Abstract
Already during early embryogenesis, before sex-specific hormone production is initiated, sex differences in embryonic development have been observed in several mammalian species. Typically, female embryos develop more slowly than their male siblings. A similar phenotype has recently been described in differentiating murine embryonic stem cells, where a double dose of the X-chromosome halts differentiation until dosage-compensation has been achieved through X-chromosome inactivation. On the molecular level, several processes associated with early differentiation of embryonic stem cells have been found to be affected by X-chromosome dosage, such as the transcriptional state of the pluripotency network, the activity pattern of several signal transduction pathways and global levels of DNA-methylation. This review provides an overview of the sex differences described in embryonic stem cells from mice and discusses a series of X-linked genes that are associated with pluripotency, signalling and differentiation and their potential involvement in mediating the observed X-dosage–dependent effects.
This article is part of the themed issue ‘X-chromosome inactivation: a tribute to Mary Lyon’.
Keywords: X-chromosome, pluripotency, signalling pathways, development, embryonic stem cells
1. Sex differences in early embryos
The majority of sexual dimorphisms arise from hormonal differences between the genders. However, already during early embryogenesis, before the development of the gonads, which will initiate the production of fetal hormones, sex differences are observed, which are attributed to dosage differences of the sex chromosomes [1,2]. For several mammalian species it was found that male pre-implantation embryos develop faster than females. This sex difference could either be attributed to the presence of a Y-chromosome in males or to a double dosage of X-linked genes in female embryos. These two alternatives can be distinguished by including aneuploid female embryos with an XO genotype in the analysis, which carry only a single X-chromosome, but no Y. A careful quantitative comparison of pre- and post-implantation embryos with XX, XY and XO genotypes revealed that the Y-chromosome (of certain genetic backgrounds) can speed up development prior to implantation, while the presence of two X-chromosomes delays development right after implantation [3,4].
Interestingly, the developmental time window, when the presence of two X-chromosomes appears to slow down embryogenesis, coincides with the onset of X-chromosome inactivation (XCI). During XCI one randomly chosen X-chromosome in each female cell is epigenetically silenced to ensure dosage compensation for X-linked genes between the sexes. Therefore, most X-chromosomal genes are present at a double dose in female embryos only during early development. In mice, the time window, when both X-chromosomes are active, seems to last for only 1–2 days around the time of implantation during the transition from the blastocyst to the epiblast [5,6]. This window might be particularly short in mice, because they have evolved an imprinted form of X-inactivation, where the paternal X-chromosome is inactivated shortly after zygotic genome activation and the silenced state is then maintained in all extra-embryonic tissues [5–7]. Only in the inner cell mass (ICM) of the blastocyst, which will give rise to the embryo proper, is the paternal X-chromosome reactivated, such that the embryo can undergo random X-inactivation 1–2 days later. Since the in vitro system of mouse embryonic stem cells (mESCs) resembles the ICM, female ESCs carry two active X-chromosomes and thus express twofold higher levels for most X-linked genes compared to male or XO ESCs [8]. mESCs can thus serve as a model to understand the role of X-chromosome dosage during early development.
Since imprinted X-inactivation seems to be absent in other eutherian mammals, the time window(s) with double X-dosage in female human embryos still remains to be defined. To date several studies have analysed the status of the X-chromosomes in human embryos up to the blastocyst and found that by day 7 of development X-inactivation has not yet occurred [9,10]. The study of human embryos past that point of development is difficult because in vitro culture is only possible for pre-implantation stages and studies of human embryos in vivo cannot be conducted for obvious ethical reasons. Interestingly, Xist, the master regulator of X-inactivation, which is normally expressed from the inactive X-chromosome and mediates chromosome-wide gene silencing, is already expressed in human pre-implantation embryos, albeit from both X-chromosomes, and fails to initiate gene silencing during this stage of development. A recent study that used single-cell transcriptomics to quantify gene expression in early human embryos suggested that dosage compensation between the sexes occurs even in the absence of X-inactivation [10]. Instead of silencing a single X-chromosome, the expression of X-linked genes seems to be dampened by about 50% from both chromosomes in female pre-implantation embryos. So far it remains unknown whether bi-allelic Xist expression and the potentially associated dampening of gene expression is reverted at a later stage of development before random X-inactivation is initiated, which is observed in somatic tissues.
