Abstract
We have previously shown that male mice living in groups of 12 males establish and maintain stable linear social hierarchies with each individual having a defined social rank. However, it is not clear which social cues mice use to signal and recognize their relative social status within their hierarchy. In this study, we investigate how individual social status both in pairs and in groups affects the levels of major urinary proteins (MUPs) and specifically MUP20 in urine. We housed groups of adult outbred CD1 male mice in a complex social environment for three weeks and collected urine samples from all individuals repeatedly. We found that dominant males produce more MUPs than subordinates when housed in pairs and that the production of MUPs and MUP20 is significantly higher in alpha males compared with all other individuals in a social hierarchy. Furthermore, we found that hepatic mRNA expression of Mup3 and Mup20 is significantly higher in alpha males than in subordinate males. We also show that alpha males have lower urinary creatinine levels consistent with these males urinating more than others living in hierarchies. These differences emerged within one week of animals being housed together in social hierarchies. This study demonstrates that as males transition to become alpha males, they undergo physiological changes that contribute to communication of their social status that may have implications for the energetic demands of maintaining dominance.
Keywords: social dominance, social hierarchy, major urinary protein, creatinine, MUP20
1. Introduction
In their natural ecology, the ancestral subspecies of laboratory mice (Mus musculus, Mus domesticus, Mus castaneus and Mus moloisha) live in large social groups organized into dominance hierarchies [1]. Mus species are characterized by a high reproductive skew with elevated levels of inter-male competition leading to the formation of territories [2,3]. Wild and laboratory male mice mark territories and advertise their quality to females by depositing urine throughout their environment [4–8]. Dominant males respond to these urine scent marks by countermarking over them with their own urine marks [5,6,8].
We have recently shown that groups of up to 30 outbred CD1 male mice living in complex housing vivaria form highly linear social hierarchies in the laboratory [9–14]. Within 4–5 days of group, housing all animals can be ranked based on their relative wins and losses against all other individuals. We have shown that after 5 days of co-housing, every male is highly directionally consistent in their behaviour, only exhibiting aggressive behaviour towards animals of relatively lower social status and consistently yielding when approached by animals of relatively higher social status. We have also shown that animals of lower ranks respond to changes in social context, recognizing when alpha males are inactive or absent from the hierarchy and consequently increase their aggressive behaviour [12,13]. In this group-housing system, the most dominant male in a group patrols the entire housing system and monopolizes the food and water areas and a few nest-boxes. Other individuals spend the majority of their time in other nest-boxes that the alpha male favours relatively less. We also have shown that non-alpha males avoid their aggressive behaviours while the alpha male is actively patrolling [12]. These behavioural characteristics are consistent with previous findings from other research groups using wild-caught or laboratory outbred mice, suggesting that those animals are capable of exhibiting appropriate social behaviours depending on different social contexts if given sufficient space and enrichment [2,15–18].
The dynamics of dominant and subordinate behaviour in social groups suggests that mice use cues to signal and perceive the ranks of other individuals within a social hierarchy. Although it is possible that individuals use a combination of visual, auditory and olfactory cues to signal relative social status, the majority of inter-male mouse social behaviour is mediated by chemosensory signals [5]. In particular, mice use both volatile and non-volatile components in urine to communicate social information. Previous studies have identified chemical differences in the volatile components of urine between dominant and subordinate males. For instance, the volatiles E,E-α-farnesene, E-β-farnesene and 2-sec-butyl-4,5-dihydrothiazole (thiazole) (SBT), 3,4-dehydro-exo-brevicomin (brevicomin) (DHB) and hexadecanol, and 1-hexadecanol acetate are higher in dominant compared with subordinate mice housed or repeatedly tested in pairs or triads [19–22].
Major urinary proteins (MUPs) constitute the majority of the non-volatile component of urine. The production of these lipocalin proteins is testosterone and growth hormone-dependent, occurring in the liver with subsequent transport via kidney into urine [5], tears, saliva and other secretions [23]. MUPs are detected by vomeronasal sensory neurons within the olfactory system [24], are elevated in males compared with females and are found at higher levels in urine following social interaction [25]. Furthermore, MUPs can also show high affinity to and bind with small volatile molecules such as E,E-α-farnesene, E-β-farnesene, SBT, 6-hydroxy-6-methyl-3-heptanone (HMH) and DHB [26–29]. MUPs have been shown to induce behavioural changes in individuals who detect these proteins including promoting female mate preference, social learning and lactational aggression [30–32]. Furthermore, total MUPs and specifically MUP3 and MUP20 have been shown to invoke inter-male aggression [24,33] and to facilitate self-recognition of own urine marks in dominant males [33]. MUP20 levels have been shown to be higher in dominant compared to subordinate inbred mice housed individually and tested daily in pairs [34] as well as in dominant outbred males housed in groups [35]. In wild mice, total MUP levels have also been shown to be higher in dominant male mice exposed to each other for 10 days on either side of a barrier [36] and higher in breeding males with territories compared with breeding males without territories or singly housed males [37]. It is unclear whether MUP levels prior to social interaction predict future dominance as pretest MUP levels have been reported to be both associated and not associated with future dominance status in pairs and small social groups [34,35].
