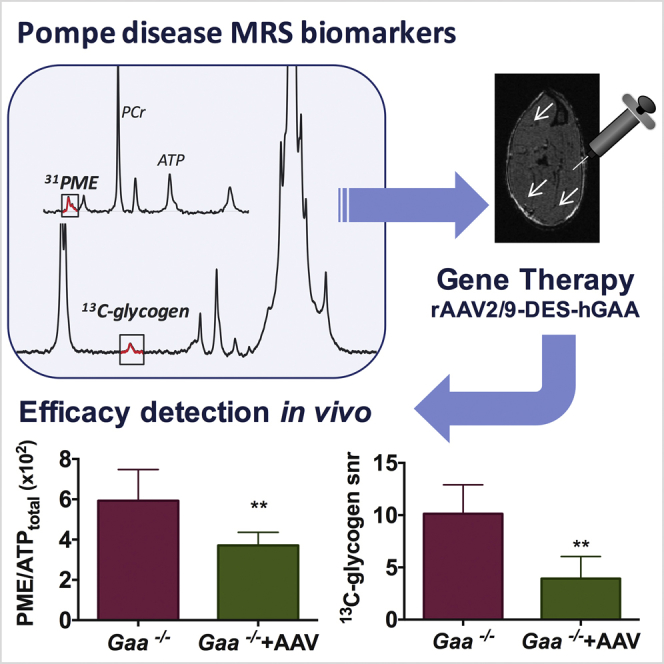
Abstract
The development of therapeutic clinical trials for glycogen storage disorders, including Pompe disease, has called for non-invasive and objective biomarkers. Glycogen accumulation can be measured in vivo with 13C MRS. However, clinical implementation remains challenging due to low signal-to-noise. On the other hand, the buildup of glycolytic intermediates may be detected with 31P MRS. We sought to identify new biomarkers of disease progression in muscle using 13C/31P MRS and 1H HR-MAS in a mouse model of Pompe disease (Gaa−/−). We evaluated the sensitivity of these MR biomarkers in vivo after treatment using an adeno-associated virus vector 2/9 encoding hGAA driven by the desmin promotor. 31P MRS showed significantly elevated phosphomonoesters (PMEs) in Gaa−/− compared to control at 2 (0.06 ± 0.02 versus 0.03 ± 0.01; p = 0.003), 6, 12, and 18 months of age. Correlative 1H HR-MAS measures in intact gastrocnemius muscles revealed high glucose-6-phosphate (G-6-P). After intramuscular AAV injections, glycogen, PME, and G-6-P were decreased within normal range. The changes in PME levels likely partly resulted from changes in G-6-P, one of the overlapping phosphomonoesters in the 31P MR spectra in vivo. Because 31P MRS is inherently more sensitive than 13C MRS, PME levels have greater potential as a clinical biomarker and should be considered as a complementary approach for future studies in Pompe patients.
Keywords: gene therapy, MR spectroscopy, Pompe disease, glycogen storage disorder, MRI, AAV, glucose-6-phosphate, glycogen, carbon, phosphorus
Graphical Abstract
Introduction
Glycogen storage type II, or Pompe disease, is a progressive metabolic disorder caused by a defect in the expression of acid α-glucosidase (GAA), the enzyme responsible for glycogen degradation within the lysosome. The disease is characterized by glycogen accumulation in many organs including the liver, heart, diaphragm, and skeletal muscles. Dependent upon the severity of the mutation, patients are mainly affected by profound cardiac and skeletal muscle weakness and, ultimately, respiratory insufficiency and fatal heart disease.1 The current standard of care consists of the management of lysosomal glycogen through enzyme replacement therapy (ERT).2 Although ERT has been successful in reducing cardiac involvement and extending life expectancy, many patients still require assisted ventilation.2 In addition, bi-weekly systemic infusion of recombinant cell-derived GAA is a major burden on patients. An alternative and potentially more efficacious approach is gene therapy, which targets the underlying cause of the disease and aims to restore sustained expression of the missing gene.
Gene therapy is gaining momentum, with several ongoing clinical trials in dystrophies, including a trial recently initiated in glycogen storage disorder type II (GSDII) patients aiming at restoring GAA activity.3 The current outcome measures are functional tests (6-min walking test [6MWT] and forced vital capacity [FVC])4 and biochemical evaluation of biopsy samples. However, functional tests are highly dependent on subject compliance and limit the study population to ambulatory patients. Biopsies are extremely invasive and cannot capture the possible heterogeneous response to treatment throughout the muscle. Accordingly, there is an urgent need for relevant, non-invasive, and sensitive biomarkers able to probe local metabolic changes in response to treatment for Pompe disease.
In vivo nuclear magnetic resonance (NMR) has long been used in animal models and in humans for quantification of glycogen concentration and metabolism in liver and muscle in vivo.5, 6, 7 In particular, NMR techniques such as 1H and 13C spectroscopy (MRS) have been applied in the liver and muscles of patients with various glycogen storage disorders.8, 9, 10, 11 However, 13C sensitivity is limited by the low natural abundance and low gyromagnetic ratio of the 13C nuclei compared to 1H, and the technique is still only primarily used in research settings. 13C detection in preclinical models, such as the genetically modified mouse models of Pompe disease (Gaa−/−),12, 13 presents even greater challenges due to the low muscle mass of the animals, and there are no reports of in vivo natural abundance 13C MRS glycogen in mouse skeletal muscle. Developing more sensitive 13C MRS protocols and investigating alternative MR markers can potentially fulfill an unmet clinical need in glycogen storage diseases.
While GAA deficiency and subsequent increase in glycogen concentration are the primary features of the disease, recent preclinical findings demonstrated a more extensive alteration of glycogen metabolism in the Gaa−/− model.14 Specifically, elevation of hexokinase, glycogen synthase (GS), glycogenin, and glucose-6-phosphate (G-6-P) have all been observed. This has led to the development of adjuvant substrate deprivation therapies targeting GS and glycogenin in preclinical models.15, 16, 17 Therefore, the molecules involved in these metabolic processes may serve as markers of disease progression and treatment efficacy in Pompe disease. Intermediates of glucose/glycogen metabolism such as G-6-P and other high-energy phosphorylated metabolites can be quantified in vivo using 31P MRS. In the past, 31P MRS has been used to study glucose transport and energy metabolism in both liver18 and muscle disorders.11, 19, 20, 21, 22 This technique presents advantages over 13C MRS, in that the 31P has a higher sensitivity than 13C and the NMR visible isotope is 100% naturally abundant.