2. Sex differences in murine embryonic stem cells
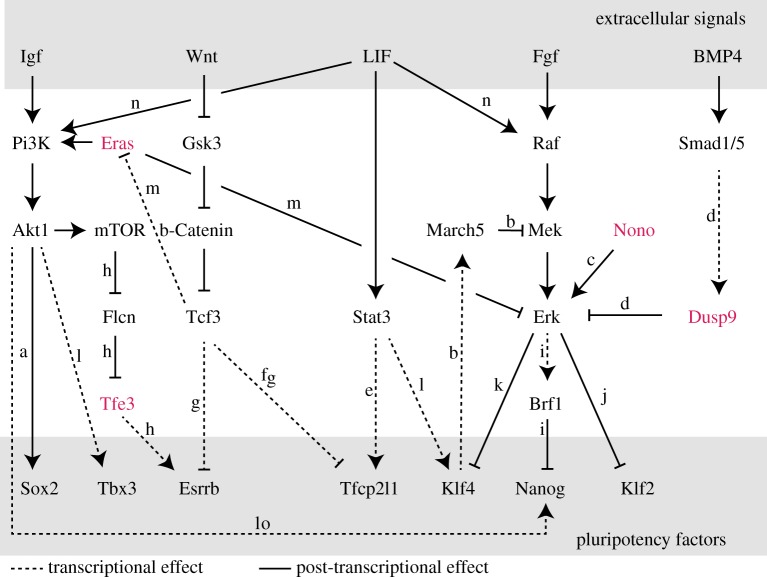
Embryonic stem cells are maintained in the pluripotent state by a complex gene regulatory network, where a series of transcription factors stabilize each others' expression (reviewed in [11]). At the core of this network lie the two essential pluripotency factors Oct4 and Sox2, which control the pluripotency associated transcriptional programme mostly acting as a hetero-dimer. Their expression is tightly controlled by a collection of other transcription factors, such as Nanog, Esrrb, Klf2, Klf4 and Tfcp2l1 that stabilize the pluripotent state in a partially redundant fashion, depending on the culture conditions. These factors integrate cues from a series of signalling pathways that either promote or destabilize the pluripotent state (figure 1). Most prominently, leukaemia inhibitory factor (LIF), which signals predominantly through Stat3, is essential for self-renewal of embryonic stem cells in conventional serum-based culture conditions. The central pluripotency-destabilizing pathway is the MAPK pathway, stimulated by Fgf4, which is expressed in an autocrine fashion by the ES cells themselves. Through the activation of Mek and Erk, Fgf4 leads to the downregulation of Nanog at the transcriptional level, but also destabilizes Klf2 and Klf4 protein through Erk-dependent phosphorylation [22,23]. Moreover, Gsk3, which can mediate Wnt signalling has a differentiation promoting effect by repressing several pluripotency factors, such as Esrrb, Nanog and Klf2, via β-Catenin and Tcf3. Finally, Pi3 K/Akt signalling promotes pluripotency and blocks differentiation, potentially by inhibiting differentiation-promoting pathways [28].
Figure 1.
The mESC signalling network. Schematic representation of several pluripotency- and differentiation associated signalling pathways and their interactions with pluripotency factors. Solid lines represent post-transcriptional and dashed lines transcriptional regulation. The regulators coloured in pink are encoded on the X-chromosome. The presented interactions are based on the following studies: a [12,13], b [14], c [15], d [16], e [17], f [18], g [19], h [20], i [21], j [22], k [23], l [24], m [25], n [26], o [27].
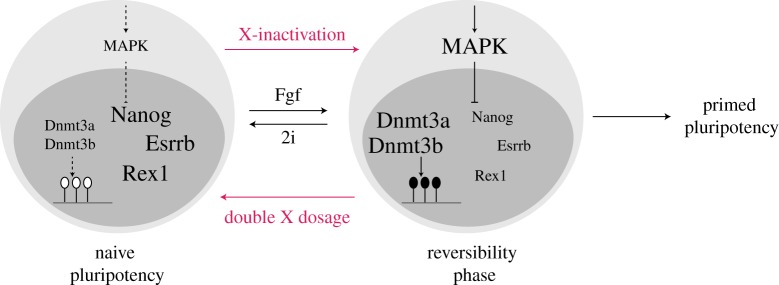
Since the pluripotency network is easily destabilized by the differentiation-promoting Mek/Erk and Gsk3/β-Catenin pathways, chemical inhibition of these signalling pathways can lock mESCs in the naive pluripotent state (2i culture conditions). In contrast to culture in the presence of these two inhibitors, conventional ESC culture in serum and LIF permits the cells to undergo an initial, still reversible step towards differentiation that is marked by the downregulation of a subset of pluripotency factors like Nanog and Esrrb (figure 2). As a consequence serum-grown ESCs consist of a heterogeneous mixture of cells expressing high and low levels of Nanog and other pluripotency factors [29–31]. In population measurements, these pluripotency factors appear therefore to be expressed at increased levels in 2i conditions. In addition, also several genes involved in the control of DNA methylation are differentially expressed between ESCs grown in conventional and 2i conditions, such as Dnmt3a and Dnmt3b, which encode for de novo DNA methyltransferases [32]. The reduced levels of Dnmt3a/b in 2i conditions contribute to the markedly reduced DNA methylation levels that are observed in 2i in a genome-wide manner [32–34]. In addition, also the maintenance of DNA methylation after DNA replication seem to be less efficient in 2i due to reduced protein expression of UHRF1, which targets the maintenance DNA methyltransferase Dnmt1 to replication foci [35]. Taken together, blocking two differentiation-associated signal transduction pathways can lock mESCs in the naive pluripotent ground state, where they express homogenously high levels of all pluripotency factors and are globally DNA hypomethylated (figure 2).
Figure 2.
X-chromosome dosage shifts mESC towards the ground state of pluripotency. When cultured in conventional serum-containing medium, mESCs can reversibly shift from the naive pluripotent ground state to a slightly more differentiated phenotype, characterized by the downregulation of some pluripotency factors such as Nanog, Esrrb and Rex1. 2i culture conditions shift cells towards naive pluripotency, which is associated with inhibition of MAPK signalling, high levels of pluripotency factors, low levels of Dnmt3a/b and global DNA hypomethylation. Double X-dosage can shift the cells to the naive state, while reduction of X-dosage by X-chromosome inactivation promotes differentiation.