In this study, we address critical questions regarding the association between MUP levels and social status and the predictive role of this biological marker in future dominance status: (i) are urinary levels of total MUPs and MUP20 associated with relative social dominance within dyads and a social hierarchy? (ii) Can future social rank in a social hierarchy be predicted by total urinary MUPs and MUP20 levels prior to social group formation and (iii) is social rank associated with individual markers of Mup mRNA expression and production in the liver?
2. Material and methods
(a). Animals and housing
A total of 72 male outbred CD1 mice aged seven weeks were purchased from Charles River Laboratories (Wilmington, MA, USA) and housed in pairs for 11–15 days in standard sized cages (27 × 17 × 12 cm) with pine shaving bedding. A total of 35 pairs were used as one pair was excluded. We assigned unique IDs to individuals and marked them accordingly by dying their fur with blue, non-toxic markers (Stoelting Co., Wood Dale, IL, USA). Following this, subjects were housed in social groups of 11–12 males with unfamiliar individuals for 20–21 days (N = 6 groups) (see electronic supplementary material for more information). We weighed and placed mice in large custom-built mouse vivaria (electronic supplementary material, figure S1; 150 × 80 cm and 220 cm high; Mid-Atlantic, Hagerstown, MD, USA). Mice were introduced to group housing sequentially, and the order was randomly assigned. All mice were introduced to each vivarium within 10 min. The vivarium was constructed as previously described [10,11]. Briefly, each vivarium was comprised an upper level with multiple shelves (36000 cm2 = 3 floor × 150 cm × 80 cm) and a lower level with five nest-boxes (2295 cm2 = 5 cages × 27 cm × 17 cm) connected by tubes, consisting of total surface of approximately 62 295 cm2, providing approximately 5191.25 cm2 per mouse. All surfaces of the vivarium were covered with pine shaving bedding. Standard chow and water were provided ad libitum at the top of the vivarium to encourage movement between of all the shelves. Mice could access each level of the vivarium via a system of ramps and tunnels. We monitored if any individual exhibited a sign of pain or injury every day during the pair and group-housing periods. As we expected heightened aggression on the first day of group housing because they engage in aggressive interactions to establish social hierarchy, we monitored animals more frequently on group-housing day (GD) 1/2. There was no animal that had a severe wound and we did not need to remove any individual from the experiment because of injury. All experiments were conducted with approval from the Columbia University Institutional Animal Care and Use Committee (IACUC protocol: AC-AAAP5405).
(b). Urine collection and analysis
We collected urine from all individuals during pair housing (three times, PD = pair-housing day; PD2/7/15) and group housing (GD 1/7/9/11/13/15/17/19). Following collection, urine samples were subjected to a Bradford assay for total MUP concentration, creatinine assay for creatinine concentration and SDS-PAGE for relative MUP20 concentration (see the electronic supplementary material). Previous studies [30,34,35] have demonstrated that MUP20 bands could be separated from other proteins in urine and we were able to replicate and separate MUP20 bands. Total urinary MUP and MUP20 levels presented are corrected with urine dilution (creatinine levels) and represent the total amount of protein each individual produced on each day of urine collection. Urinary protein concentration values refer to uncorrected data and represent the amount of protein per unit volume of urine that each male produces. Following observations on GD19 animals were sacrificed, and livers were extracted and stored at −80°C until gene expression analysis via quantitative real-time PCR.
