Abstract
Animals have evolved different defensive strategies to survive predation, among which chemical defences are particularly widespread and diverse. Here we investigate the function of chemical defence diversity, hypothesizing that such diversity has evolved as a response to multiple enemies. The aposematic wood tiger moth (Arctia plantaginis) displays conspicuous hindwing coloration and secretes distinct defensive fluids from its thoracic glands and abdomen. We presented the two defensive fluids from laboratory-reared moths to two biologically relevant predators, birds and ants, and measured their reaction in controlled bioassays (no information on colour was provided). We found that defensive fluids are target-specific: thoracic fluids, and particularly 2-sec-butyl-3-methoxypyrazine, which they contain, deterred birds, but caused no aversive response in ants. By contrast, abdominal fluids were particularly deterrent to ants, while birds did not find them repellent. Our study, to our knowledge, is the first to show evidence of a single species producing separate chemical defences targeted to different predator types, highlighting the importance of taking into account complex predator communities in studies on the evolution of prey defence diversity.
Keywords: predator–prey interactions, chemical defences, aposematism, pyrazines
1. Introduction
Predation is a key agent of natural selection in prey species [1]. In order to survive in a multi-predator world, animals have evolved different defensive strategies that vary in their nature and efficacy in relation to predator sensory abilities and attack tactics [2–4]. Which strategy, or set of strategies, is used as a defence depends on the benefits granted and the costs incurred. However, the strategy employed must ultimately aim to prevent the completion of a predation event as early as possible in the predation sequence (i.e. detection, identification, approach, subjugation and consumption sensu; Endler [2]).
Aposematic organisms gain protection from predators by displaying colourful warning signals, which are coupled with some form of unprofitability [5]. This unprofitability is frequently related to the possession of chemical defences that can be unpalatable or even toxic [1,5–7]. Predators learn to associate the warning signal with a bad experience when tasting the prey, and remember it in subsequent encounters (e.g. [7–11]), leading to an aversive behaviour towards that particular prey.
Chemical defences in aposematic species can also vary in composition, quantity, and quality and, although this variation is relatively common [12–20], it has been addressed much less frequently than variation in warning signals [21]. Because these defences are usually effective during the subjugation and/or consumption stages of the predation sequence [2], chemical defences are often referred to as secondary defences. They can deter predators in a variety of ways, including volatile irritation, distastefulness or even toxicity [12]. Chemical defences can be costly [22–24], as they involve processes ranging from the sequestration of active compounds, either with or without subsequent modifications, through to their synthesis de novo [12,24]. Therefore, these defences are expected to evolve only if needed, and to be effective against a wide array of predators [14].
The wood tiger moth (Arctia (formerly Parasemia) plantaginis [25]) is an aposematic arctiid species distributed across the Holarctic region [26]. Males display either white or yellow hind wings (except for the Caucasus, where males are mostly red), whereas females present a hindwing coloration that varies continuously from yellow through to red. This warning coloration is coupled with the possession of two types of seemingly distasteful chemical secretions [27,28]. One type (hereafter ‘neck fluids’) is secreted from the prothoracic (cervical) glands, and the other one (hereafter ‘abdominal fluids’) is released from the abdominal tract. These fluids are released under different circumstances (i.e. seldom simultaneously). While abdominal fluids can be released in response to subtle disturbances, and mostly (if not only) during the early stages of adult life, neck fluids are most frequently secreted in response to the active ‘squeezing’ of the prothoracic glands (i.e. a bird attack; see the electronic supplementary material, video ESM1). The exact compounds in the defensive fluids of wood tiger moths have not yet been fully identified, but many other arctiids are well known for their chemical defences, which include pyrrolizidine alkaloids, methoxypyrazines and iridoid glycosides, among others [17–20]. Given the possible costs associated with insect chemical defences [12,24], it is intriguing that wood tiger moths are able to afford two different types of fluids.
Here, we test the hypothesis that these moths have two different types of chemical defences because they are targeted towards different predator types. We collected defensive fluids from laboratory-reared males, analysed their chemical composition and examined the reaction of two biologically relevant predators, birds and ants. We first show that the two defensive fluids are chemically distinct, and demonstrate that birds and invertebrate predators react to them differently. Following the results of these assays we identified a compound, 2-sec-butyl-3-methoxypyrazine (SBMP), which explains the target-specific nature of the thoracic defence fluid.
2. Material and methods
(a). Study species and collection of defensive fluids
The wood tiger moth, Arctia plantaginis, is an arctiid species distributed across the Holarctic region [26]. They are polyphagous and capital breeders [29], feeding only while larvae. Adults have a short lifespan (two to three weeks for males, less than one week for females) and produce only one generation per year in the wild. Under laboratory conditions, wood tiger moths can be relatively easily bred and kept on a diet consisting mostly of dandelion (Taraxacum sp.) leaves, and can produce three generations per year. The individuals used in the present experiments were obtained from two laboratory stocks, established in 2010 and 2011, from wild moths collected from central and southern Finland, and reared at the University of Jyväskylä (Finland) under natural light conditions and a temperature ca 23°C.
Fluids for the bird experiments were collected in 2012 from approximately 120 males, 60 white and 60 yellow, taken from the laboratory stock founded in 2011. Fluids for the ant experiments were collected in 2014 from 45 males from the same stock (see details about collection of defensive fluids in the electronic supplementary material, S2). There are no differences between wild and laboratory-reared moths in the volume of their defensive fluids, which appear to be produced de novo [30].
(b). Chemical analyses
For the preliminary chemical analysis, neck and abdominal fluids from five individuals were pooled. Five hundred microlitres of dichloromethane (DCM) was added to thoracic fluids and vortexed, and 20 µl of the abdominal fluid was pipetted into 500 µl DCM. The DCM was then evaporated under constant nitrogen flow and the dried samples re-dissolved with 250 µl pyridine and 250 µl silylation reagent (BSTFA + 1% TMCS, Regisil). Extracted fluid samples were analysed with an Agilent 6890 gas chromatograph–5973 mass spectrometer (GC/MS) system. A sample volume of 1 µl from both thoracic and abdominal fluid samples was injected into the injector using a pulsed, splitless mode and the temperature was set to 290°C. Compounds were separated with a HP-5 ms column (30 m × 0.25 mm internal diameter with a film thickness of 0.25 µm; J&W Scientific Inc.). Helium was used as a carrier gas at a constant flow (1 ml min−1). The oven temperature was programmed as follows: 2 min at 80°C, then ramped to 180°C at the rate of 8°C min−1 and from 180°C to 290°C at the rate of 7°C min−1, and kept at that temperature for an additional 10 min. Electron ionization (70 eV) mass spectra were used for identification. Chromatograms and mass spectra were evaluated using Agilent Chemstation (version G1701CA) software, and the Wiley 7th edition mass spectral database.