Not only the culture conditions affect the stability of mouse ESCs, but also their sex chromosome composition. In non-2i culture conditions, where mESCs exist in a dynamic equilibrium between the naive pluripotent ground state and a slightly more differentiated state, the two active X-chromosomes present in female cells promote the transition towards the pluripotent ground state (figure 2) [8]. In population measurements, female XX ESCs therefore express higher levels of those pluripotency factors that are downregulated during the early reversible differentiation step, such as Nanog and Esrrb. Moreover, the signal transduction network adopts a distinct state in female ESCs with increased activity of the pluripotency associated Pi3 K/Akt pathway, and reduced output from the differentiation promoting Mek/Erk and Gsk3/β-Catenin pathways [8,36]. Since these signalling pathways are known to control pluripotency factor expression, the observed changes in signalling activity could account for down-stream effects on the pluripotency network. Interestingly target genes of Mek/Erk signalling are clearly reduced in mESCs with two X-chromosomes, but Mek/Erk phosphorylation levels exhibit the opposite trend [8,36]. This suggests that X-chromosome dosage inhibits MAPK signalling downstream of Erk, leading to reduced target gene expression, but increased Mek and Erk phosphorylation due to negative feedback regulation. These sex differences in mESCs can be clearly attributed to X-dosage since male XY ESCs resemble female XO cells regarding pluripotency factor expression and signalling activity pattern. Moreover, artificial inactivation of one X-chromosome in female ESCs induces changes in pluripotency factor expression and signalling activity towards the XO phenotype [8].
Since double X-dosage shifts mESCs towards the pluripotent ground state, which is associated with DNA hypomethylation, female ESCs exhibit lower levels of DNA methylation compared to males as well as reduced expression of Dnmt3a and Dnmt3b [37]. While 60–80% of all CpG dinucleotides in the genome are methylated in males ESCs grown in non-2i media, only about 20% are methylated in females [33,36,37]. Interestingly global DNA hypomethylation of female ESCs is even maintained in 2i medium, where levels in male cells drop to 20%, while in females they are reduced to a few per cent [33]. DNA methylation seems to respond very sensitively to the MAPK signalling activity since the reduction in methylation levels appears to be much less severe if 0.4 µM of Mek inhibitor are used compared to the ‘standard’ 1 µM, albeit this observation could also be due to the usage of different cell lines in the different studies [38]. With the reduced dose of Mek inhibitor, male ESCs maintain 50% methylation and females 20%. The observation that even in 2i conditions some of the sex differences in mESCs persist suggests either that X-dosage further inhibits some remaining low activity of MAPK signalling or that MAPK-independent mechanisms also contribute to the sex differences. Although the difference in methylation levels is quite large, this appears to have only small effects on the gene expression state in agreement with observations in ESCs devoid of DNA methylation [39].
Since X-chromosome dosage decreases the propensity of mESCs to acquire more differentiated states, female ESCs exhibit a delayed downregulation of pluripotency factors during in vitro differentiation [8]. Only when the cells have undergone X-inactivation and have thus neutralized the sex differences in signalling and pluripotency factor expression, can they completely exit the pluripotent state. Consequently, speeding up or blocking X-inactivation genetically also increased and decreased the differentiation speed, respectively [8]. A recent study using single-cell transcriptomics showed that even after four passages of differentiation towards epiblast-like cells, female ESCs still mostly cluster with undifferentiated ESCs in contrast to male cells, which have already acquired a much more differentiated transcriptome at this stage [40]. Only after another three passages, when they had achieved dosage compensation for X-linked genes, the female cells started to resemble their male counterparts. Also in that study, female ESCs maintained higher levels of pluripotency factors compared to males and showed differential expression of genes associated with MAPK and Wnt signalling in a KEGG pathway analysis. The X-inactivation status thus appears to be intimately linked to cellular differentiation, since double X-dosage delays differentiation, while downregulation of pluripotency factors is known to trigger XCI [41].
3. The pluripotency-associated signalling network
As described in the previous sections the activity state of the mESC signalling network appears to be affected by X-dosage. As a basis for a discussion of how this X-dosage effect might be mediated on the molecular level, this section will give an overview over how different signalling pathways control the pluripotency network (figure 1).
(a). Differentiation-associated pathways
As already described above, the two main signalling pathways that promote the exit from the pluripotent state and that are thus inhibited in 2i culture conditions, are the Mek/Erk and the Gsk3/β-Catenin pathways. The Mek/Erk pathway is stimulated by autocrine Fgf4, which is a well characterized target gene of Oct4/Sox2 thus forming a pluripotency-destabilizing negative feedback loop [11]. Fgf will trigger the MAP kinase cascade via the FGF receptor, leading to successive phosphorylation and activation of the Raf, Mek and Erk kinases. This will trigger the translocation of Erk into the nucleus, where it phosphorylates several transcription factors to control target gene expression. It remains poorly understood which molecular mechanisms link Erk to the pluripotency network, but some progress has been made recently. Two pluripotency factors, Klf2 and Klf4 have been shown to be directly phosphorylated by Erk, which promotes their degradation [22,23]. Klf4 in turn induces the E3 ubiquitin ligase March5, which promotes the pluripotent state by inhibiting the Mek/Erk cascade [14]. Moreover, the AU-rich element mRNA binding protein Brf1 has been identified as an Erk target gene in mESCs and has been shown to bind and destabilize the mRNAs of several pluripotency-associated genes, including Nanog [21]. This mechanism might mediate the long known Erk-dependent downregulation of Nanog [42]. In addition Nanog has also been identified as a direct phosphorylation target of Erk in vitro, but the functional consequences of this observation in vivo remain unknown [43]. Interestingly, Erk also associates with chromatin, probably to directly phosphorylate chromatin-bound substrates. It has been shown to bind bivalent promoters and to phosphorylate RNA polymerase II at Ser5, thereby promoting initiation of transcription [44]. Bivalency is characterized by the co-occurrence of the repressive and activating histone marks (H3K27me3 and H3K4me3) and is thought to poise developmental genes for upregulation upon exit from the naive pluripotent state. At a subset of bivalent promoters, Erk interacts with the X-linked factor Nono, which promotes Erk activity [15]. Also in human ES cells, Erk has been identified as a chromatin-bound factor and shown to co-localize with Elk1, a classic transcription factor downstream of Erk, at a subset of Elk1 target genes [45]. Although it is commonly thought that Erk phosphorylation is only mediated by Mek1/2, a recent study suggests that Erk might have Mek-independent functions in mESCs [46]. The study finds that, in contrast to pharmacological inhibition of Mek, which promotes mESC self-renewal, mESCs deficient for Erk1/2 are not viable due to genomic instability. The molecular basis of this observation, however, remains to be further investigated. In summary, Erk is a central player in the transition from the naive to the primed pluripotent state and mediates its effects through multiple downstream effectors.
