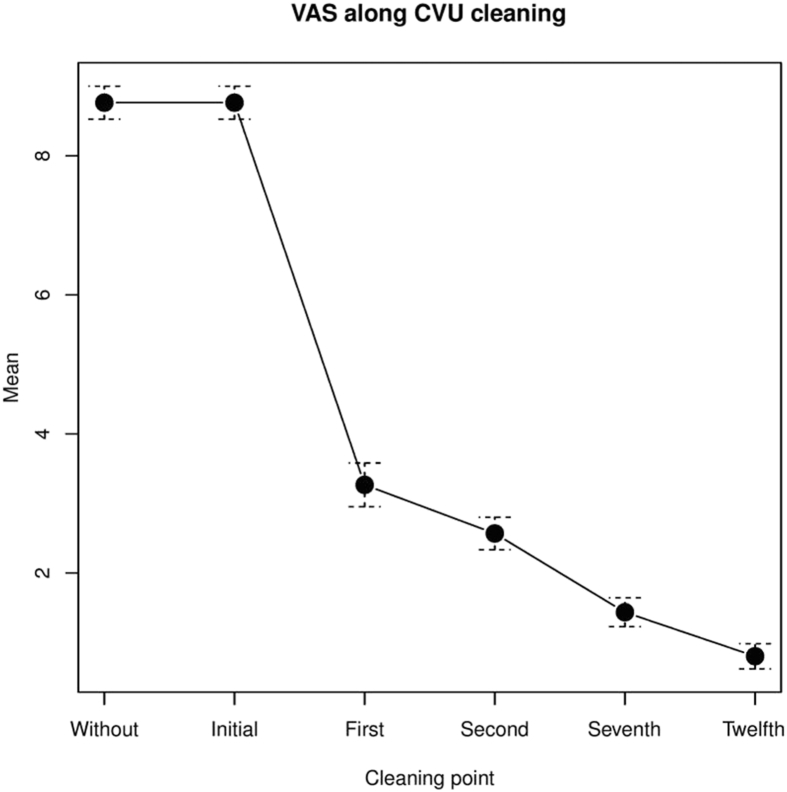
Initial mean VAS was 8.8 ± 1.3 points in the previous and the initial cleanings without sevoflurane. Pain diminished from the first cleaning with sevoflurane. By the twelfth cleaning mean VAS was 0.8 ± 1 points (p = .001) (Figure 1, Figure 2). Latency time varied between 2 and 7 minutes (mean 3.9 ± 1.5 minutes). Duration ranged between 8 and 18 hours (mean 12 ± 2.9 hours).
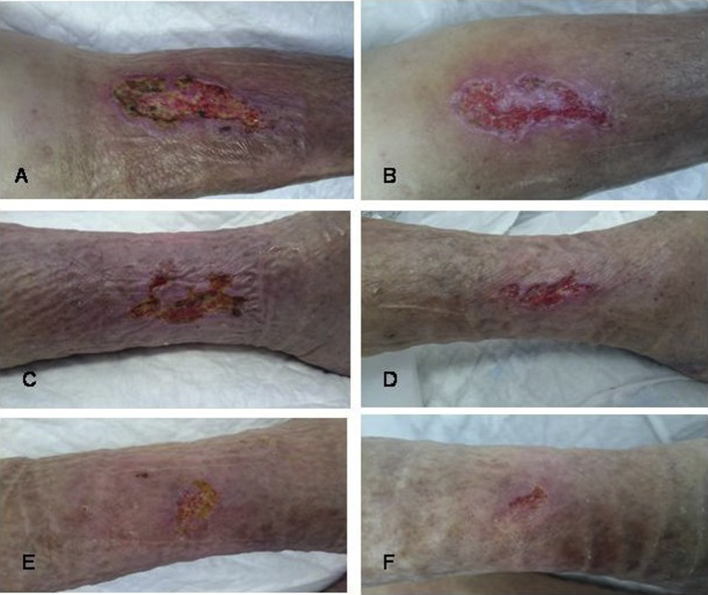
Figure 1.
Chronic venous ulcers (A, C, E) before and (B, D, F) after sevoflurane treatment.
Figure 2.
Visual analog scale along cleaning. Note. CVU = chronic venous ulcers.