A further chemical analysis was performed at TU Branschweig. The samples were collected using Supelco Red (100 µm Polydimethylsiloxane, PDMS) and Black (75 µm Carboxen™/PDMS) solid phase micro extraction (SPME) fibres with neck fluids (1–10 µl) of freshly eclosed moths. Fibres were placed into the neck fluid and immediately transferred to the injection port of the GC/MS system. GC/MS analyses were carried out on an Agilent GC 7890B system connected to a 5977A mass-selective detector (Agilent) fitted with a HP-5 MS fused-silica capillary column (30 m × 0.25 mm i.d., 0.22 µm film; Hewlett–Packard). Conditions were as follows: carrier gas (He): 1.2 ml min−1; injector: 250°C; transfer line from injector to column: 300°C. The gas chromatograph was programmed as follows: 50°C (5 min isothermal), increased at 5°C min−1 to 320°C, and operated in splitless mode. The identification of compounds was performed by comparison of mass spectra and retention times with those of reference compounds (see the electronic supplementary material, S3).
(c). Bird response to moths' chemical defences
Blue tits (Cyanistes caeruleus) were observed through a mesh-covered window in one of the experimental cage's sides, and video-recorded with a digital camera (Sony DSC-HX1). The experimental cages were placed in a dark room, such that the observer was not noticeable to the birds (see details on bird housing and training in the electronic supplementary material, S2). Each bird was randomly assigned to one of five different groups, each with 13 birds. Groups were tested with either abdominal (A) fluids from yellow (Y) or white (W) moths; and neck (N) fluids from yellow or white moths. The fifth and final group was a control (C), tested with water only.
Each assay consisted of five trials, the first and last of which were done with water-soaked oats to ensure that the birds were feeding at the beginning of the experiment, and were not satiated at the end; in trials 2, 3 and 4 the birds were offered the treatment oats, which contained one type of the defence fluids. Therefore, only trials 2, 3 and 4 were included in the analysis. Each of these three trials was carried out with 25 µl of a specific blend of the fluids of three males of the same colour (see the electronic supplementary material, S2 for details on fluid collection) mixed with distilled water. Each blend was used twice (i.e. for two different birds). The 25 µl of fluids (or water, in case of the control group (C)), were distributed among three oat flakes, which were presented simultaneously to the birds, each of which had been food-deprived for a period no longer than two hours in order to ensure motivation to feed. During the experiment we recorded the ‘latency to approach’, defined as the time taken by the bird to approach and peck/eat the oats after seeing them, and recorded the number of oats eaten by the bird in a maximum trial duration of 5 min. The duration of the trial, taken as the time taken by the bird to finish the oats, was recorded in those cases where the birds ate all the oat flakes before the 5 min limit.
We ran two separate statistical analyses, one to test for differences in bird reaction towards the abdominal (A) or neck (N) fluids in comparison to the controls (C), and a second one to compare bird reactions to the defence fluids of white (W) and yellow (Y) morphs. For the first analysis the differences in bird latency to approach the oats among treatments were analysed using a mixed-effects Cox model. The time before the bird started to eat the oats (i.e. time to event) was used as the response variable, and type of fluid (C, N or A), trial number and the interaction between the two were taken as explanatory variables, with bird identity (ID) as a random factor. Then, we ran a generalized linear mixed model (GLMM) with a Poisson distribution including the total number of oats eaten as response variable, using the same predictor variables as mentioned above. Trial duration was included as a covariate to account for the time it took for the birds to consume the oats, and bird ID was entered again as a random factor. Once we confirmed that bird reaction to the moths' chemical defences was different from that of controls, we ran the second analysis excluding the individuals from the control (C) group, using the same models described above, but with moth colour rather than fluid type as an explanatory variable. In order to see whether bird reaction changed over the course of the experiment, we compared trials 3 and 4 to trial 2, as birds were exposed for the first time to the moths’ defences during trial 2. Model simplification (see the electronic supplementary material, S2) was done on the basis of differences in Akaike information criterion (AIC).
(d). Ant response to moths' chemical defences
The assays with ants were done in September 2014 in a forest patch in the vicinity of Jyväskylä (62.193 N, 25.699 E), Finland. We identified 15 ant nests (Formica sp.) and their associated trails; two different trails per nest were chosen on the basis of their traffic (number of ants following the trail) in order to test ant response to the two different chemical defences of A. plantaginis following a protocol modified from previous studies [31,32]. Once a trail was chosen, an acetate disc of approximately 9 cm diameter was placed on the ground, making sure that the ants would walk over it. Three drops of 10 µl each were added to the disc at similar distances from each other, two containing a blend of chemical fluids coming from three different males of the same colour, mixed with a 20% sugar solution (sucrose), and one with only the sugar solution, acting as a control. Using a sugar solution combined with a blend (in a 10% concentration) of the chemical defences ensured that the ants would have the motivation to drink despite the bad taste. We drew marks on the acetate disc with three different randomly assigned colours to identify the fluid type in each droplet. Two discs were used for each nest, one for each type of chemical defence. Both discs had fluids from both colour morphs plus a control droplet (i.e. NY, NW and C were presented simultaneously in one disc, and AY, AW and C were presented in the other one).
Ants were allowed to come to the disc and drink from the droplets for 5 min after which the disc was removed. Each assay was filmed with a digital camera (SONY DSC-HX1), and the videos were analysed in detail after the final experiment. For each disc we counted the number of drinking events (an ant approaches the droplet and drinks from it) and rejections (an ant approaches the droplet, tastes it and leaves immediately) in each droplet. With this we calculated an ‘acceptance score’ as the number of drinking events divided by the sum of drinking events and rejections, where values closer to 0.5 mean the ants have no preference or repulsion, values closer to 1 mean the ants drank the fluid more than they rejected it, and values close to 0 indicate that ants reject the fluid more than they drink it. Additionally, we did scans every 30 s to count the number of ants drinking from each droplet, and on the disc, and took the maximum number of ants over the 5 min period as a proxy for ant traffic.
We ran a GLMM with binomial distribution where the acceptance score was the response variable, and the interaction between morph and type of fluid was included as the explanatory variable. We also included ant traffic as a covariate, and nest ID as a random factor. Main effects were not included, as neck and abdominal fluid were not presented to the ants simultaneously and, therefore, are not directly comparable. For this and all other analyses we took a full-model approach. The variance explained by random effects was calculated following [33]. This and all statistical analyses were carried out with the software R Studio [34], using the packages coxme [35] and lmer4 [36].
(e). Bird and ant response to pure pyrazine
Following the results of the second chemical analysis (see below) we performed a second assay with ants (June 2016) and birds (November 2016) to determine whether the pyrazine detected in the neck fluids was capable of eliciting aversive reactions on its own, and in the concentrations found. The procedures followed the protocols described above for each predator type. For details on the methods of these assays see the electronic supplementary material, S2.