Although Fgf is clearly the main trigger of the pathway, Erk has also been shown to be activated by LIF signalling [24]. This observation is somewhat puzzling since LIF is the central signal to maintain the pluripotent state. In addition, a third signalling pathway has recently been shown to affect Erk activity in mESCs. The pluripotency-associated growth factor BMP4, which activates Smad1/5 has been shown to upregulate the X-linked factor Dusp9. Dusp9 encodes for a dual-specificity phosphatase (Dusp) which dephosphorylates and thereby de-activates Erk [16].
The second differentiation-promoting pathway that is inhibited in 2i conditions is Gsk3/β-Catenin signalling. This pathway is known to mediate Wnt signals, but some discrepancies between the effects of Wnt and the Gsk3 inhibitor suggest that the situation might be more complex [11]. Gsk3 phosphorylates β-Catenin and thereby induces its degradation. β-Catenin associates with the ESC-specific Tcf/Lef factor Tcf3/Tcf7l1 and inhibits its DNA binding activity, which otherwise represses several pluripotency factors such as Esrrb and Tfcp2l1 [18,19]. In this way, Gsk3 activity reduces β-Catenin levels, which leads to activation of Tcf3/Tcf7l1, which will repress pluripotency-associated genes and thereby destabilize the pluripotent state.
(b). Pluripotency-associated pathways
The central pluripotency-promoting signal is LIF, which primarily activates the Jak/Stat pathway resulting in phosphorylation of Stat3. Stat3 will thus dimerize and translocate to the nucleus, where it binds many pluripotency-associated genes often together with Oct4, Sox2 and Nanog [47]. Klf4 and Tfcp2l1 have been identified as main Stat3 target genes [17,24]. Apart from Stat3, LIF appears to also induce phosphorylation of Erk and of Akt [24,26]. The functional relevance of these observations, however, remains unclear.
Akt is activated by Pi3 kinase (Pi3 K), which is part of the insulin-like growth factor (Igf) signalling pathway. In embryonic stem cells, however, the Pi3 K/Akt pathways can also be activated independent of Igf by the constitutively active Ras-like GTPase Eras, which is encoded by an X-linked gene and binds and thus activates Pi3 K [28]. Interestingly, Eras is expressed at high levels in serum-grown ES cells, but is strongly downregulated in 2i conditions because it is repressed by Tcf3, which mediates Gsk3 inhibition [25]. The downstream effects of Pi3 K/Akt signalling are not well understood, but Nanog and Tbx3 seem to be transcriptional target genes [24,27]. Moreover, Akt can directly phosphorylate Sox2, thereby stabilizing this core pluripotency factor [12,13]. The effects of Pi3 K/Akt activation are in part mediated by the mTOR pathway, which has been suggested to phosphorylate and thereby inhibit Follinculin (Flcn). Flcn in turn promotes cytoplasmic localization of the X-linked transcription factor Tfe3, which otherwise blocks differentiation by inducing Esrrb [20]. In this way, activation of mTOR will promote nuclear localization of Tfe3, which induces the pluripotency factor Esrrb.
4. X-linked modulators of pluripotency
As described in detail above, mESCs with a double dosage of X-linked genes are shifted towards the naive pluripotent state and their differentiation is partially blocked until they have undergone X-inactivation [8]. Consequently, X-dosage appears to increase the expression of some pluripotency factors such as Nanog and Esrrb, but does not affect expression of the core factors Oct4 and Sox2. Moreover, double X-dosage increases activity of the Pi3 K/Akt pathway and inhibits expression of target genes of the Gsk3 and Mek/Erk pathways, albeit accompanied by an increase in Mek and Erk phosphorylation. In addition double X-dosage appears to decrease global DNA methylation levels. These X-dosage dependent effects are likely to be mediated by one or several X-linked genes, which are present at a higher dose in female XX compared to male XY cells. In this section, several X-linked modulators of the pluripotency network and of the mESC signalling network will be discussed in detail with respect to their potential for mediating the observed X-dosage dependent effects (summarized in table 1).
Table 1.