Quality of life
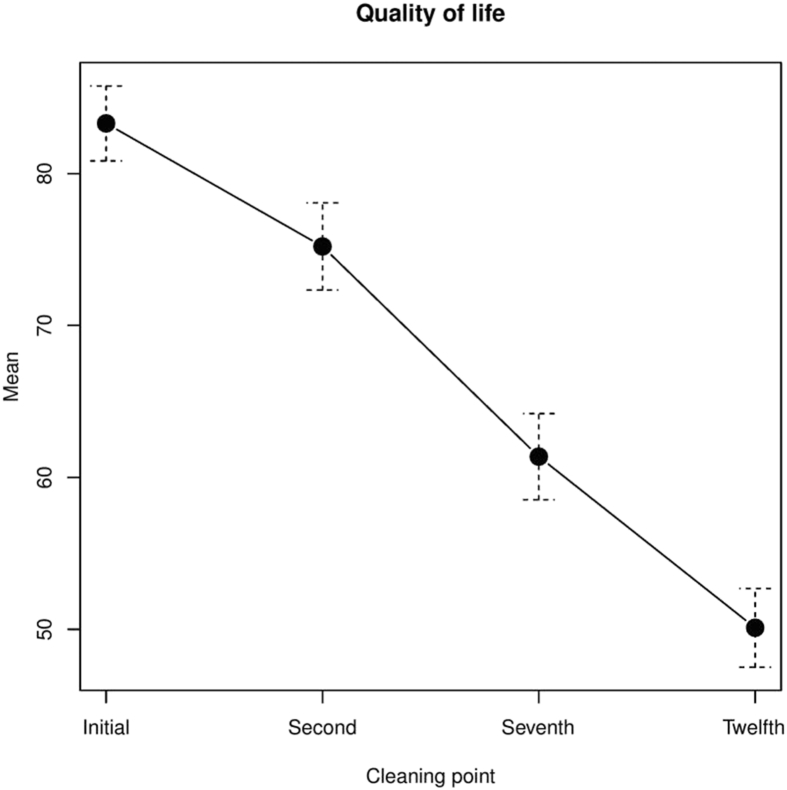
Initial mean CCVUQ score was 83 ± 14 points. There was a statistically significant improvement in quality of life from the second cleaning with sevoflurane. At the twelfth cleaning with sevoflurane mean CCVUQ score was 50 ± 14 points (p = .001) (Fig. 3).
Figure 3.
Quality of life with Charing Cross Venous Leg Ulcer Questionnaire.
Functional capacity
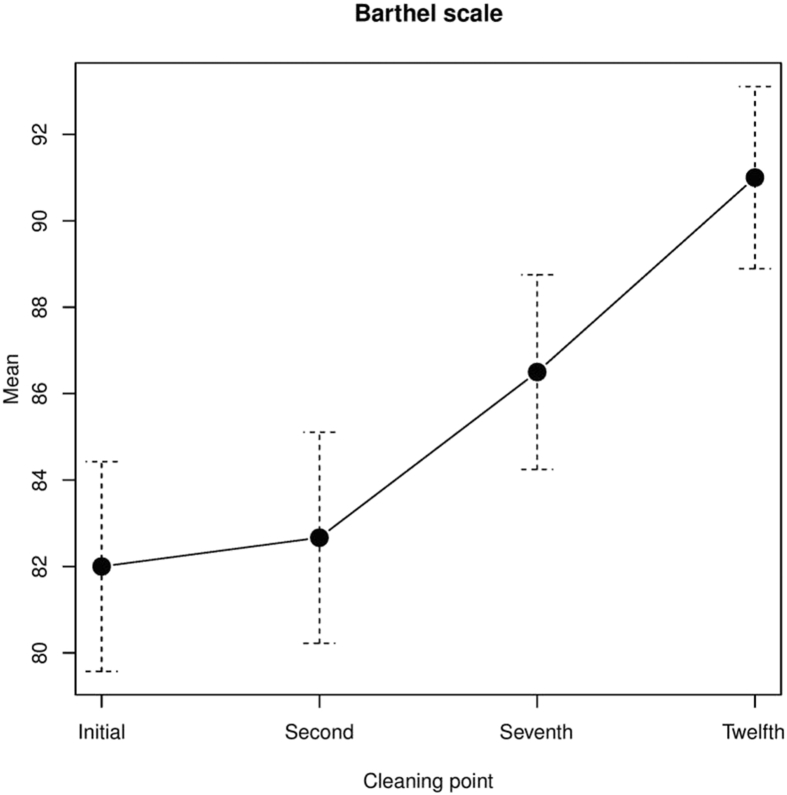
Initial median Barthel index was 82 ± 13.3 points. There was a significant increase in Barthel Index at the twelfth cleaning compared with the first, with a median Barthel index of 91 ± 11.6 points (p = .001) (Fig. 4).
Figure 4.
Barthel index.
Adverse effects
The main local adverse effects were mild and transient, including pruritus in five patients (16.7%), erythema in two (6.7%), heat in three (10%), and irritative dermatitis in one (3.3%). No systemic adverse effects or sensitisation were detected.
Discussion
An intense analgesic effect of topical sevoflurane has been reported in a few chronic vascular leg ulcer, surgical wound, malignant leg ulcer, and epidural abscess with cutaneous fistulisation case reports.7, 8, 9, 10, 11, 12, 13, 14 There were no VAS results. The latency period varied between 1 and 10 minutes and the duration ranged between 7 and 24 hours.