3. Results
(a). Preliminary chemical analysis
We found that the two types of defensive fluids differ in their composition (electronic supplementary material, S4). In addition to containing a greater number of peaks, the peak areas obtained from the neck fluids were essentially larger (electronic supplementary material, S4a) compared to abdominal fluids (electronic supplementary material, S4b). The main compound groups in neck defensive fluids were amino and carboxylic acids (see table S1 in the electronic supplementary material, S2). The methods used in this first analysis did not allow for the identification of highly volatile compounds because it aimed to identify as many compounds as possible using a silylation derivatising step, in which the very volatile compounds are lost.
(b). Bird response to moths' chemical defences
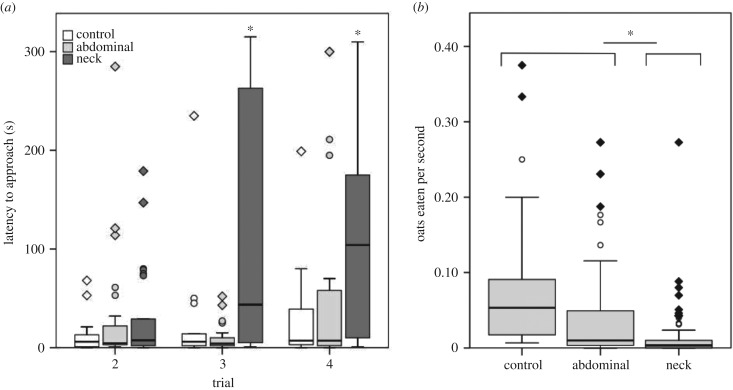
Birds were overall significantly more deterred by the neck fluids than by the abdominal ones. This was reflected in a higher latency to approach oats soaked with neck fluids compared to control oats across trials (table 1; figure 1a), whereas no differences were found between the latency to approach oats soaked with abdominal fluids and controls (table 1).
Table 1.
GLMM showing the effect of fluid type on bird latency to approach during the three trials with defensive fluids (fluid C and trial 2 are included in the intercept). (A = abdominal, N = neck, C = control (only water). Numbers in bold denote significant parameters at the p < 0.05 level.)
| variable | estimate ± s.e. | z | p |
|---|---|---|---|
| fluid (A) | −0.577 ± 0.53 | −1.08 | 0.280 |
| fluid (N) | −0.511 ± 0.52 | −0.98 | 0.330 |
| trial 3 | −0.328 ± 042 | −0.77 | 0.440 |
| trial 4 | −0.524 ± 0.42 | −1.25 | 0.210 |
| fluid (A): trial 3 | 0.867 ± 0.52 | 1.66 | 0.098 |
| fluid (N): trial 3 | −1.182 ± 0.54 | −2.20 | 0.028 |
| fluid (A): trial 4 | 0.200 ± 0.52 | 0.38 | 0.700 |
| fluid (N): trial 4 | −1.051 ± 0.35 | −1.97 | 0.049 |
Figure 1.
(a) Latency to approach (time taken for blue tits to start eating the oat flakes) is higher in response to neck fluids; and (b) birds eat oats soaked in neck fluids at a significantly lower rate. Asterisks indicate significant differences. Boxes show the median and the 25th and 75th percentiles of data distribution. Vertical lines indicate data range. Diamonds and circles denote extremes and outliers in data distribution, respectively.
Likewise, birds ate oats soaked with neck fluids at a significantly lower rate than controls (i.e. either took longer to finish the three oats presented, or ate less of them within the maximum length (5 min) of each trial; estimate ± s.e. = −0.409 ± 0.152, z = −2.689, p = 0.007; figure 2b), and then oats soaked with abdominal fluids (estimate ± s.e. = −0.317 ± 0.131, z = −2.408, p = 0.016; figure 2b); however, there was no difference between the number of oats eaten when soaked with abdominal fluids and water (estimate ± s.e. = −0.092 ± 0.124, z = −0.740, p > 0.05; figure 2b). Oat eating rate did not differ either between trial 3 (estimate ± s.e. = −0.058 ± 0.124, z = −0.473, p > 0.05) or trial 4 (estimate ± s.e. = −0.031 ± 0.125, z = −0.247, p > 0.05) and trial 2.
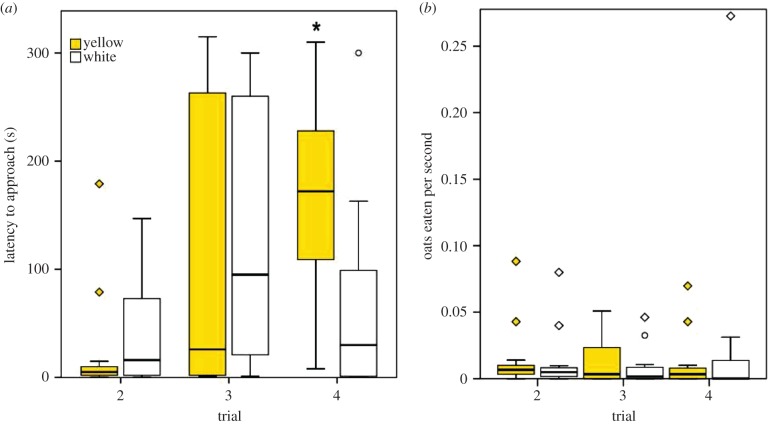
Figure 2.
(a) Latency to approach oats soaked in neck fluids (time taken for blue tits to start eating the fluid-soaked oat flakes) increases with time for neck fluids coming from yellow males; and (b) oat flakes are eaten at similar rates when soaked with neck fluids of yellow or white males. Asterisk indicates significant differences. Boxes show the median and the 25th and 75th percentiles of data distribution. Vertical lines indicate data range. Diamonds and circles denote extremes and outliers in data distribution, respectively.
Having found that neck fluids repel birds whereas abdominal fluids do not, we checked with a second analysis whether there were differences between the colour morphs in the efficiency of their neck defensive fluids. This analysis revealed a significant interaction between moth colour and trial, so that the latency to approach in the fourth trial was significantly higher in response to the neck fluids of yellow males than to those of white males (morph (Y) × trial (4): estimate ± s.e. = −2.057 ± 0.128, z = −3.16, p = 0.002; figure 2a; table S2 in the electronic supplementary material, S2), indicating that latency increases with time in response to fluids of yellow males (figure 2a), but not in response to white males' fluids. The rate at which birds presented with neck fluids ate oats was not affected by moth colour (estimate ± s.e. = 0.057 ± 0.265, z = −0.215, p > 0.05; figure 2b).