X-linked regulators of the pluripotency signalling network. For six X-linked genes that have been implicated in transcriptional regulation and signalling in mESCs the table summarizes the consequences of loss and/or gain of function on pluripotency factors (Nanog, Esrrb), signalling pathways, DNA methylation levels and differentiation of mESC. The bottom row summarizes known X-dosage-dependent effects. Entries coloured in red indicate that increased dose of the respective gene resembles the XX phenotype (n.a. = not affected). (Online version in colour.)
| pluripotency factors | Mek Gsk target genes | pErk | Akt activity | DNA methylation | differentiation | |
|---|---|---|---|---|---|---|
| Eras [25] | Nanog n.a. | pErk ↑ | pAkt1 ↑ | promoted | ||
| Nono [15] | Nanog ↓ | Mek ↑ | pErk ↑ | promoted | ||
| Dusp9 [16,36] | Nanog ↑ | Mek ↓ Gsk3 ↓ |
pErk ↓/↑a | Akt target genes ↑ | 5mC ↑ | blocked |
| Tfe3 [20] | Nanog n.a. Esrrb ↑ |
Mek n.a. Gsk3 n.a. |
blocked | |||
| Nr0b1/Dax1 [48,49] | Esrrb n.a. Nanog n.a. |
pErk n.a. | blocked | |||
| Zfx [50] | Nanog n.a. Tcl1 ↑ |
blocked | ||||
| X dosage | Nanog ↑ Esrrb ↑ Tcl1 ↑ |
Mek ↓ Erk ↓ Gsk3↓ |
pErk ↑ | Akt1 ↑ | 5mC ↑ | blocked |
aConflicting data.
(a). Eras
Since Eras is an activator of the Pi3 K/Akt pathway, an increased dose of Eras in female ESCs could potentially increase Akt phosphorylation and the expression of Akt target genes. In addition, Eras has been shown to increase phosphorylation of Erk [25] and could thus in principle be responsible for the increased levels of Erk phosphorylation in female ESCs compared to males [8]. However, Nanog is not affected by Eras knock-down or over-expression and Eras seems to rather promote, not block differentiation [25]. Therefore, Eras could be responsible for the observed effects on the signalling network, but not for the differentiation phenotype.
(b). Nono
Nono has been found to act as an Erk cofactor at a subset of bivalent genes. In Nono deficient mESCs reduced Erk phosphorylation is observed [15], suggesting that an increased dose of Nono in female ESCs could account for the observed elevated levels of pErk [8]. However, Nono deficient mESCs exhibit increased levels of Nanog and reduced differentiation propensity and are defective in the expression of Erk target genes [15]. Overall, an increased dose of Nono would thus be expected to rather shift female ESCs towards differentiation, and not towards naive pluripotency.
(c). Dusp9
Dusp9 is an X-linked Erk phosphatase that has been shown to be upregulated by BMP4 in mESCs [16,51]. Through inhibition of the Erk pathway, overexpression of Dusp9 results in a reduction of Erk target genes and increased Nanog expression and Dusp9 knock-down promotes differentiation [16]. Moreover, Dusp9 has also been shown to reduce DNA methylation levels and Akt target genes, while increasing Gsk3 and MAPK targets [36]. Therefore, an increased dose of Dusp9 in female mESC could explain the majority of observed X-dosage-dependent effects. With Dusp9 being an Erk phosphatase its over-expression is expected to decrease Erk phosphorylation, which has indeed been observed [16]. In a more recent study by contrast, the opposite response to Dusp9 over-expression has been described, resembling the increase in Erk phosphorylation observed in female mESCs [36]. It thus remains to be determined, whether the effects of Dusp9 are mediated by substrates other than Erk or whether other X-linked genes are responsible for the observed elevated phosphorylation levels of pErk in female mESCs.
(d). Tfe3
Overexpression and forced nuclear localization of Tfe3 has been shown to block ESC differentiation and to increase expression of Esrrb [20], thus partially pheno-copying the observed X-dosage effect. However, neither Nanog expression nor Mek and Gsk3 target genes were affected by forced nuclear translocation of Tfe3 [20]. Thus, Tfe3 could partially account for the differentiation phenotype of female ESCs, but probably not for the effects on the signalling network.
(e). Nr0b1/Dax1
Nr0b1/Dax1 is an orphan nuclear hormone receptor that co-occupies many gene promoters together with other pluripotency factors, such as Nanog, Sox2 and Klf4 [52]. Nr0b1/Dax1 overexpression blocks differentiation, but does neither affect expression of other pluripotency factors such as Nanog and Esrrb, nor phosphorylation levels of Erk [48,49]. In addition, it does not exhibit the twofold elevated levels in female ESCs that have been found for the majority of X-linked genes [8]. Instead it seems to be dosage-compensated by a gene-specific mechanism. Therefore, Nr0b1/Dax1 is rather unlikely to mediate the observed shift towards the naive state.
(f). Zfx
Zfx is a zinc finger transcription factor that is required for self-renewal of mESCs and its overexpression can block differentiation [50]. While Nanog is unaffected by Zfx overexpression, other pluripotency-associated factors such as Tbx3 and Tcl1 are increased. Therefore, Zfx could in principle account for some of the X-dosage effects on the pluripotency network, but is not expected to affect the signalling network.
Among the X-linked factors discussed above, Dusp9 overexpression most closely resembles the double X-dosage phenotype. In theory an X-linked inhibitor of the Mek/Erk pathway, such as Dusp9 would be expected to reduce the expression of Mek/Erk target genes, to block differentiation and to increase expression of Nanog and Esrrb. If the inhibition occurred down-stream of Erk it would potentially increase Mek and Erk phosphorylation through negative feedback regulation as observed upon pharmacological inhibition of Mek [8,53]. Since Dusp9 is an Erk phosphatase, it is, however, expected to reduce Erk phosphorylation, while increasing pMek through the negative feedback. Since there are conflicting reports whether Dusp9 augments or reduces pErk its mechanism of action remains to be clarified [16,36]. Since Mek inhibition also reduces expression of Gsk3 target genes and leads to an increase in Akt phosphorylation, an inhibitor of the Mek/Erk pathway that acts downstream of Erk could potentially account for all observed X-dosage-dependent effects [8]. It is, however, likely that a combination of different X-linked genes shapes the X-dosage phenotype.