Considering that the buildup of both glycogen and glycogenesis intermediates occur in Pompe skeletal muscle, we sought to implement both 13C and 31P MRS measurements in the Gaa−/− mouse model of the disease. We hypothesized that 31P-MRS of phosphorylated metabolites can provide complementary biomarkers of Pompe disease progression and treatment efficacy. We implemented in vivo MRS at high field (11.1T) and non-invasively characterized the 13C and 31P MRS signatures of disease progression in Gaa−/− muscle. In addition, we evaluated the changes in MRS-detected metabolite levels in response to the expression of GAA following gene transfer using an adeno-associated virus vector 2/9 encoding hGAA driven by the desmin promotor (rAAV2/9-DES-hGAA).23 The results were compared to ex vivo NMR analysis of muscle samples with 1H high resolution magic angle spinning (1H HR-MAS) spectroscopy and biochemical assays on tissue homogenates to validate the in vivo measurements.
Results
31P MRS Metabolic Signature of Disease in Gaa−/− Skeletal Muscle
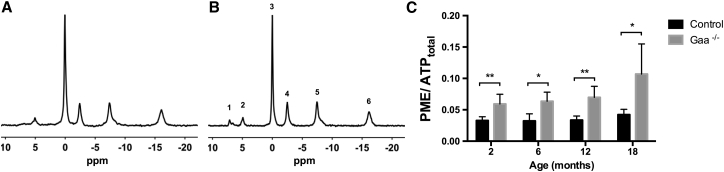
Typical 31P MR spectra obtained from the lower leg of a 6-month-old Gaa−/− mouse and an age-matched control are shown in Figures 1A and 1B. 31P MRS data from resting muscle allowed for the determination of phosphorylated metabolite ratios and intracellular pH (pHi), thereby providing an index of the cellular energetic state. The PME signal relative to the total ATP signal (PME/ATPtotal) was significantly higher in Gaa−/− mice compared to controls at 2 months (p = 0.003), 6 months (p = 0.04), 12 months (p = 0.006), and 18 months of age (p = 0.024) (Table 1; Figure 1C). In control, PME/ATPtotal was not significantly different between age groups, while in Gaa−/− animals it was significantly different between the 2- and 18-month-old time-points (p = 0.008). All other metabolic indices—inorganic phosphates (Pi) to phosphocreatine (PCr) ratio (Pi/PCr) ratio, pHi, and PCr signal relative to the total ATP content (PCr/ATPtotal)—did not show significant differences between animal groups and across age groups (Table 1).
Figure 1.
In Vivo 31P MRS
Representative spectra from the lower leg muscle of (A) a control animal and (B) a Gaa−/− animal. The different peaks were assigned to (1) PME, (2) Pi, (3) PCr, (4) adenosine triphosphate γ (ATPγ), (5) ATPα, and (6) ATPβ. (C) PME levels determined by peak signal integration and expressed as PME/ATPtotal were significantly higher compared to age-matched controls by a factor of 2 or more in all age groups (2, 6, 12, 18 months old). Results are presented as mean ± standard deviation. *p < 0.05; **p < 0.01.
Table 1.
In Vivo 31P MRS Measures of Metabolites Ratios and pHi for Controls, Gaa−/− Untreated, and Gaa−/− Treated with Intramuscular Injections of rAAV2/9-DES-hGAA
| No Treatment |
rAAV Treatment |
||||
|---|---|---|---|---|---|
| 2 Months Old | 6 Months Old | 12 Months Old | 18 Months Old | 2 Months Old | |
| pH | |||||
| Control | 7.13 ± 0.03 | 7.10 ± 0.02 | 7.05 ± 0.04 | 7.10 ± 0.03 | NA |
| Gaa−/− | 7.11 ± 0.02 | 7.05 ± 0.03 | 7.04 ± 0.02 | 7.09 ± 0.06 | 7.12 ± 0.06 |
| PCr/ATPtotal | |||||
| Control | 0.90 ± 0.09 | 0.80 ± 0.04 | 0.68 ± 0.02 | 0.82 ± 0.11 | NA |
| Gaa−/− | 0.86 ± 0.11 | 0.81 ± 0.10 | 0.63 ± 0.04 | 0.7 ± 0.07 | 0.74 ± 0.10 |
| Pi/(Pi + PCr) | |||||
| Control | 0.09 ± 0.02 | 0.08 ± 0.02 | 0.10 ± 0.03 | 0.09 ± 0.02 | NA |
| Gaa−/− | 0.10 ± 0.02 | 0.09 ± 0.03 | 0.12 ± 0.01 | 0.13 ± 0.03 | 0.08 ± 0.01 |
| PME/ATPtotal | |||||
| Control | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.04 ± 0.01 | NA |
| Gaa−/− | 0.06 ± 0.02** | 0.06 ± 0.01* | 0.07 ± 0.02** | 0.11 ± 0.05* | 0.04 ± 0.01# |
No difference in PCr/ATPtotal, Pi/(Pi + PCr), or pHi were found between groups. PME/ATPtotal was significantly higher in Gaa−/− compared to control for all age groups (2, 6, 12, 18 months old). PME/ATPtotal were significantly lower in Gaa−/− after treatment compared to untreated legs. *p < 0.05 compared to age-matched control; **p < 0.006 compared to age-matched control; #p = 0.03 compared to untreated leg.
rAAV2/9-DES-hGAA Induces High GAA Activity and Glycogen Clearance in Gaa−/− Gastrocnemius
Twenty-eight days after unilateral intramuscular injection of rAAV2/9-DES-hGAA in 2 month-old Gaa−/−mice (Gaa−/− + AAV), glycogen content was evaluated using three different types of measurements: in vivo 13C MRS, ex vivo 1H HR-MAS, and quantitative determination of glycogen content with biochemical assay on muscle extracts. Examples of 13C MRS spectra obtained in vivo from an untreated Gaa−/− mouse and a naive wild-type control are shown in Figure 2.
Figure 2.