(c). Ant response to moths' chemical defences
Ants reacted in a different way to the two types of moth fluids. Compared to the controls, neck fluids had a higher acceptance score, whereas abdominal fluids had a lower one (figure 3). As expected, there was no significant difference between the acceptance score of the controls in the discs containing abdominal fluids and those of discs containing neck fluids (fluid (A) × morph (C): estimate ± s.e. = 0.07 ± 0.24, z = 0.30, p = 0.77; figure 3). Nest ID accounted only for 5.3% of the variance in acceptance score. There was a significant interaction between the type of fluid and colour morph indicating that, compared to controls, abdominal fluids of both colour morphs are rejected more often than neck fluids (fluid (A) × morph (W): estimate ± s.e. = −1.09 ± 0.16, z = −6.77, p < 0.001; fluid (A) × morph (Y): estimate ± s.e. = −1.40 ± 0.17, z = −8.31, p < 0.001; figure 3). Taking a closer look at the disks of each fluid type, we found that the abdominal fluids of yellow males are rejected more often than those of white males (estimate ± s.e. = −0.459 ± 0.14, z = −3.26, p = 0.001; figure 3), whereas no significant differences in acceptance score were found between the neck fluids of white males and those of yellow males (estimate ± s.e. = −0.459 ± 0.14, z = −3.26, p = 0.001; figure 3). Neck fluids of white males, however, were accepted significantly more than the pure sugar solution contained in controls (estimate ± s.e. = 0.505 ± 0.22, z = 2.27, p = 0.023; figure 3).
Figure 3.
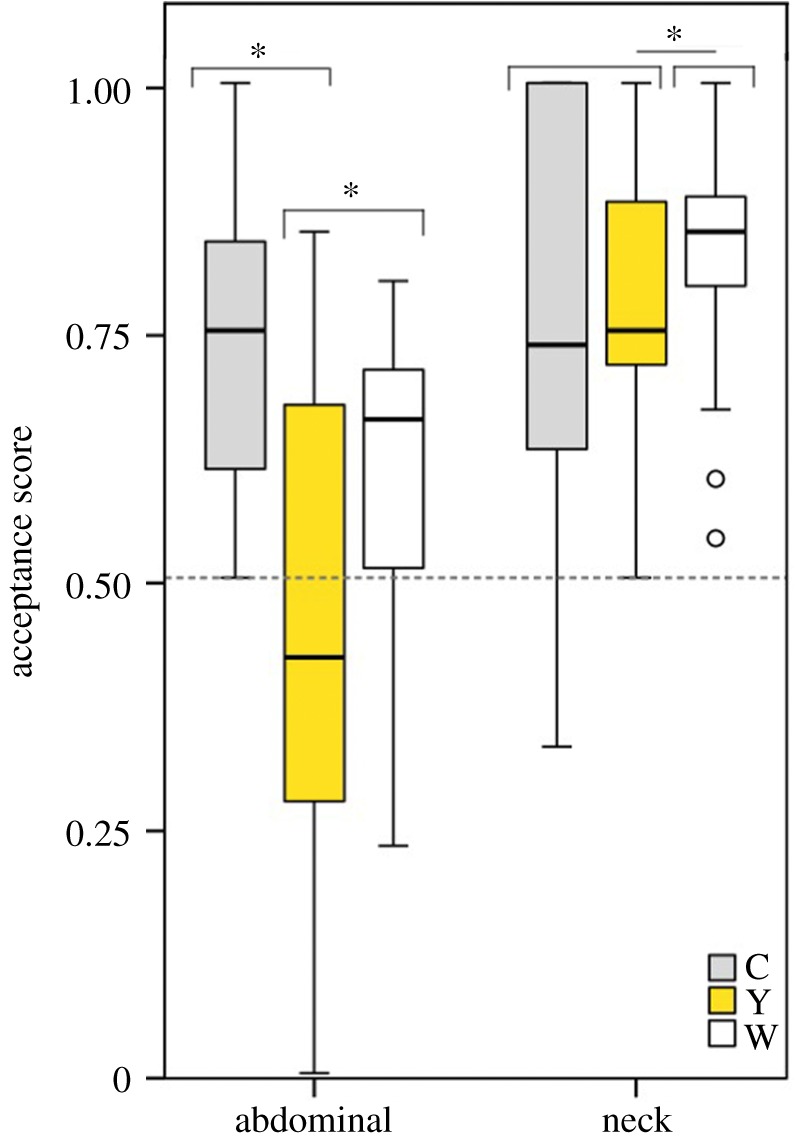
Acceptance score of ants (see methods section for details on calculation) is lower for abdominal fluids, especially from yellow males, which tend to be more rejected than accepted. The variation in the acceptance score of abdominal fluids from yellow males, however, is the greatest. Boxes show the median and the 25th and 75th percentiles of data distribution. Vertical lines indicate data range, circles denote outliers and asterisks highlight statistically significant differences.
(d). Further chemical analysis
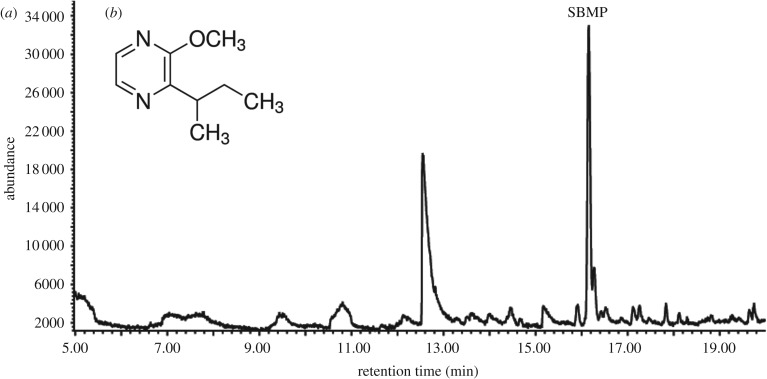
Further chemical analysis of the neck fluids by SPME without derivatisation proved the presence of the volatile SBMP (figure 4), which was not detected in abdominal fluids. The SBMP concentration in individual samples of neck fluids ranged from 0.1 to 1 ng µl−1. As methoxypyrazines are known to be deterrent for birds [37], and they are commonly found in the defensive fluids of lepidopterans [38], we further tested bird reaction to oats coated with SBMP.
Figure 4.
(a) Results of GC-MS analysis monitoring ions m/z 124, 138 and 151 (electronic supplementary material, figure S3); and (b) structure of 2-sec-butyl-3-methoxypyrazine (SBMP), the compound responsible for bird deterrence towards wood tiger moths' neck fluids.
(e). Bird and ant response to pure pyrazine
Birds (n = 10) showed a strong aversion to pure SBMP even at the lowest concentration (0.1 ng µl−1), reflected in the significantly lower amount of oats eaten when soaked with the pyrazine than with water (estimate ± s.e.: −0.560 ± 0.177, t = −3.163, p = 0.005; electronic supplementary material, S5a). Birds exposed to pyrazine-soaked oats also showed a tendency to hesitate for a longer time before approaching than did birds exposed to controls (estimate ± s.e. = −1.143, 0.604, z = −1.89, p = 0.059; electronic supplementary material, S5b). By contrast, we did not find pure SBMP to have a deterrent effect on ants. There were no differences in acceptance score between a sugar solution containing 1 ng µl−1 SBMP and the control solution (estimate ± s.e. = 0.139 ± 0.235; z = 0.589; p > 0.056; electronic supplementary material, S5c).