Dámaso Fernández-Ginés et al. reported 34 of 36 (94.4%) patients treated with topical sevoflurane for a mean of 10.5 months (range 3–24 months).15 Excellent control of basal pain with rapid, intense, and lasting relief (2–48 hours) was found. An improvement in quality of life and a significant reduction in the consumption of analgesics were seen, but there were no results to report.
A prospective observational study assessed the efficacy and safety of topical sevoflurane for a period of 90 days.16 Patients were randomly assigned to two groups: standard wound care with (n = 10) or without (n = 5) sevoflurane. In the sevoflurane group the mean VAS score was 7.36 ± 1.56 points at baseline and 0.59 ± 0.48 points at 90 days. At the start, patients were taking 102.7 ± 36.8 mg/day morphine which was reduced to 20.0 ± 5.4 mg/day after treatment with sevoflurane. The mean ulcer area was 12.5 ± 2.2 cm2 at baseline and 6.1 ± 2.67 cm2 at 90 days. The mean latency time was 3.2 ± 1.2 minutes and the mean duration was 9.6 ± 4.7 hours. In four patients a mild localised reddening and pruritus in the area surrounding ulcers occurred.
Sevoflurane improves the quality of life when it is used in the subsequent cleanings. The results showed that topical sevoflurane may cause a favourable change in functional capacity as the pain relief made ambulation easier.
Sevoflurane's mechanism of action is unknown. It is suggested that a vasodilatory effect increases vascular flow, improving the microcirculation. Topical sevoflurane may have a direct inhibitory effect on vascular smooth muscle independently of endothelium.19 A central but not peripheral effect of inhaled sevoflurane has been clinically demonstrated. It is also suggested that a reversible peripheral concentration-dependent analgesic effect is probably caused by sufficient partial pressure in the peripheral nociceptors, which blocks the transmission of the pain stimulus.20, 21, 22, 23, 24 The absence of harmful effects is probably due to no or negligible slow and incomplete systemic absorption.
Limitations include the retrospective nature of the study, the lack of randomisation and blindness, and the relatively small number of patients. Randomised clinical trials and larger prospective studies with longer follow-up are needed to better assess the efficacy and safety of topical sevoflurane.
Conflict of interest
None.
Funding
None.
References
- 1.Nemeth K.A., Harrison M.B., Graham I.D., Burke S. Pain in pure and mixed etiology venous leg ulcers: a three-phase point prevalence study. J Wound Care. 2003;12:336–340. doi: 10.12968/jowc.2003.12.9.26532. [DOI] [PubMed] [Google Scholar]
- 2.Guarnera G., Tinelli G., Abeni D., Di Pietro C., Sampogna F., Tabolli S. Pain and quality of life in patients with vascular leg ulcers: an Italian multicentre study. J Wound Care. 2007;16:347–351. doi: 10.12968/jowc.2007.16.8.27856. [DOI] [PubMed] [Google Scholar]
- 3.Duque M.I., Yosipovitch G., Chan Y.H., Smith R., Levy P. Itch, pain, and burning sensation are common symptoms in mild to moderate chronic venous insufficiency with an impact on quality of life. J Am Acad Dermatol. 2005;53:504–508. doi: 10.1016/j.jaad.2005.04.079. [DOI] [PubMed] [Google Scholar]
- 4.Valencia I.C., Falabella A., Kirsner R.S., Eaglstein W.H. Chronic venous insufficiency and venous leg ulceration. J Am Acad Dermatol. 2001;44:401–421. doi: 10.1067/mjd.2001.111633. [DOI] [PubMed] [Google Scholar]
- 5.Kelechi T.J., Johnson J.J., Yates S. Chronic venous disease and venous leg ulcers: an evidence-based update. J Vasc Nurs. 2015;33:36–46. doi: 10.1016/j.jvn.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Behne M., Wilke H.J., Harder S. Clinical pharmacokinetics of sevoflurane. Clin Pharmacokinet. 1999;36:13–26. doi: 10.2165/00003088-199936010-00002. [DOI] [PubMed] [Google Scholar]
- 7.Lafuente-Urrez R.F., Gilaberte Y. Sevoflurane: a valid alternative for the treatment of vascular ulcers? Actas Dermosifiliogr. 2014;105:202–203. doi: 10.1016/j.ad.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Gerónimo-Pardo M., Martínez-Monsalve A., Martínez-Serrano M. Analgesic effect of topical sevoflurane on venous ulcer intractable pain. Phlebologie. 2011;40:95–97. [Google Scholar]