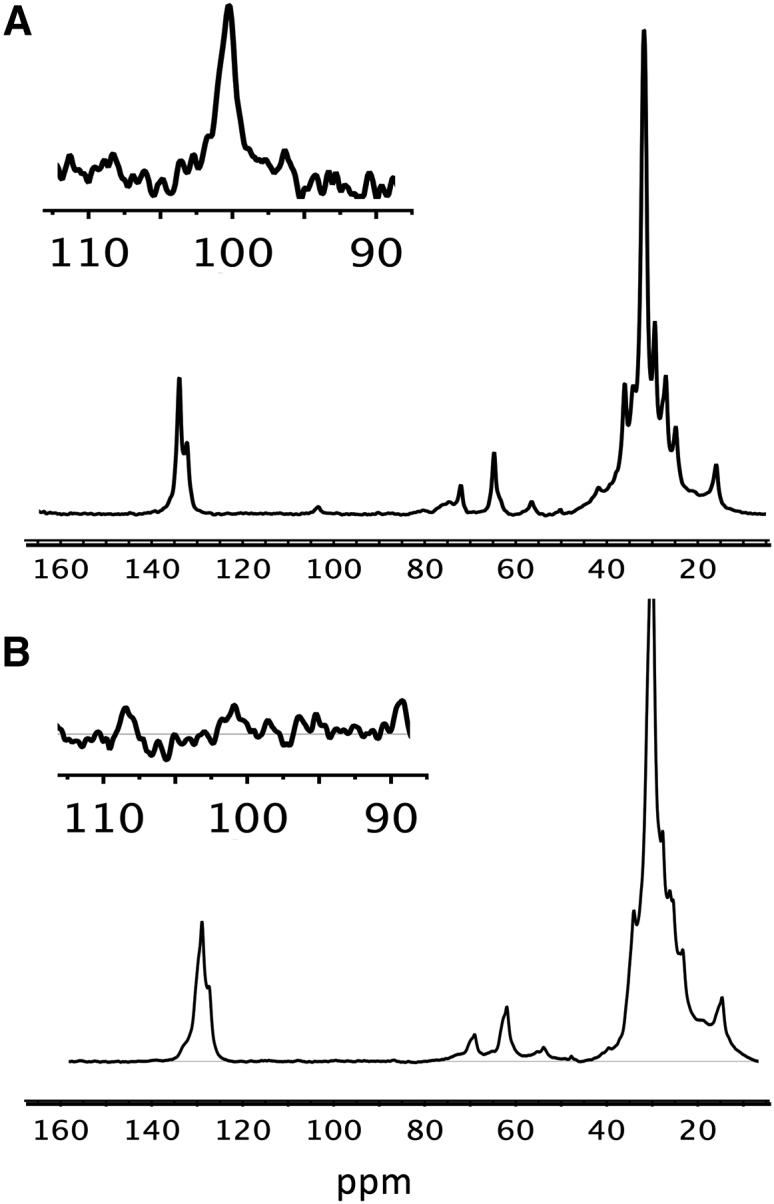
In Vivo 13C MRS
Representative in vivo 13C MR spectra acquired from the lower leg muscles of a Gaa−/− mouse (A) and a control mouse (B) in vivo. The inserts show a zoom on the 90 to 110 ppm region, with the C1 glycogen resonance at 100.5 ppm. No signal could be detected in control animals due to very low glycogen concentrations. The other peaks are largely dominated by signal from the lipids overlapping with other resonances such as other glycogen carbons, creatine, taurine, and other sugars.
Analysis of the glycogen peak signal-to-noise at the 13C1 position (100.5 ppm) showed significantly lower glycogen levels in the treated leg compared to the untreated Gaa−/− (Gaa−/− + AAV, 3.9 ± 2.1; Gaa−/−, 10.1 ± 2.7, p = 0.0005; Figure 3A). This observation was consistent with 1H HR-MAS NMR measurements of the glycogen peak relative to the total metabolites signal in the corresponding harvested gastrocnemius muscles (Gaa−/−+ AAV, 0.14 ± 0.07; Gaa−/−, 0.40 ± 0.25, p = 0.04; Figure 3B). These results were further confirmed by biochemical assessment, where glycogen concentration in the treated leg was significantly lower as compared to the untreated leg (Gaa−/− + AAV, 20.2 ± 9.1; Gaa−/−, 56.4 ± 14.1 μg/mL; p = 0.0001; Figure 3C) but was not significantly different from control values (control, 9.5 ± 5.0 μg/mL).
Figure 3.

Glycogen Measurements
Measures of glycogen levels (A) with 13C MRS in vivo, (B) with 1H HR-MAS of intact muscles, and (C) with quantitative biochemical assays of muscle extracts in μg/mL. All three types of measurements showed significantly higher glycogen levels in Gaa−/− compared to controls and significantly lower glycogen level after rAAV2/9-DES-hGAA treatment (Gaa−/− + AAV) as compared to untreated Gaa−/−. Data are presented as individual data points and box plots indicating the lower and upper quartiles and the median. The bars indicate the minimum and maximum values. *p < 0.05; **p < 0.01; ***p < 0.001.
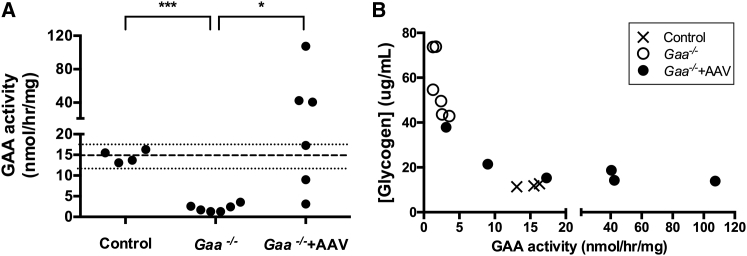
rAAV2/9-DES-hGAA injections resulted in higher GAA enzymatic activity compared to the untreated leg, with values ranging from 3 to 107 nmol/hr/mg (Figure 4A). Activity levels were significantly lower in Gaa−/− (p < 0.0001) as compared to control values and significantly higher in Gaa−/− + AAV as compared to Gaa−/− (p = 0.03).
Figure 4.
Effect of rAAV2/9-DES-hGAA Treatment on GAA Activity and Glycogen Concentration
Glycogen concentration in μg/mL determined by biochemical assay on muscle homogenates for control, Gaa−/−, and Gaa−/− + AAV (A) and as a function of GAA activity (B). The dashed lines represent the average control value and the upper and lower 95% confidence interval. GAA activity of 10 nmol/hr/mg was sufficient to induce glycogen clearance. GAA activity above that value did not induce lower glycogen concentrations than found in control animals. *p = 0.03, ***p < 0.0001.