4. Discussion
Many animals are prey to multiple species, spread across numerous taxa. This predator diversity poses a significant problem for the effectiveness of anti-predator defences, as different taxa have different sensory capabilities, tolerances, and hunting strategies. Thus, different predator types may produce differential selection pressures on the same prey [7,39], which may explain why defence chemicals vary so greatly between and within species [21]. This variation in selection pressures could even result in prey evolving defences targeted at particular predators. Our experiments reveal a case of animal target-specific chemical defences. Wood tiger moths produce two types of defensive fluids, which differ in function and composition. While neck fluids successfully deter birds, abdominal fluids repel ants. In both cases, the chemical defences of yellow individuals elicited a stronger aversion than those of white males.
Previous studies on the chemical defences of several lepidopteran species have revealed that their active compounds, mostly pyrrolizidine alkaloids, cardenolides and cardiac glycosides [17,18,40–46], are unpalatable to a wide array of predators, including ants [31,47], spiders [48], bats [49], and birds [50–52]. Our findings suggest, however, that having only one type of chemical defence would not be enough to deter all the different predator types that wood tiger moths could encounter.
The two defence types found in A. plantaginis seem well suited for the different contexts in which these moths may encounter avian and invertebrate predators. Because neck fluids are secreted when the prothoracic glands are compressed, birds could be exposed to these chemicals when attacking the moth, regardless of whether the moth is flying or resting on the vegetation. Additionally, previous observations have revealed that birds tend to attack the moths by their heads, which means an almost immediate exposure to the neck fluids (see the electronic supplementary material, S1). Abdominal fluids, on the other hand, may be particularly useful for protection from terrestrial predators (i.e. ants) at moments when the moths are resting on the vegetation (especially females; J. Mappes 2013, personal observation), or when fleeing is difficult, for example when the moth is coming out of the pupa and its wings are not yet fully extended, or when the temperature is too low to initiate flight. Indeed, the abdominal fluids may not be produced solely for adult defence against predators, but might rather be the remains of the pupae liquid (i.e. meconium), and hence available primarily at the very early stages of adult life, when individuals are most vulnerable. Laboratory observations support this idea, as abdominal fluid is typically (but not always) produced during the first few days of adulthood, and individuals frequently release it if disturbed (E. Burdfield-Steel 2015, personal observation).
Ants were, as expected, motivated to drink from the three droplet types, presumably because of their content of sucrose, which they prefer over other sugar kinds [53]. However, the clear differences in acceptance scores show that not only are abdominal fluids distasteful, but also that neck fluids tend to be more accepted than the control solution. It is possible that neck fluids have valuable nutrients for the ants in addition to sugar. For instance, some ant species find a mixed solution of sugar and a blend of amino acids more appealing than a pure sugar solution [53]. Indeed, our preliminary chemical analysis showed high levels of amino acids, particularly in the neck fluids (table S2 in the electronic supplementary material, S2; electronic supplementary material, S4a), as is the case for some zygaenid moths [15]. Future research into the wood tiger moth defences could therefore focus on understanding why they invest in such costly products not related to the defence, or whether those are instead just by-products of the haemolymph.
While the initial chemical analysis shows that the abdominal fluids contain fewer compounds and are generally more dilute, it also shows that many of the major components of the two fluids are the same. These included many acids, such as citric acid. However, the pH of the fluids is close to neutral (E. Burdfield-Steel 2015, personal observation), suggesting that acidity is unlikely to be contributing to the predator response. Although there do appear to be some compounds present in the abdominal fluids that are missing from the neck fluids, mostly notably glutamic acid, it is still not clear what compound is responsible for the deterrent effect against ants.
Birds were significantly more deterred by neck fluids than by abdominal fluids. Furthermore, their latency towards neck fluids from yellow individuals was the highest by the end of the three trials (figure 2a). Because in our experiment bird predators did not have information on prey coloration, their response was based purely on the odour and taste of the chemicals they were exposed to. This might indicate that the odour of neck fluids from yellow males is more of a deterrent than that of white males. While warning colours are always ‘on’, taste and smell are hidden to predators until they come closer to the prey and/or attack them, in a similar fashion to ultrasonic clicks emitted by tiger moths in response to echolocating bats [54].
As our initial chemical analysis did not detect any clear source of the strong odour and taste associated with the neck fluids, we performed a second analysis to identify volatile candidate compounds that may be contributing to the predator aversive response. This resulted in the discovery of SBMP. Pyrazines, most specifically methoxypyrazines, have been previously found in the chemical defences of some arctiids [38,55], and we believe SBMP is one of the major components explaining the anti-predator effect of the neck fluids. It has been suggested that the odour of methoxypyrazines, which are responsible for some of the strongest and most haunting odours known [56], could serve a warning function towards predators which use smell to locate prey, in the same way that certain colours or colour patterns would work as warning signals for visual predators [38]. Previous studies have indeed convincingly shown that odours from methoxypyrazines can reinforce aversive responses of predators to certain colours [37,57], or elicit taste-avoidance learning on their own [58]. Domestic chicks have even been shown to be able to detect the methoxypyrazine odour from a distance and to associate such smell with a bitter taste provoking an aversive reaction [56]. However, there is little prior evidence that methoxypyrazines are in themselves strongly aversive to birds. Here we demonstrate that birds exposed to pure SBMP indeed find it very repellent, even at the lower end of the concentration range detected from the moths defences.
By contrast, much less is known about the role of pyrazines in invertebrate signalling (but see [59] for an illustration of the deterrent effect of SBMP against tropical invertebrate predators). We therefore also tested the effect of pure SBMP on ants and found, in keeping with the results from the neck fluid trials, that it did not deter them. Thus, SBMP seems the key behind the target-specific nature of the neck fluids, effective against bird predators, but not against insect predators such as ants.
Neck fluids of yellow males appear to be more effective than those of white males. Stronger defences in white males would have indicated a trade-off between warning signal efficacy and the strength of chemical defences that would help explain why, against theoretical expectations, white and yellow males can coexist in the same populations. With a more efficient warning signal [28] and somewhat better chemical defences (i.e. neck fluids that elicit bird increasing latency to approach with time (figure 2a), and abdominal fluids that are more often rejected than accepted by invertebrate predators (figure 3)), the reason(s) why the yellow morph has not reached fixation remains puzzling. These between-morphs differences in chemical defence quality are unlikely to be because of differences in larval diet between the two morphs, as larvae present no detectable differences in food choice (K. Suisto 2014, personal observation). Recent studies suggest that variation in the composition in predator communities [60], combined with differential mating success [61] and sufficient gene flow [61,62], could contribute to the maintenance of this colour polymorphism. Further research should thus assess the relative importance of warning signals versus chemical defences in wood tiger moths, and evaluate whether either defence overrides the other, or whether they have a synergistic effect and form a redundant multimodal display (sensu Partan & Marler [63]).