The fact that X-dosage affects pluripotency and differentiation has so far only been shown in mice since they are the prime model system for mammalian development. In addition, delayed in vivo development has been observed for female embryos in several other species such as cows, rats and humans [1]. For more detailed mechanistic studies, embryonic stem cells from these species will be valuable model systems. The use of human ESCs for studying X-dosage-dependent effects has so far been hampered by the fact that most cell lines adopt a state where one X-chromosome is at least partially silenced [54]. A recent study has characterized the X-inactivation status and the differentiation potential of a series of female human ESC lines in detail [55]. The finding that lines with two active X-chromosomes maintain this state during differentiation, shows that double X-dosage in human ESCs does not completely block differentiation. However, the study also showed that the extent of X-inactivation correlates with differentiation propensity of the cells line, such that XCI appears to facilitate differentiation also in human ESCs.
Acknowledgements
I am grateful to Oriana Genolet for critical reading of the manuscript.
Data accessibility
This article has no additional data.
Competing interests
I have no competing interests.
Funding
This work is supported by the Max Planck Research Group leader programme and by the German Ministry of Science and Education (BMBF) through the grant E:bio Module III - Xnet.
References
- 1.Mittwoch U. 1993. Blastocysts prepare for the race to be male. Hum. Reprod. 8, 1550–1555. ( 10.1093/oxfordjournals.humrep.a137889) [DOI] [PubMed] [Google Scholar]
- 2.Gardner DK, Larman MG, Thouas GA. 2010. Sex-related physiology of the preimplantation embryo. Mol. Hum. Reprod. 16, 539–547. ( 10.1093/molehr/gaq042) [DOI] [PubMed] [Google Scholar]
- 3.Burgoyne PS, Thornhill AR, Boudrean SK, Darling SM, Bishop CE, Evans EP, Capel B, Mittwoch U. 1995. The genetic basis of XX-XY differences present before gonadal sex differentiation in the mouse [and discussion]. Phil. Trans. R. Soc. Lond. B 350, 253–261. ( 10.1098/rstb.1995.0159) [DOI] [PubMed] [Google Scholar]
- 4.Burgoyne PS. 1993. A Y-chromosomal effect on blastocyst cell number in mice. Development 117, 341–345. [DOI] [PubMed] [Google Scholar]
- 5.Okamoto I, Otte AP, Allis CD, Reinberg D, Heard E. 2004. Epigenetic dynamics of imprinted X inactivation during early mouse development. Science 303, 644–649. ( 10.1126/science.1092727) [DOI] [PubMed] [Google Scholar]
- 6.Mak W, Nesterova TB, de Napoles M, Appanah R, Yamanaka S, Otte AP, Brockdorff N. 2004. Reactivation of the paternal X chromosome in early mouse embryos. Science 303, 666–669. ( 10.1126/science.1092674) [DOI] [PubMed] [Google Scholar]
- 7.Deng Q, Ramsköld D, Reinius B, Sandberg R. 2014. Single-cell RNA-seq reveals dynamic, random monoallelic gene expression in mammalian cells. Science 343, 193–196. ( 10.1126/science.1245316) [DOI] [PubMed] [Google Scholar]
- 8.Schulz EG, et al. 2014. The two active X chromosomes in female ESCs block exit from the pluripotent state by modulating the ESC signaling network. Cell Stem Cell 14, 203–216. ( 10.1016/j.stem.2013.11.022) [DOI] [PubMed] [Google Scholar]
- 9.Okamoto I, et al. 2011. Eutherian mammals use diverse strategies to initiate X-chromosome inactivation during development. Nature 472, 370–374. ( 10.1038/nature09872) [DOI] [PubMed] [Google Scholar]
- 10.Petropoulos S, et al. 2016. Single-cell RNA-seq reveals lineage and X chromosome dynamics in human preimplantation embryos. Cell 165, 1012–1026. ( 10.1016/j.cell.2016.03.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martello G, Smith AG. 2014. The nature of embryonic stem cells. Annu. Rev. Cell Dev. Biol. 30, 647–675. ( 10.1146/annurev-cellbio-100913-013116) [DOI] [PubMed] [Google Scholar]
- 12.Fang L, Zhang L, Wei W, Jin X, Wang P, Tong Y, Li J, Du JX, Wong J. 2014. A methylation-phosphorylation switch determines Sox2 stability and function in ESC maintenance or differentiation. Mol. Cell 55, 537–551. ( 10.1016/j.molcel.2014.06.018) [DOI] [PubMed] [Google Scholar]
- 13.Jeong C-H, et al. 2010. Phosphorylation of Sox2 cooperates in reprogramming to pluripotent stem cells. Stem Cells 28, 2141–2150. ( 10.1002/stem.540) [DOI] [PubMed] [Google Scholar]
- 14.Gu H, et al. 2015. Mitochondrial E3 ligase March5 maintains stemness of mouse ES cells via suppression of ERK signalling. Nat. Commun. 6, 7112 ( 10.1038/ncomms8112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma C, et al. 2016. Nono, a bivalent domain factor, regulates Erk signaling and mouse embryonic stem cell pluripotency. Cell Rep. 17, 997–1007. ( 10.1016/j.celrep.2016.09.078) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Z, et al. 2012. BMP4 signaling acts via dual-specificity phosphatase 9 to control ERK activity in mouse embryonic stem cells. Cell Stem Cell 10, 171–182. ( 10.1016/j.stem.2011.12.016) [DOI] [PubMed] [Google Scholar]