Taking advantage of the range of GAA activity achieved post-gene-transfer, we looked at the relationship between the glycogen measures normalized to the maximum measured value and enzymatic activity for each technique. We observed that glycogen levels were highly correlated to GAA activity and rapidly decreased with increasing GAA activity (biochemical assay—Spearman r = −0.96, p < 0.0001; 1H HR-MAS—r = −0.85, p = 0.0006; 13C MRS—r = −0.71, p = 0.013; Figures 4B and 5). Glycogen content determined by biochemical assay could be fitted to an exponential decay as a function of GAA activity as shown on Figure 5. Enzymatic activity above 10 nmol/hr/mg did not induce a larger decrease and all three types of measures plateaued at wild-type control values.
Figure 5.
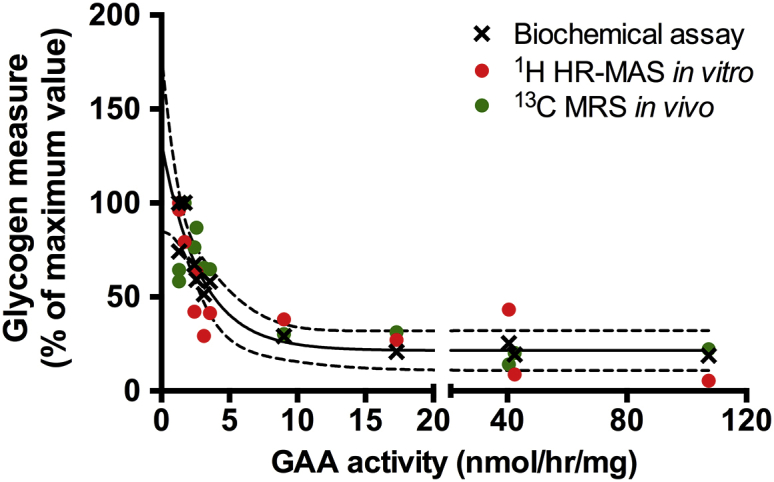
Relationship between Glycogen Levels and GAA Activity
Overlay of the results obtained from the three different glycogen measurements as a function of the corresponding GAA concentration. The solid black line represents the result of a fit of the quantitative measurements by biochemical assay to a monoexponential decay, and the dashed lines represent the 95% confidence interval of the fit. Values are normalized to their maximum for display purposes.
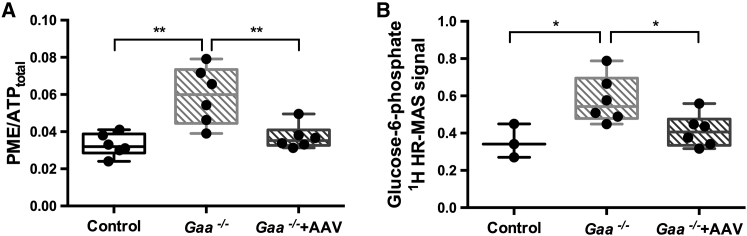
PME/ATPtotal and G-6-P Signal Decrease in Response to rAAV2/9-DES-hGAA
31P MRS measurements showed that PME/ATPtotal was significantly lower in Gaa−/− + AAV than in naive Gaa−/− mice (Gaa−/− + AAV, 0.037 ± 0.006; Gaa−/−, 0.059 ± 0.015, p = 0.03). In addition, Gaa−/− + AAV values were not statistically different from control (0.032 ± 0.006; ns) (Figure 6). G-6-P signal detected by 1H HR-MAS relative to the total signal was significantly higher in Gaa−/− when compared to controls (0.58 ± 0.12 versus 0.35 ± 0.09; p = 0.03). Similar to PME, G-6-P levels were lower in Gaa−/− + AAV compared to Gaa−/− (0.41 ± 0.03; p = 0.03) and not different from controls (p = 0.33).
Figure 6.
Effect of rAAV2/9-DES-hGAA Treatment on 31P MRS PME Resonances In Vivo and 1H HR-MAS Glucose-6-Phosphate in Isolated Muscles
Both in vivo PME levels and glucose-6-phosphate levels from intact muscles were significantly elevated in Gaa−/− mice compared to control. rAAV2/9-DES-hGAA induced a significant decrease in both PME (A) and glucose-6-phosphate (B). Data are presented as individual data points and box plots indicating the lower and upper quartiles and the median. The bars indicate the minimum and maximum values. *p < 0.05; **p < 0.005.
Discussion
For the first time, we have demonstrated the significance of MRS to detect highly concentrated phosphorylated metabolites for the non-invasive evaluation of disease progression and treatment efficacy in the muscles of a validated preclinical model of Pompe disease. First, we observed that the phosphomonoester (PME) 31P MRS signal was elevated in the skeletal muscle of Gaa−/− mice and throughout the lifespan of the animal. Second, we showed that restoration of normal to high levels of GAA activity using an AAV-based approach resulted in a decrease in 31P MRS PME signal. These changes were concomitant to the glycogen clearance expected with increased GAA activity, as measured by 13C MRS in vivo, 1H HRMAS of muscle samples, and by the biochemical determination of glycogen content in muscle extracts. In addition, we showed that the changes in PME signal detected in vivo could be potentially explained by changes in glucose-6-phosphate, one of the contributors to the 31P PME signal.
A major finding of this study is that elevated 31P MRS PME signal can be detected in vivo in Gaa−/− mice skeletal muscle. As early as 2 months of age, PME levels were 60% higher in Gaa−/− as compared to control muscles, and remained elevated throughout the 18 months of our study. Multiple overlapping resonances contribute to the MR signal in the PME region, including phosphocholine, phosphoethanolamine and glycolytic intermediates (glucose-6-P, fructose-6-P), that could not be resolved by 31P MRS in vivo. Therefore, we performed ex vivo analysis using 1H high resolution magic angle spectroscopy (1H HR-MAS), which allows for the acquisition of high resolution NMR spectra in intact tissues and can be used specifically for the determination of phosphate sugars.24 With this technique, we found a significantly higher G-6-P signal in the spectra, suggesting a large contribution of G-6-P to the change in PME signal in vivo. G-6-P is at a key intersection of glucose utilization pathways, namely glycolysis, pentose-phosphate, and glycogenesis pathways. It is also a direct product of glycogenolysis. Accordingly, high G-6-P levels are compatible with the high cytoplasmic glycogen concentration in Pompe disease.25, 26 Since G-6-P is known as an activator of GS,27 it is possible that the increase in GAA activity with rAAV2/9-DES-hGAA treatment initiated a cycle in which glycogen clearance results in lower G-6-P levels and, in turn, in lower glycogen production through GS. These multiple roles of G-6-P make PME levels an attractive marker of the metabolic changes associated with Pompe disease progression and treatment that can be detected non-invasively by in vivo 31P MRS.