Chemical defences can vary in several ways, yet this has not been studied as thoroughly as variation in coloration [21]. Here we demonstrate that the existence of two different, seemingly costly ([28]; K. Suisto et al. 2011, unpublished; E. Burdfield-Steel et al. 2015, unpublished), defensive fluids is justified by their predator specificity. Although the mechanisms by which these chemicals are produced are not yet known, our findings will hopefully stimulate research on the possible life-history trade-offs and fitness-related consequences faced by species with one type of chemical defences versus those faced by species with two (or more). Comparative phylogenetic analyses could be a useful and interesting approach to track the origin and evolution of general versus specific chemical defences. We also show that there are differences between yellow and white males in chemical defence quality. This aspect of variation in chemical defences is not trivial for aposematic species [64]. Experiments are needed where the probability of survival of individuals with different levels of chemical defence is recorded, in order to gain a better understanding of the mechanisms underlying intraspecific variation in chemical defences.
Our study not only highlights the largely overlooked importance of invertebrate predators as selective agents on prey defences [65], despite their abundance in nature, but also stresses the need to choose relevant predator species when studying the efficacy of chemical defences, and drawing conclusions about the selective agent shaping prey defences. The presence of enemy-specific chemical defences in a same prey animal hints at the importance of predator community in shaping prey evolution, and suggests that selection on chemical defence may be far more complex than we have previously assumed.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We are indebted to Helinä Nisu for help with birds, to the greenhouse workers at the University of Jyväskylä for moth rearing; to Janne Valkonen and Sebastiano De Bona for statistical advice; and to Catherine Soler and Morgan Brain for help with assays. J.V. and S.D.B. filmed the bird attack. J.V., Rose Thorogood, Candy Rowe and three anonymous referees provided thoughtful comments that greatly improved the manuscript.
Ethics
Wild birds were used with permission from the Central Finland Centre for Economic Development, Transport and Environment and licence from the National Animal Experiment Board (ESAVI/9114/04.10.07/2014) and the Central Finland Regional Environment Centre (VARELY/294/2015), and used according to the ASAB guidelines for the treatment of animals in behavioural research and teaching.
Data accessibility
The datasets have been uploaded as part of the electronic supplementary material.
Authors' contributions
Study design: B.R., E.B.-S., K.S., J.M.; implementation of bioassays: B.R., E.B.-S., K.S. Chemical analyses: H.P., E.B.-S., S.S., M.M., K.S.; video analyses: B.R.; statistical analyses and first draft of the paper: B.R., E.B.-S.; all co-authors contributed to final editing, and approved the submitted version of the manuscript.
Competing interests
We have no competing interests to declare.
Funding
Centre of Excellence in Biological Interactions (Academy of Finland, project no. 284666 to J.M.).
References
- 1.Edmunds M. 1974. Defence in animals: a survey of antipredator defences. New York, NY: Longman. [Google Scholar]
- 2.Endler JA. 1986. Defense against predators. In Predator prey relationships. Perspectives and approaches from the study of lower vertebrates (ed. Feder MELGV.), pp. 109–134. Chicago, IL: University of Chicago Press. [Google Scholar]
- 3.Hoverman JT, Relyea RA. 2007. The rules of engagement: how to defend against combinations of predators. Oecologia 154, 551–560. ( 10.1007/s00442-007-0847-3) [DOI] [PubMed] [Google Scholar]
- 4.Sih A, Englund G, Wooster D. 1998. Emergent impacts of multiple predators on prey. Trends Ecol. Evol. 13, 350–355. ( 10.1016/S0169-5347(98)01437-2) [DOI] [PubMed] [Google Scholar]
- 5.Poulton EB. 1890. The colours of animals: their meaning and use, pp. 558–612. London, UK: Kegan Paul, Trench, Trubner. [Google Scholar]
- 6.Cott HB. 1940. Adaptive coloration in animals. London, UK: Methuen. [Google Scholar]
- 7.Ruxton GD, Sherratt TN, Speed MP. 2004. Avoiding attack: the evolutionary ecology of crypsis, warning signals and mimicry, i p Oxford, UK: Oxford University Press. [Google Scholar]
- 8.Alatalo RV, Mappes J. 1996. Tracking the evolution of warning signals. Nature 382, 708–710. ( 10.1038/382708a0) [DOI] [Google Scholar]
- 9.Guilford T. 1990. The secrets of aposematism: unlearned responses to specific colors and patterns. Trends Ecol. Evol. 5, 323 ( 10.1016/0169-5347(90)90177-f) [DOI] [Google Scholar]
- 10.Mappes J, Marples N, Endler JA. 2005. The complex business of survival by aposematism. Trends Ecol. Evol. 20, 598–603. ( 10.1016/j.tree.2005.07.011) [DOI] [PubMed] [Google Scholar]
- 11.Skelhorn J, Halpin CG, Rowe C. 2016. Learning about aposematic prey. Behav. Ecol. 27, 955–964. ( 10.1093/beheco/arw009) [DOI] [Google Scholar]