- 17.Martello G, Bertone P, Smith AG. 2013. Identification of the missing pluripotency mediator downstream of leukaemia inhibitory factor. EMBO J. 32, 2561–2574. ( 10.1038/emboj.2013.177) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ye S, Li P, Tong C, Ying Q-L. 2013. Embryonic stem cell self-renewal pathways converge on the transcription factor Tfcp2l1. EMBO J. 32, 2548–2560. ( 10.1038/emboj.2013.175) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martello G, Sugimoto T, Diamanti E, Joshi A, Hannah R, Ohtsuka S, Göttgens B, Niwa H, Smith AG. 2012. Esrrb is a pivotal target of the Gsk3/Tcf3 axis regulating embryonic stem cell self-renewal. Cell Stem Cell 11, 491–504. ( 10.1016/j.stem.2012.06.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Betschinger J, Nichols J, Dietmann S, Corrin PD, Paddison PJ, Smith AG. 2013. Exit from pluripotency is gated by intracellular redistribution of the bHLH transcription factor Tfe3. Cell 153, 335–347. ( 10.1016/j.cell.2013.03.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan FE, Elowitz MB. 2014. Brf1 posttranscriptionally regulates pluripotency and differentiation responses downstream of Erk MAP kinase. Proc. Natl Acad. Sci. USA 111, E1740–E1748. ( 10.1073/pnas.1320873111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yeo J-C, et al. 2014. Klf2 is an essential factor that sustains ground state pluripotency. Cell Stem Cell 14, 864–872. ( 10.1016/j.stem.2014.04.015) [DOI] [PubMed] [Google Scholar]
- 23.Kim MO, et al. 2012. ERK1 and ERK2 regulate embryonic stem cell self-renewal through phosphorylation of Klf4. Nat. Struct. Mol. Biol. 19, 283–290. ( 10.1038/nsmb.2217) [DOI] [PubMed] [Google Scholar]
- 24.Niwa H, Ogawa K, Shimosato D, Adachi K. 2009. A parallel circuit of LIF signalling pathways maintains pluripotency of mouse ES cells. Nature 460, 118–122. ( 10.1038/nature08113) [DOI] [PubMed] [Google Scholar]
- 25.Zhao Z-A, Yu Y, Ma H-X, Wang X-X, Lu X, Zhai Y, Zhang X, Wang H, Li L. 2015. The roles of ERAS during cell lineage specification of mouse early embryonic development. Open Biol. 5, 150092 ( 10.1098/rsob.150092) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paling NRD, Wheadon H, Bone HK, Welham MJ. 2004. Regulation of embryonic stem cell self-renewal by phosphoinositide 3-kinase-dependent signaling. J. Biol. Chem. 279, 48 063–48 070. ( 10.1074/jbc.M406467200) [DOI] [PubMed] [Google Scholar]
- 27.Storm MP, Kumpfmueller B, Thompson B, Kolde R, Vilo J, Hummel O, Schulz H, Welham MJ. 2009. Characterization of the phosphoinositide 3-kinase-dependent transcriptome in murine embryonic stem cells: identification of novel regulators of pluripotency. Stem Cells 27, 764–775.( 10.1002/stem.3) [DOI] [PubMed] [Google Scholar]
- 28.Welham MJ, Kingham E, Sanchez-Ripoll Y, Kumpfmueller B, Storm MP, Bone H. 2011. Controlling embryonic stem cell proliferation and pluripotency: the role of PI3 K- and GSK-3-dependent signalling. Biochem. Soc. Trans. 39, 674–678. ( 10.1042/BST0390674) [DOI] [PubMed] [Google Scholar]
- 29.Chambers I, et al. 2007. Nanog safeguards pluripotency and mediates germline development. Nature 450, 1230–1234. ( 10.1038/nature06403) [DOI] [PubMed] [Google Scholar]
- 30.Toyooka Y, Shimosato D, Murakami K, Takahashi K, Niwa H. 2008. Identification and characterization of subpopulations in undifferentiated ES cell culture. Development 135, 909–918. ( 10.1242/dev.017400) [DOI] [PubMed] [Google Scholar]
- 31.Hayashi K, Lopes SMC, Tang F, Surani MA. 2008. Dynamic equilibrium and heterogeneity of mouse pluripotent stem cells with distinct functional and epigenetic states. Cell Stem Cell 3, 391–401. ( 10.1016/j.stem.2008.07.027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leitch HG, et al. 2013. Naive pluripotency is associated with global DNA hypomethylation. Nat. Struct. Mol. Biol. 20, 311–316. ( 10.1038/nsmb.2510) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Habibi E, et al. 2013. Whole-genome bisulfite sequencing of two distinct interconvertible DNA methylomes of mouse embryonic stem cells. Cell Stem Cell 13, 360–369. ( 10.1016/j.stem.2013.06.002) [DOI] [PubMed] [Google Scholar]
- 34.Ficz G, et al. 2013. FGF signaling inhibition in ESCs drives rapid genome-wide demethylation to the epigenetic ground state of pluripotency. Cell Stem Cell 13, 351–359. ( 10.1016/j.stem.2013.06.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meyenn F, et al. 2016. Impairment of DNA methylation maintenance is the main cause of global demethylation in naive embryonic stem cells. Mol. Cell 62, 848–861. ( 10.1016/j.molcel.2016.04.025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi J, et al. 2017. DUSP9 modulates DNA hypomethylation in female mouse pluripotent stem cells. Cell Stem Cell 20, 706–719. ( 10.1016/j.stem.2017.03.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zvetkova I, Apedaile A, Ramsahoye B, Mermoud JE, Crompton LA, John R, Feil R, Brockdorff N. 2005. Global hypomethylation of the genome in XX embryonic stem cells. Nat. Genet. 37, 1274–1279. ( 10.1038/ng1663) [DOI] [PubMed] [Google Scholar]