The primary hallmark of Pompe disease progression is the increase in lysosomal and cytosolic glycogen concentration. Because the C1 glycogen peak detected by 13C MRS can be unequivocally identified at 100.5 ppm, and with the availability of higher field strength clinical scanner,28 natural abundance 13C MRS has become a method of choice for clinical research studies of glycogen storage disorders. However, it remains challenging to perform such measurements in transgenic mouse models because of the small size of the animal, especially in skeletal muscles where glycogen content is much lower than in the liver. Here, we were able to detect glycogen in the lower leg of Gaa−/− mice using in vivo MRS and a simple surface coil set-up at 11.1T. However, unlike PME levels, glycogen could not be reliably measured in control mice due to low signal-to-noise ratio. Even at 11.1T, long acquisition times were necessary even in Gaa−/− mice with high glycogen concentration (1 hr). To address this limitation, future 13C MRS preclinical studies may benefit from the use of a higher magnetic field strength, the development of more sensitive RF coils,29 dynamic polarization, and possibly the use of 13C enriched substrates. Nonetheless, we were able to detect changes in glycogen level in vivo after AAV treatment, and all glycogen measures showed a significant and consistent response to treatment 28 days after AAV injection, with an average decrease of 61%, 66% and 64% for MRS, HR-MAS, and biochemical assays, respectively.
We previously reported that intra-muscular injection of rAAV2/9-DES-hGAA could restore GAA expression in the TA muscle.23 Here, we successfully modified this protocol to treat a larger part of the leg, including the TA and both heads of the gastrocnemius. We found that GAA activity was restored to control values at or above endogenous levels in the gastrocnemius, where muscle wet weight is typically more than twice that of the TA. Significant glycogen clearance was achieved within 28 days post AAV delivery. This strategy was also motivated by the fact that the MRS acquisitions performed in this study were not spatially selective. The entire leg contributed to the observed signal 31P and 13C signal, with a larger contribution of the posterior compartment (i.e gastrocnemius and soleus). This limitation is specific to small animal studies and can easily be overcome in human studies, where 31P chemical shift imaging has been implemented, including in muscular dystrophies.22, 30, 31
From a technical standpoint, the increasing availability of high field multinuclei hardware in research centers combined with the much higher SNR achievable in human compared to mice makes the clinical translation of these MR measures readily feasible. Indeed, the 31P MRS measurement of muscle phosphodiesters (PDE) has been shown to be a biomarker for Duchenne and Becker muscular dystrophy.22, 32 A recent study by Le Guiner et al. in dystrophic dogs showed that Pi/ATP and PDE levels were sensitive to intramuscular delivery of rAAV2/8 encoding a canine microdystrophin.33 However, a limitation of our approach is that 13C and 31P markers are modulated by both AAV transduction success and heterogeneity of treatment distribution within a given volume of tissue. This is inherent to any volume-averaged measure and cannot be easily overcome. In addition, the ideal surrogate biomarker for Pompe disease should have a high and preferably close to linear sensitivity to moderate changes in either or both glycogen concentration and GAA activity. Further investigation is therefore warranted to determine the exact level of sensitivity of the proposed 13C and 31P markers within the specific range of values observed in humans.
In conclusion, our findings show that metabolic changes involving phosphorylated metabolites in parallel to glycogen accumulation can be probed in vivo in Gaa−/− mice using 31P MRS. Specifically, elevated PME levels are a characteristic of the disease and sensitive to the response to rAAV2/9-DES-hGAA intramuscular injections. Because the sensitivity of 31P MRS is superior to that of 13C MRS, PME levels have great potential as a clinical biomarker and should be considered as a complementary approach for future studies in Pompe patients.
Materials and Methods
Animals
All procedures were performed in accordance with the U.S. Government Principle for the Utilization and Care of Vertebrate Animals and were approved by the University of Florida’s Institutional Animal Care & Use Committee.
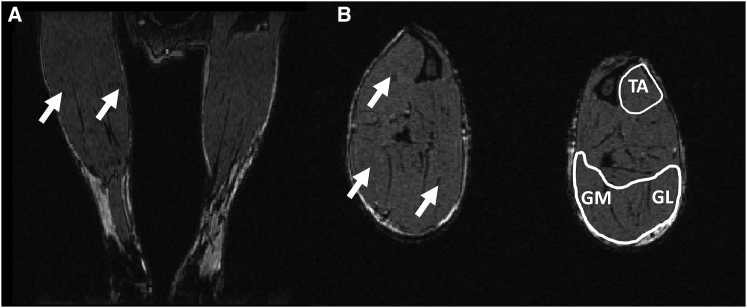
Male and female Gaa−/− mice (Taconic, Germantown, NY) originally developed by Raben et al.13 were outbred to a 129SVE background. Gaa−/− mice and age-matched control wild-type 129SVE mice, (Taconic, Germantown, NY) were studied at 2 months (Gaa−/−, n = 6; control, n = 6), 6 months (Gaa−/−, n = 3; control, n = 3), 12 months (Gaa−/−, n = 5; control, n = 4), and 18 months of age (Gaa−/−, n = 8; control, n = 4). A separate group of 6 Gaa−/− animals received unilateral intramuscular injections of rAAV2/9-DES-hGAA at three different locations in the lower leg (Gaa−/− + AAV). The gastrocnemius medial, the gastrocnemius lateral head, and the tibialis anterior each received a dose of 5 × 1014 vg/kg of tissue diluted in 50 μL, 50 μ, and 25 μL, respectively (Figure 7). The tibialis anterior and gastrocnemius were assumed to be representative of 0.1% and 0.3% of the animal body weight, respectively.
Figure 7.
Description of the rAAV2/9-DES-hGAA Injection Sites
Representative coronal (A) and axial (B) images from a 3D T1 weighted MR image of the lower legs of a Gaa−/− animal are shown. The fascia appear in black on the images. The muscles are delineated in white, and the white arrows indicate the three rAAV2/9-DES-hGAA injection sites (TA, tibialis anterior; GM, medial gastrocnemius; GL, lateral gastrocnemius). 3D T1 weighted images were obtained using a gradient echo sequence on with 4.7T Agilent scanner (Agilent, Inc., Palo Alto, CA) and a custom build 3 cm-diameter 1H volume coil. TR/TE = 50/7 ms, field of view = 1.5 × 1.8 × 1.8 cm3, matrix size = 256 × 192 × 96.