- 9.Martinez Monsalve A., Gerónimo Pardo M. Sevoflurano como anestésico local en herida isquémica de paciente cardiópata con insuficiencia respiratoria secundaria a morfina. Heridas y cicatrización. 2011;6:46–49. [Google Scholar]
- 10.Rueda-Martínez J.L., Gerónimo-Pardo M., Martínez-Monsalve A., Martínez-Serrano M. Topical sevoflurane and healing of a post-operative surgical site superinfected by multi-drug-resistant Pseudomonas aeruginosa and susceptible Staphylococcus aureus in an immunocompromised patient. Surg Infect Larchmt. 2014;15:843–846. doi: 10.1089/sur.2013.079. [DOI] [PubMed] [Google Scholar]
- 11.Gerónimo Pardo M., Martinez Serrano M., Martínez Molsalve A., Rueda Martínez J.L. Usos alternativos del sevoflurano. Efecto analgésico tópico. Rev Electron AnestesiaR. 2012;4:181. [Google Scholar]
- 12.Imbernón A., Blázquez C., Puebla A., Churruca M., Lobato A., Martínez M. Chronic venous ulcer treatment with topical sevoflurane. Int Wound J. 2016;13:1060–1062. doi: 10.1111/iwj.12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernández F.D., Cortiñas M., Fernández C., Morales J.A. Sevoflurano tópico: una nueva opción terapéutica paliativa en las úlceras cutáneas. Med Paliativa. 2015;180:5. [Google Scholar]
- 14.Ferrara P., Domingo-Chiva E., Selva-Sevilla C., Campos-García J., Gerónimo-Pardo M. Irrigation with liquid sevoflurane and healing of a postoperative, recurrent epidural infection: a potential cost-saving alternative. World Neurosurg. 2016;90 doi: 10.1016/j.wneu.2016.02.079. 702.e1–5. [DOI] [PubMed] [Google Scholar]
- 15.Dámaso Fernández-Ginés F., Cortiñas-Sáenz M., Navajas-Gómez de Aranda A., Yoldi Bocanegra R., Sierra-García F. Reply: to chronic venous ulcer treatment with topical sevoflurane by Imbernón et al. Int Wound J. 2017;14:591. doi: 10.1111/iwj.12709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dámaso Fernández-Ginés F., Cortiñas-Sáenz M., Mateo-Carrasco H., de Aranda A.N., Navarro-Muñoz E., Rodríguez-Carmona R. Efficacy and safety of topical sevoflurane in the treatment of chronic skin ulcers. Am J Health Syst Pharm. 2017;74:176–182. doi: 10.2146/ajhp151008. [DOI] [PubMed] [Google Scholar]
- 17.González-Consuegra R.V., Verdú J. Quality of life in people with venous leg ulcers: an integrative review. J Adv Nurs. 2011;67:926–944. doi: 10.1111/j.1365-2648.2010.05568.x. [DOI] [PubMed] [Google Scholar]
- 18.Loewen S.C., Anderson B.A. Reliability of the modified motor assessment scale and the Barthel index. Phys Ther. 1988;68:1077–1081. doi: 10.1093/ptj/68.7.1077. [DOI] [PubMed] [Google Scholar]
- 19.Izumi K., Akata T., Takahashi S. The action of sevoflurane on vascular smooth muscle of isolated mesenteric resistance arteries (part 1): role of endothelium. Anesthesiology. 2000;92:1426–1440. doi: 10.1097/00000542-200005000-00034. [DOI] [PubMed] [Google Scholar]
- 20.Chu C.C., Wu S.Z., Su W.L., Shieh J.P., Kao C.H., Ho S.T. Subcutaneous injection of inhaled anesthetics produces cutaneous analgesia. Can J Anaesth. 2008;55:290–294. doi: 10.1007/BF03017206. [DOI] [PubMed] [Google Scholar]
- 21.Skouteri I., Staikou C., Sarantopoulos C., Siafaka I., Fassoulaki A. Local application of halothane, isoflurane or sevoflurane increases the response to an electrical stimulus in humans. Acta Anaesthesiol Belg. 2007;58:169–175. [PubMed] [Google Scholar]
- 22.Fassoulaki A., Skouteri I., Siafaka I., Sarantopoulos C. Local application of volatile anesthetics attenuates the response to a mechanical stimulus in humans. Can J Anaesth. 2005;52:951–957. doi: 10.1007/BF03022057. [DOI] [PubMed] [Google Scholar]
- 23.Matute E., Rivera-Arconada I., López-García J.A. Effects of propofol and sevoflurane on the excitability of rat spinal moto-neurones and nociceptive reflexes in vitro. Br J Anaesth. 2004;93:422–427. doi: 10.1093/bja/aeh217. [DOI] [PubMed] [Google Scholar]
- 24.Antognini J.F., Kien N.D. Potency (minimum alveolar anestheticconcentration) of isoflurane is independent of peripheral anesthetic effects. Anesth Analg. 1995;81:69–72. doi: 10.1097/00000539-199507000-00014. [DOI] [PubMed] [Google Scholar]