- 12.Bowers MD. 1992. The evolution of unpalatability and the cost of chemical defense in insects. In Insect chemical ecology. An evolutionary approach (eds Roitberg BD, Isman MB), pp. 216–244. London, UK: Chapman & Hall. [Google Scholar]
- 13.Maan ME, Cummings ME. 2012. Poison frog colors are honest signals of toxicity, particularly for bird predators. Am. Nat. 179, E1–E14. ( 10.1086/663197) [DOI] [PubMed] [Google Scholar]
- 14.Pasteels JM, Gregoire JC, Rowellrahier M. 1983. The chemical ecology of defense in arthropods. Annu. Rev. Entomol. 28, 263–289. ( 10.1146/annurev.en.28.010183.001403) [DOI] [Google Scholar]
- 15.Pentzold S, Zagrobelny M, Khakimov B, Engelsen SB, Clausen H, Petersen BL, Borch J, Møller BL, Bak S. 2016. Lepidopteran defence droplets: a composite physical and chemical weapon against potential predators. Sci. Rep. 6, 22407 ( 10.1038/srep22407) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ritland DB. 1994. Variation in palatability of queen butterflies (Danaus gilippus) and implications regarding mimicry. Ecology 75, 732–746. ( 10.2307/1941731) [DOI] [Google Scholar]
- 17.Rothschild M, Aplin RT, Cockrum PA, Edgar JA, Fairweather P, Lees R. 1979. Pyrrolizidine alkaloids in arctiid moths (Lep.) with a discussion on host plant relationships and the role of these secondary plant substances in the Arctiidae. Biol. J. Linn. Soc. 12, 305–326. ( 10.1111/j.1095-8312.1979.tb00062.x) [DOI] [Google Scholar]
- 18.Trigo JR. 2000. The chemistry of antipredator defense by secondary compounds in neotropical Lepidoptera: facts, perspectives and caveats. J. Brazil. Chem. Soc. 11, 551–561. ( 10.1590/S0103-50532000000600002) [DOI] [Google Scholar]
- 19.Triponez Y, Naisbit RE, Jean-Denis JB, Rahier M, Alvarez N. 2007. Genetic and environmental sources of variation in the autogenous chemical defense of a leaf beetle. J. Chem. Ecol. 33, 2011–2024. ( 10.1007/s10886-007-9351-9) [DOI] [PubMed] [Google Scholar]
- 20.Weller SJ, Jacobson NL, Conner WE. 1999. The evolution of chemical defences and mating systems in tiger moths (Lepidoptera: Arctiidae). Biol. J. Linn. Soc. 68, 557–578. ( 10.1111/j.1095-8312.1999.tb01188.x) [DOI] [Google Scholar]
- 21.Speed MP, Ruxton GD, Mappes J, Sherratt TN. 2012. Why are defensive toxins so variable? An evolutionary perspective. Biol. Rev. 87, 874–884. ( 10.1111/j.1469-185X.2012.00228.x) [DOI] [PubMed] [Google Scholar]
- 22.Reudler JH, Lindstedt C, Pakkanen H, Lehtinen I, Mappes J. 2015. Costs and benefits of plant allelochemicals in herbivore diet in a multi enemy world. Oecologia 179, 1147–1158. ( 10.1007/s00442-015-3425-0) [DOI] [PubMed] [Google Scholar]
- 23.Skelhorn J, Ruxton GD. 2008. Ecological factors influencing the evolution of insects’ chemical defenses. Behav. Ecol. 19, 146–153. ( 10.1093/beheco/arm115%7CISSN%201045-2249) [DOI] [Google Scholar]
- 24.Zvereva EL, Kozlov MV. 2016. The costs and effectiveness of chemical defenses in herbivorous insects: a meta-analysis. Ecol. Monogr. 86, 107–124. ( 10.1890/15-0911.1) [DOI] [Google Scholar]
- 25.Rönkä K., Mappes J, Kaila L, Wahlberg N. 2016. Putting Parasemia in its phylogenetic place: a molecular analysis of the subtribe Arctiina (Lepidoptera). Syst. Entomol. 41, 844–853. ( 10.1111/syen.12194) [DOI] [Google Scholar]
- 26.Hegna RH, Galarza JA, Mappes J. 2015. Global phylogeography and geographical variation in warning coloration of the wood tiger moth (Parasemia plantaginis). J. Biogeogr. 42, 1469–1481. ( 10.1111/jbi.12513) [DOI] [Google Scholar]
- 27.Lindstedt C, Eager H, Ihalainen E, Kahilainen A, Stevens M, Mappes J. 2011. Direction and strength of selection by predators for the color of the aposematic wood tiger moth. Behav. Ecol. 22, 580–587. ( 10.1093/beheco/arr017) [DOI] [Google Scholar]
- 28.Nokelainen O, Hegna RH, Reudler JH, Lindstedt C, Mappes J. 2012. Trade-off between warning signal efficacy and mating success in the wood tiger moth. Proc. R. Soc. B 279, 257–265. ( 10.1098/rspb.2011.0880) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tammaru T, Haukioja E. 1996. Capital breeders and income breeders among Lepidoptera: consequences to population dynamics. Oikos 77, 561–564. ( 10.2307/3545946) [DOI] [Google Scholar]
- 30.Burdfield-Steel E, Pakkanen H, Rojas B, Galarza JA, Mappes J. Submitted. De novo synthesis of chemical defences in an aposematic moth. [DOI] [PMC free article] [PubMed]
- 31.Molleman F, Whitaker MR, Carey JR. 2010. Rating palatability of butterflies by measuring ant feeding behaviour. Entomol. Bericht. 70, 52–62. [Google Scholar]
- 32.Müller C, Boevé J.-L, Brakefield PM. 2002. Host plant derived feeding deterrence towards ants in the turnip sawfly Athalia rosae. Entomol. Exp. Appl. 104, 153–157. ( 10.1046/j.1570-7458.2002.01002.x) [DOI] [Google Scholar]
- 33.Nakagawa S, Schielzeth H. 2010. Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biol. Rev. 85, 935–956. ( 10.1111/j.1469-185X.2010.00141.x) [DOI] [PubMed] [Google Scholar]
- 34.RStudio. 2015. RStudio: Integrated development environment for R (version 0.99.441) [Computer software], Boston, MA. See http://www.rstudio.com.
- 35.Therneau TM. 2015. coxme: mixed effects Cox models. (2.2-5 edn). See http://cran.r-project.org/package=coxme.