31P/13C MR Spectroscopy Acquisitions In Vivo
Acquisitions were performed in an 11.1T/470MHz Agilent system (Agilent, Inc., Palo Alto, CA) using the VnmrJ 2.3 software. Animals were placed prone on a water-heated cradle, and anesthesia was ensured through a nose cone delivering an isoflurane-oxygen mixture (2%, 1 L/min).
For phosphorus spectroscopy, a single turn 10 × 8 mm oval 31P surface coil was placed over the belly of the lower leg posterior compartment. A 12 mm diameter 1H surface coil was placed on the side of the leg, perpendicular to the 31P coil, and used for positioning and shimming. After manual voxel shimming, spectra were accumulated for 5 min at the Ernst angle calibrated on PCr with a 20 μs broad pulse and a repetition time (TR) of 1 s. Acquisition bandwidth was 10 kHz with a 2,048 point free induction decay. The carrier frequency was set to −4.5 ppm with reference to the PCr peak. A fully relaxed spectrum was acquired (TR = 15 s) to determine T1 saturation effect for each of the metabolites.
For carbon spectroscopy, a similar set up was used with a single turn 10 × 8 mm oval 13C surface coil. Spectra were acquired with a single 7.2 μs broad pulse calibrated at the Ernst angle for TR = 1 s and a WALTZ-16 1H decoupling scheme34 and accumulated for 1 hr. For each spectrum, a 1,024 point free induction decay (FID) was acquired over 33 ms leading to an acquisition bandwidth of 30 kHz.
31P/13C MR Spectroscopy Data Analysis
All spectra were processed using MestReNova version 11.0.4 (Mestrelab Research S.L., Norwich, CT). After zero filling to 4,096 points, 25 Hz exponential apodization, zero and first order phasing and baseline correction, PCr frequency was set to 0 ppm on the 31P spectra and peak integration of over the following chemical shifts was used to determine the relative concentrations of PME (7.8 to 5.8 ppm), Pi (5.6 to 3.6 ppm), PCr (1 to −1 ppm), γ- (1.5 to −3.8 ppm), α- (−6.5 to −9 ppm), and β-ATP (−14.8 to −17.8 ppm). In addition, pHi was determined using the relative chemical shift difference between Pi and PCr resonances according to the following formula pH = 6.75 + log ((3.27 − δPi)/(δPi − 5.69)).35 To account for partial saturation, the amplitude of each component was multiplied by the appropriate correction factor derived from fully relaxed spectra, and results are reported as the relative concentrations of Pi, PCr, γ-, α-, and β-ATP. 13C spectra were processed similarly. After zero filling to 2,048 points, 50 Hz exponential apodization, zero and first-order phasing and baseline correction, the glycogen peak was integrated over 2 ppm around 100.5 ppm, and results are reported as C1-glycogen peak-to-noise ratio.
1H HR-MAS Spectroscopy
Gastrocnemius tissue from 2-month-old control (n = 3), Gaa−/− (n = 3), and Gaa−/− + AAV mice (n = 6, both legs) were analyzed using high-resolution magic-angle spinning (1H HR-MAS) NMR. Material preparation and data acquisition followed the protocols from Beckonert et al.36 A portion of the tissue sample (25 ± 2 mg) was soaked in D2O and transferred into a 40 μL rotor. 1H HR-MAS data were acquired using a 4 mm probe on a 600 MHz Bruker spectrometer (Bruker, Billerica, MA) with Topspin 3.2 software. The magic angle, 54.7°, was calibrated using potassium bromide once at the beginning of the run. Pulse calibration, tune, match, and manual shimming were performed for each sample. The data was acquired using noesypr1d pulse sequence with 128 scans accumulation, spectral width of 10 ppm, 16k data points, a relaxation delay of 2 s, and mixing time of 90 ms. Spectra were processed in MestreNova 10.0 using an exponential window function with a 0.5 Hz line broadening and zero-filled to 16k. All spectra were then phased individually. Processed HR-MAS spectra were globally aligned to creatine at 3.02 ppm, and the glycogen and G-6-P resonances were identified at 5.4 and 5.22 ppm, respectively, as previously published24 and as was confirmed by “spiking” a sample using a glycogen solution and a G-6-P solution. Metabolites were quantified using the MestreNova 10.0 Simple Mixture Analysis (SMA) plugin and normalized to the total signal.
Biochemical Assays
Quantitative measurement of glycogen content was performed on gastrocnemius samples using the Glycogen Assay Kit (ab65620; Abcam, Cambridge, MA) and following the manufacturer’s instructions. GAA activity was assessed in gastrocnemius tissue lysates. The amount of 4-methylumbelliferyl-α-D-glucoside released by cleavage after 1 hr incubation at 37°C was measured using a commercially available kit (Sigma M9766; Sigma), as previously described.37
Statistical Analysis
All analyses were performed using GraphPad Prism version 6.00 for Mac OS X (GraphPad Software, La Jolla, CA). To describe the differences between Gaa−/− and age-matched controls, unpaired two-tailed t tests with Holm-Sidak correction for multiple comparisons were used. When comparing controls, Gaa−/−-treated and Gaa−/−-untreated groups, a one-way ANOVA with Holm-Sidak correction for multiple comparisons was used. To compare the results obtained from Gaa−/−-treated and -untreated legs only, a Wilcoxon matched-pairs signed-rank test was used. Correlations between measures were assessed with a Pearson r test. Significance level was set to 0.05. All results are reported as mean ± standard deviation.
Author Contributions
C.B. was responsible for the conception and design of the experiments, performed the experiments, analyzed the data, interpreted the results, and prepared the figures and manuscript. AG.T., B.L.-M., and R.S.V. performed the experiments and analyzed the data. D.J.F., B.J.B., and G.A.W. were responsible for the conception and design of the experiments, interpreted the results, and reviewed and approved the manuscript.
Acknowledgments
This work was funded by the Muscular Dystrophy Association (#MDA218585) development research grant to C.B., the National High Magnetic Field Laboratory (NHMFLP021453), the P01 HL59412 to B.J.B. and G.A.W., the Southeast Center for Integrative Metabolomics (U24 DK097209), and NIAMS (K01AR066077) to D.J.F. A portion of this work was performed in the McKnight Brain Institute at the National High Magnetic Field Laboratory’s AMRIS Facility, which is supported by National Science Foundation Cooperative Agreement No. DMR-1157490 and the State of Florida. The authors would like to thank Denise Cloutier, James Rocca, and Dr. Huadong Zeng for their technical assistance.