- 38.Shirane K, et al. 2016. Global landscape and regulatory principles of DNA methylation reprogramming for germ cell specification by mouse pluripotent stem cells. Dev. Cell 39, 87–103. ( 10.1016/j.devcel.2016.08.008) [DOI] [PubMed] [Google Scholar]
- 39.Fouse SD, Shen Y, Pellegrini M, Cole S, Meissner A, Van Neste L, Jaenisch R, Fan G. 2008. Promoter CpG methylation contributes to ES cell gene regulation in parallel with Oct4/Nanog, PcG complex, and histone H3 K4/K27 trimethylation. Cell Stem Cell 2, 160–169. ( 10.1016/j.stem.2007.12.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen G, et al. 2016. Single-cell analyses of X chromosome inactivation dynamics and pluripotency during differentiation. Genome Res. 26, 1342–1354. ( 10.1101/gr.201954.115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Navarro P, Chambers I, Karwacki-Neisius V, Chureau C, Morey C, Rougeulle C, Avner P. 2008. Molecular coupling of Xist regulation and pluripotency. Science 321, 1693–1695. ( 10.1126/science.1160952) [DOI] [PubMed] [Google Scholar]
- 42.Lanner F, Rossant J. 2010. The role of FGF/Erk signaling in pluripotent cells. Development 137, 3351–3360. ( 10.1242/dev.050146) [DOI] [PubMed] [Google Scholar]
- 43.Brumbaugh J, Russell JD, Yu P, Westphall MS, Coon JJ, Thomson JA. 2014. NANOG is multiply phosphorylated and directly modified by ERK2 and CDK1 in vitro. Stem Cell Rep. 2, 18–25. ( 10.1016/j.stemcr.2013.12.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tee W-W, Shen SS, Oksuz O, Narendra V, Reinberg D. 2014. Erk1/2 activity promotes chromatin features and RNAPII phosphorylation at developmental promoters in mouse ESCs. Cell 156, 678–690. ( 10.1016/j.cell.2014.01.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Göke J, Chan Y-S, Yan J, Vingron M, Ng H-H. 2013. Genome-wide kinase-chromatin interactions reveal the regulatory network of ERK signaling in human embryonic stem cells. Mol. Cell 50, 844–855. ( 10.1016/j.molcel.2013.04.030) [DOI] [PubMed] [Google Scholar]
- 46.Chen H, Guo R, Zhang Q, Guo H, Yang M, Wu Z, Gao S, Liu L, Chen L. 2015. Erk signaling is indispensable for genomic stability and self-renewal of mouse embryonic stem cells. Proc. Natl Acad. Sci. USA 112, E5936–E5943. ( 10.1073/pnas.1516319112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen X, et al. 2008. Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell 133, 1106–1117. ( 10.1016/j.cell.2008.04.043) [DOI] [PubMed] [Google Scholar]
- 48.Khalfallah O, Rouleau M, Barbry P, Bardoni B, Lalli E. 2009. Dax-1 knockdown in mouse embryonic stem cells induces loss of pluripotency and multilineage differentiation. Stem Cells 27, 1529–1537. ( 10.1002/stem.78) [DOI] [PubMed] [Google Scholar]
- 49.Zhang J, et al. 2014. Dax1 and Nanog act in parallel to stabilize mouse embryonic stem cells and induced pluripotency. Nat. Commun. 5, 5042 ( 10.1038/ncomms6042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Galan-Caridad JM, Harel S, Arenzana TL, Hou ZE, Doetsch FK, Mirny LA, Reizis B. 2007. Zfx controls the self-renewal of embryonic and hematopoietic stem cells. Cell 129, 345–357. ( 10.1016/j.cell.2007.03.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Caunt CJ, Keyse SM. 2013. Dual-specificity MAP kinase phosphatases (MKPs): shaping the outcome of MAP kinase signalling. FEBS J. 280, 489–504. ( 10.1111/j.1742-4658.2012.08716.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim J, Chu J, Shen X, Wang J, Orkin SH. 2008. An extended transcriptional network for pluripotency of embryonic stem cells. Cell 132, 1049–1061. ( 10.1016/j.cell.2008.02.039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fritsche-Guenther R, Witzel F, Sieber A, Herr R, Schmidt N, Braun S, Brummer T, Sers C, Blüthgen N. 2011. Strong negative feedback from Erk to Raf confers robustness to MAPK signalling. Mol. Syst. Biol. 7, 489 ( 10.1038/msb.2011.27) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pasque V, Plath K. 2015. X chromosome reactivation in reprogramming and in development. Curr. Opin. Cell Biol. 37, 75–83. ( 10.1016/j.ceb.2015.10.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Patel S, et al. 2017. Human embryonic stem cells do not change their X inactivation status during differentiation. Cell Rep. 18, 54–67. ( 10.1016/j.celrep.2016.11.054) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.