- 36.Bates D, Maechler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 37.Rowe C, Guilford T. 1996. Hidden colour aversions in domestic clicks triggered by pyrazine odours of insect warning displays. Nature 383, 520–522. ( 10.1038/383520a0) [DOI] [Google Scholar]
- 38.Rothschild M, Moore BP, Brown WV. 1984. Pyrazines as warning odour components in the monarch butterfly, Danaus plexippus, and in moths of the genera Zygaena and Amata (Lepidoptera). Biol. J. Linn. Soc. 23, 375–380. ( 10.1111/j.1095-8312.1984.tb00153.x) [DOI] [Google Scholar]
- 39.Vencl FV, Srygley RB. 2013. Enemy targeting, trade-offs, and the evolutionary assembly of a tortoise beetle defense arsenal. Evol. Ecol. 27, 237–252. ( 10.1007/s10682-012-9603-1) [DOI] [Google Scholar]
- 40.Cogni R, Trigo JR, Futuyma DJ. 2012. A free lunch? No cost for acquiring defensive plant pyrrolizidine alkaloids in a specialist arctiid moth (Utetheisa ornatrix). Mol. Ecol. 21, 6152–6162. ( 10.1111/mec.12086) [DOI] [PubMed] [Google Scholar]
- 41.Moranz R, Brower LP. 1998. Geographic and temporal variation of cardenolide-based chemical defenses of queen butterfly (Danaus gilippus) in Northern Florida. J. Chem. Ecol. 24, 905–932. ( 10.1023/A:1022329702632) [DOI] [Google Scholar]
- 42.Rothschild M, Euw JV, Reichstein T. 1973. Cardiac glycosides (heart poisons) in the polka-dot moth Syntomeida Epilais Walk. (Ctenuchidae: Lep.) with some observations on the toxic qualities of Amata (=Syntomis) phegea (L.). Proc. R. Soc. Lond. B 183, 227–247. ( 10.1098/rspb.1973.0015) [DOI] [PubMed] [Google Scholar]
- 43.Hartmann T, Theuring C, Beuerle T, Bernays EA, Singer MS. 2005. Acquisition, transformation and maintenance of plant pyrrolizidine alkaloids by the polyphagous arctiid Grammia geneura. Insect Biochem. Mol. Biol. 35, 1083–1099. ( 10.1016/j.ibmb.2005.05.011) [DOI] [PubMed] [Google Scholar]
- 44.Hartmann T, Theuring C, Beuerle T, Ernst L, Singer MS, Bernays EA. 2004. Acquired and partially de novo synthesized pyrrolizidine alkaloids in two polyphagous arctiids and the alkaloid profiles of their larval food-plants. J. Chem. Ecol. 30, 229–254. ( 10.1023/B:JOEC.0000017975.16399.c3) [DOI] [PubMed] [Google Scholar]
- 45.von Nickisch-Rosenegk E, Wink M. 1993. Sequestration of pyrrolizidine alkaloids in several arctiid moths (Lepidoptera, Arctiidae). J. Chem. Ecol. 19, 1889–1903. ( 10.1007/BF00983794) [DOI] [PubMed] [Google Scholar]
- 46.Roque-Albelo L, Schroeder FC, Conner WE, Bezzerides A, Hoebeke ER, Meinwald J, Eisner T. 2002. Chemical defense and aposematism: the case of Utetheisa galapagensis. Chemoecology 12, 153–157. ( 10.1007/s00012-002-8341-6) [DOI] [Google Scholar]
- 47.Molleman F, Kaasik A, Whitaker MR, Carey JR. 2012. Partitioning variation in duration of ant feeding bouts can offer insights into the palatability of insects: experiments on African fruit-feeding butterflies. J. Res. Lepidopt. 45, 65–75. [Google Scholar]
- 48.Carrell JE. 2001. Response of predaceous arthropods to chemically defended larvae of the pyralid moth Uresiphita reversalis (Guenée) (Lepidoptera: Pyralidae). J. Kansas Entomol. Soc. 74, 128–135. [Google Scholar]
- 49.Hristov N, Conner WE. 2005. Effectiveness of tiger moth (Lepidoptera, Arctiidae) chemical defenses against an insectivorous bat (Eptesicus fuscus). Chemoecology 15, 105–113. ( 10.1007/s00049-005-0301-0) [DOI] [Google Scholar]
- 50.Brower LP, Ryerson WN, Coppinger LL, Glazier SC. 1968. Ecological chemistry and the palatability spectrum. Science 161, 1349–1350. ( 10.1126/science.161.3848.1349) [DOI] [PubMed] [Google Scholar]
- 51.Cardoso MZ. 1997. Testing chemical defence based on pyrrolizidine alkaloids. Anim. Behav. 54, 985–991. ( 10.1006/anbe.1997.0505) [DOI] [PubMed] [Google Scholar]
- 52.Massuda K, Trigo J. 2009. Chemical defence of the warningly coloured caterpillars of Methona themisto (Lepidoptera: Nymphalidae: Ithomiinae). Eur. J. Entomol. 106, 253–259. ( 10.14411/eje.2009.033) [DOI] [Google Scholar]
- 53.Blüthgen N, Fiedler K. 2004. Preferences for sugars and amino acids and their conditionality in a diverse nectar-feeding ant community. J. Anim. Ecol. 73, 155–166. ( 10.1111/j.1365-2656.2004.00789.x) [DOI] [Google Scholar]
- 54.Ratcliffe JM, Nydam ML. 2008. Multimodal warning signals for a multiple predator world. Nature 455, 96 ( 10.1038/nature07087) [DOI] [PubMed] [Google Scholar]
- 55.Moore BP, Brown WV, Rothschild M. 1990. Methylalkylpyrazines in aposematic insects, their hostplants and mimics. Chemoecology 1, 43–51. ( 10.1007/bf01325227) [DOI] [Google Scholar]
- 56.Guilford T, Nicol C, Rothschild M, Moore BP. 1987. The biological roles of pyrazines: evidence for a warning odour function. Biol. J. Linn. Soc. 31, 113–128. ( 10.1111/j.1095-8312.1987.tb01984.x) [DOI] [Google Scholar]
- 57.Lindström L, Rowe C, Guilford T. 2001. Pyrazine odour makes visually conspicuous prey aversive. Proc. R. Soc. Lond. B 268, 159–162. ( 10.1098/rspb.2000.1344) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roper TJ, Marples NM. 1997. Odour and colour as cues for taste-avoidance learning in domestic chicks. Anim. Behav. 53, 1241–1250. ( 10.1006/anbe.1996.0384) [DOI] [PubMed] [Google Scholar]
- 59.Vencl FV, Ottens K, Dixon MM, Candler S, Bernal XE, Estrada C, Page RA. 2016. Pyrazine emission by a tropical firefly: an example of chemical aposematism? Biotropica 48, 645–655. ( 10.1111/btp.12336) [DOI] [Google Scholar]
- 60.Nokelainen O, Valkonen J, Lindstedt C, Mappes J. 2014. Changes in predator community structure shifts the efficacy of two warning signals in Arctiid moths. J. Anim. Ecol. 83, 598–605. ( 10.1111/1365-2656.12169) [DOI] [PubMed] [Google Scholar]
- 61.Gordon SP, Kokko H, Rojas B, Nokelainen O, Mappes J. 2015. Colour polymorphism torn apart by opposing positive frequency-dependent selection, yet maintained in space. J. Anim. Ecol. 84, 1555–1564. ( 10.1111/1365-2656.12416) [DOI] [PubMed] [Google Scholar]
- 62.Galarza JA, Nokelainen O, Ashrafi R, Hegna RH, Mappes J. 2014. Temporal relationship between genetic and warning signal variation in the aposematic wood tiger moth (Parasemia plantaginis). Mol. Ecol. 23, 4939–4957. ( 10.1111/mec.12913) [DOI] [PubMed] [Google Scholar]
- 63.Partan S, Marler P. 1999. Communication goes multimodal. Science 283, 1272–1273. ( 10.1126/science.283.5406.1272) [DOI] [PubMed] [Google Scholar]
- 64.Rowland HM, Ihalainen E, Lindström L, Mappes J, Speed MP. 2007. Co-mimics have a mutualistic relationship despite unequal defences. Nature 448, 64–67. ( 10.1038/nature05899) [DOI] [PubMed] [Google Scholar]
- 65.Pekár S, Petráková L, Bulbert MW, Whiting MJ, Herberstein ME. 2017. The golden mimicry complex uses a wide spectrum of defence to deter a community of predators. Elife 6, e22089 ( 10.7554/eLife.22089) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets have been uploaded as part of the electronic supplementary material.