References
- 1.Hirschhorn R., Reuser A.J.J. Glycogen storage disease type II: acid alpha-glucosidase (acid maltase) deficiency. In: Scriver C.R., Sly W.S., Childs B., Beaudet A.L., Valle D., Kinzler K.W., Vogelstein B., editors. Vol. 3. McGraw-Hill; 2001. pp. 3389–3420. (The Metabolic and Molecular Bases of Inherited Disease). [Google Scholar]
- 2.Byrne B.J., Kishnani P.S., Case L.E., Merlini L., Müller-Felber W., Prasad S., van der Ploeg A. Pompe disease: design, methodology, and early findings from the Pompe Registry. Mol. Genet. Metab. 2011;103:1–11. doi: 10.1016/j.ymgme.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Smith B.K., Collins S.W., Conlon T.J., Mah C.S., Lawson L.A., Martin A.D., Fuller D.D., Cleaver B.D., Clément N., Phillips D. Phase I/II trial of adeno-associated virus-mediated alpha-glucosidase gene therapy to the diaphragm for chronic respiratory failure in Pompe disease: initial safety and ventilatory outcomes. Hum. Gene Ther. 2013;24:630–640. doi: 10.1089/hum.2012.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lachmann R., Schoser B. The clinical relevance of outcomes used in late-onset Pompe disease: can we do better? Orphanet J. Rare Dis. 2013;8:160. doi: 10.1186/1750-1172-8-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shulman R.G., Rothman D.L. 13C NMR of intermediary metabolism: implications for systemic physiology. Annu. Rev. Physiol. 2001;63:15–48. doi: 10.1146/annurev.physiol.63.1.15. [DOI] [PubMed] [Google Scholar]
- 6.Garlick P.B., Pritchard R.D. Absolute quantification and NMR visibility of glycogen in the isolated, perfused rat heart using 13C NMR spectroscopy. NMR Biomed. 1993;6:84–88. doi: 10.1002/nbm.1940060113. [DOI] [PubMed] [Google Scholar]
- 7.Jue T., Rothman D.L., Tavitian B.A., Shulman R.G. Natural-abundance 13C NMR study of glycogen repletion in human liver and muscle. Proc. Natl. Acad. Sci. USA. 1989;86:1439–1442. doi: 10.1073/pnas.86.5.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beckmann N., Seelig J., Wick H. Analysis of glycogen storage disease by in vivo 13C NMR: comparison of normal volunteers with a patient. Magn. Reson. Med. 1990;16:150–160. doi: 10.1002/mrm.1910160114. [DOI] [PubMed] [Google Scholar]
- 9.Roser W., Beckmann N., Wiesmann U., Seelig J. Absolute quantification of the hepatic glycogen content in a patient with glycogen storage disease by 13C magnetic resonance spectroscopy. Magn. Reson. Imaging. 1996;14:1217–1220. doi: 10.1016/s0730-725x(96)00243-3. [DOI] [PubMed] [Google Scholar]
- 10.Wary C., Laforêt P., Eymard B., Fardeau M., Leroy-Willig A., Bassez G., Leroy J.P., Caillaud C., Poenaru L., Carlier P.G. Evaluation of muscle glycogen content by 13C NMR spectroscopy in adult-onset acid maltase deficiency. Neuromuscul. Disord. 2003;13:545–553. doi: 10.1016/s0960-8966(03)00069-5. [DOI] [PubMed] [Google Scholar]
- 11.Wary C., Nadaj-Pakleza A., Laforêt P., Claeys K.G., Carlier R., Monnet A., Fleury S., Baligand C., Eymard B., Labrune P., Carlier P.G. Investigating glycogenosis type III patients with multi-parametric functional NMR imaging and spectroscopy. Neuromuscul. Disord. 2010;20:548–558. doi: 10.1016/j.nmd.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 12.Bijvoet A.G., van de Kamp E.H., Kroos M.A., Ding J.H., Yang B.Z., Visser P., Bakker C.E., Verbeet M.P., Oostra B.A., Reuser A.J., van der Ploeg A.T. Generalized glycogen storage and cardiomegaly in a knockout mouse model of Pompe disease. Hum. Mol. Genet. 1998;7:53–62. doi: 10.1093/hmg/7.1.53. [DOI] [PubMed] [Google Scholar]
- 13.Raben N., Nagaraju K., Lee E., Kessler P., Byrne B., Lee L., LaMarca M., King C., Ward J., Sauer B., Plotz P. Targeted disruption of the acid alpha-glucosidase gene in mice causes an illness with critical features of both infantile and adult human glycogen storage disease type II. J. Biol. Chem. 1998;273:19086–19092. doi: 10.1074/jbc.273.30.19086. [DOI] [PubMed] [Google Scholar]
- 14.Taylor K.M., Meyers E., Phipps M., Kishnani P.S., Cheng S.H., Scheule R.K., Moreland R.J. Dysregulation of multiple facets of glycogen metabolism in a murine model of Pompe disease. PLoS ONE. 2013;8:e56181. doi: 10.1371/journal.pone.0056181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Douillard-Guilloux G., Raben N., Takikita S., Batista L., Caillaud C., Richard E. Modulation of glycogen synthesis by RNA interference: towards a new therapeutic approach for glycogenosis type II. Hum. Mol. Genet. 2008;17:3876–3886. doi: 10.1093/hmg/ddn290. [DOI] [PubMed] [Google Scholar]
- 16.Douillard-Guilloux G., Raben N., Takikita S., Ferry A., Vignaud A., Guillet-Deniau I., Favier M., Thurberg B.L., Roach P.J., Caillaud C., Richard E. Restoration of muscle functionality by genetic suppression of glycogen synthesis in a murine model of Pompe disease. Hum. Mol. Genet. 2010;19:684–696. doi: 10.1093/hmg/ddp535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clayton N.P., Nelson C.A., Weeden T., Taylor K.M., Moreland R.J., Scheule R.K., Phillips L., Leger A.J., Cheng S.H., Wentworth B.M. Antisense oligonucleotide-mediated suppression of muscle glycogen synthase 1 synthesis as an approach for substrate reduction therapy of Pompe disease. Mol. Ther. Nucleic Acids. 2014;3:e206. doi: 10.1038/mtna.2014.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oberhaensli R., Rajagopalan B., Galloway G.J., Taylor D.J., Radda G.K. Study of human liver disease with P-31 magnetic resonance spectroscopy. Gut. 1990;31:463–467. doi: 10.1136/gut.31.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siciliano G., Rossi B., Martini A., Angelini C., Martinuzzi A., Lodi R., Zaniol P., Barbiroli B., Muratorio A. Myophosphorylase deficiency affects muscle mitochondrial respiration as shown by 31P-MR spectroscopy in a case with associated multifocal encephalopathy. J. Neurol. Sci. 1995;128:84–91. doi: 10.1016/0022-510x(94)00207-5. [DOI] [PubMed] [Google Scholar]
- 20.Barnes P.R., Kemp G.J., Taylor D.J., Radda G.K. Skeletal muscle metabolism in myotonic dystrophy A 31P magnetic resonance spectroscopy study. Brain. 1997;120:1699–1711. doi: 10.1093/brain/120.10.1699. [DOI] [PubMed] [Google Scholar]
- 21.de Haan J.H., Klomp D.W., Tack C.J., Heerschap A. Optimized detection of changes in glucose-6-phosphate levels in human skeletal muscle by 31P MR spectroscopy. Magn. Reson. Med. 2003;50:1302–1306. doi: 10.1002/mrm.10630. [DOI] [PubMed] [Google Scholar]
- 22.Hooijmans M.T., Niks E.H., Burakiewicz J., Verschuuren J.J., Webb A.G., Kan H.E. Elevated phosphodiester and T2 levels can be measured in the absence of fat infiltration in Duchenne muscular dystrophy patients. NMR Biomed. 2017;30:30. doi: 10.1002/nbm.3667. [DOI] [PubMed] [Google Scholar]
- 23.Todd A.G., McElroy J.A., Grange R.W., Fuller D.D., Walter G.A., Byrne B.J., Falk D.J. Correcting neuromuscular deficits with gene therapy in Pompe disease. Ann. Neurol. 2015;78:222–234. doi: 10.1002/ana.24433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diserens G., Vermathen M., Gjuroski I., Eggimann S., Precht C., Boesch C., Vermathen P. Direct determination of phosphate sugars in biological material by (1)H high-resolution magic-angle-spinning NMR spectroscopy. Anal. Bioanal. Chem. 2016;408:5651–5656. doi: 10.1007/s00216-016-9671-0. [DOI] [PubMed] [Google Scholar]
- 25.Garancis J.C. Type II glycogenosis. Biochemical and electron microscopic study. Am. J. Med. 1968;44:289–300. doi: 10.1016/0002-9343(68)90160-5. [DOI] [PubMed] [Google Scholar]
- 26.Thurberg B.L., Lynch Maloney C., Vaccaro C., Afonso K., Tsai A.C., Bossen E., Kishnani P.S., O’Callaghan M. Characterization of pre- and post-treatment pathology after enzyme replacement therapy for Pompe disease. Lab. Invest. 2006;86:1208–1220. doi: 10.1038/labinvest.3700484. [DOI] [PubMed] [Google Scholar]
- 27.Adeva-Andany M.M., González-Lucán M., Donapetry-García C., Fernández-Fernández C., Ameneiros-Rodríguez E. Glycogen metabolism in humans. BBA Clin. 2016;5:85–100. doi: 10.1016/j.bbacli.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heinicke K., Dimitrov I.E., Romain N., Cheshkov S., Ren J., Malloy C.R., Haller R.G. Reproducibility and absolute quantification of muscle glycogen in patients with glycogen storage disease by 13C NMR spectroscopy at 7 Tesla. PLoS ONE. 2014;9:e108706. doi: 10.1371/journal.pone.0108706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kovacs H., Moskau D., Spraul M. Cryogenically cooled probes—a leap in NMR technology. Prog. Nucl. Magn. Reson. Spectrosc. 2005;46:131–155. [Google Scholar]
- 30.Valkovič L., Chmelík M., Meyerspeer M., Gagoski B., Rodgers C.T., Krššák M., Andronesi O.C., Trattnig S., Bogner W. Dynamic (31) P-MRSI using spiral spectroscopic imaging can map mitochondrial capacity in muscles of the human calf during plantar flexion exercise at 7 T. NMR Biomed. 2016;29:1825–1834. doi: 10.1002/nbm.3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilhelm T., Bachert P. In vivo 31P echo-planar spectroscopic imaging of human calf muscle. J. Magn. Reson. 2001;149:126–130. doi: 10.1006/jmre.2001.2288. [DOI] [PubMed] [Google Scholar]
- 32.Wokke B.H., Hooijmans M.T., van den Bergen J.C., Webb A.G., Verschuuren J.J., Kan H.E. Muscle MRS detects elevated PDE/ATP ratios prior to fatty infiltration in Becker muscular dystrophy. NMR Biomed. 2014;27:1371–1377. doi: 10.1002/nbm.3199. [DOI] [PubMed] [Google Scholar]
- 33.Le Guiner C., Servais L., Montus M., Larcher T., Fraysse B., Moullec S., Allais M., François V., Dutilleul M., Malerba A. Long-term microdystrophin gene therapy is effective in a canine model of Duchenne muscular dystrophy. Nat. Commun. 2017;8:16105. doi: 10.1038/ncomms16105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shaka A.J., Keeler J., Frenkiel T., Freeman R. An improved sequence for broadband decoupling: WALTZ-16. J. Magn. Reson. 1983;52:335–338. [Google Scholar]
- 35.Taylor D.J., Bore P.J., Styles P., Gadian D.G., Radda G.K. Bioenergetics of intact human muscle. A 31P nuclear magnetic resonance study. Mol. Biol. Med. 1983;1:77–94. [PubMed] [Google Scholar]
- 36.Beckonert O., Coen M., Keun H.C., Wang Y., Ebbels T.M., Holmes E., Lindon J.C., Nicholson J.K. High-resolution magic-angle-spinning NMR spectroscopy for metabolic profiling of intact tissues. Nat. Protoc. 2010;5:1019–1032. doi: 10.1038/nprot.2010.45. [DOI] [PubMed] [Google Scholar]
- 37.Falk D.J., Mah C.S., Soustek M.S., Lee K.Z., Elmallah M.K., Cloutier D.A., Fuller D.D., Byrne B.J. Intrapleural administration of AAV9 improves neural and cardiorespiratory function in Pompe disease. Mol. Ther. 2013;21:1661–1667. doi: 10.1038/mt.2013.96. [DOI] [PMC free article] [PubMed] [Google Scholar]