(c). Statistical analysis
All statistical analyses were undertaken in R v. 3.3.1 [38]. Using win–loss data from each group, we determined the hierarchical organization of each group by calculating Landau's linearity index (h′), the directional consistency index (DCI) and triangle transitivity (ttri), and the ranks of individual animals using the I & SI and Glicko rating methods. The relationship between pair-housing social status and final rank and wins and losses during group housing was tested using randomization tests and generalized linear mixed models (GLMMs). Protein, creatinine and gene expression levels were analysed using GLMMs and linear mixed models (LMMs) with the lme4, lmerTest, glmmADMB and MASS R packages [39–42]. Main and interaction effects of day and social status (pair-housing status, final I & SI rank or group-housing status as appropriate) were tested for all models, except for models with only main effects of social status. Random variables included were the pair-ID or group-ID of each subject. Only significant interaction effects are reported. All models were tested for whether random slopes improved fit and included them where appropriate. GLMMs used were for data with gamma distribution and GLMMPQL was used for data with lognormal distribution after considering the distribution of both data and residuals from fitted models. We used Akaike (AIC) and Schwarz (BIC) information criteria to test the fit of every model as well as to test the distribution of dependent variables and residuals. All significance values from multiple hypothesis tests were corrected to control for false discovery rate (FDR) using a Benjamini–Hochberg method [43].
3. Results
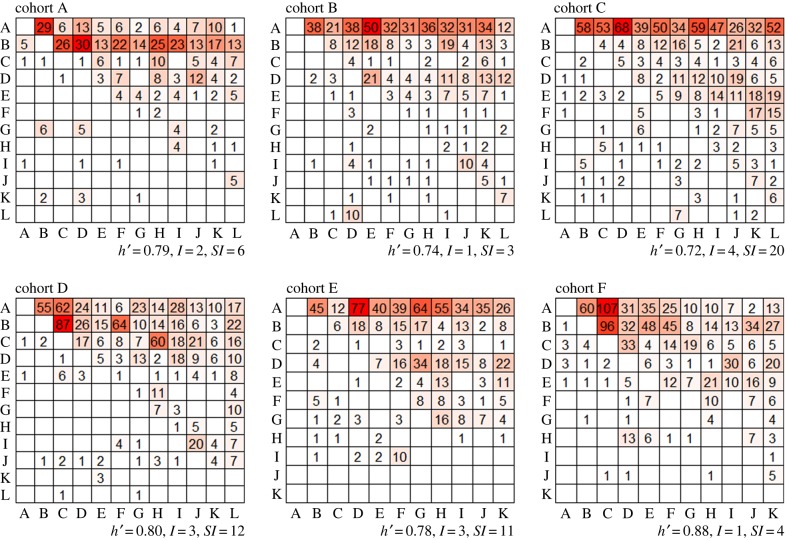
(a). Behaviour in dyads and social groups
All animals in pairs (N = 35 dyads) could be identified as dominant or subordinate based on relative wins and losses when observed between PD13 and PD15. In social groups, the total number of agonistic interactions recorded ranged from 475 to 1158 across the six cohorts with a mean of 849 contests recorded per social group. The frequency of wins and losses between each animal is shown in sociomatrices in figure 1. All social groups resulted in a significantly linear social dominance hierarchy (h′ = 0.78 ± 0.06, mean ± s.e.; all p < 0.001) with significantly high directional consistency of aggressive behaviour (DCI = 0.90 ± 0.02, all p < 0.001) and triangle transitivity (ttri = 0.84 ± 0.09, range 0.69–0.93, all p < 0.001). Body weight prior to vivarium housing, at the end of vivarium housing and the difference in body weight between these two time points was not significantly related to dominance rank in any social group (Spearman's rank correlation tests, controlled for FDR, all p > 0.76). All cohorts had significant h′ values and ttri by the end of GD5 (electronic supplementary material, figure S2). Animals that were dominant in pairs were not significantly more likely to have higher final social dominance ranks within each hierarchy than those animals characterized as subordinate in pairs (electronic supplementary material, figure S3; randomization tests, all p > 0.11). Congruently, dominance status in pair housing was not significantly associated with total wins or total losses during group housing (wins: p = 0.130; losses: p = 0.760). However, animals that were dominant during pair housing engaged in significantly more aggressive interactions (electronic supplementary material, figure S4; sum of wins and losses: b = −0.65 ± 0.19, n = 70, p < 0.001; wins only: b = −0.96 ± 0.41, n = 70, p = 0.020; losses only: b = −0.28 ± 0.15, n = 70, p = 0.069) during the first 2 days of group housing (GD1/2) than males which were subordinate in pairs.
Figure 1.
Win–loss sociomatrices showing total wins and losses by each individual in group housing. Cells are colour-indexed from white (no wins) to darker colours (highest number of wins). Winners of each contest are listed in rows and losers are listed in columns according to I & SI rank order. All six cohorts formed a significantly linear social dominance hierarchy. Empty cells indicate that the frequency of aggressive interactions was 0. (Online version in colour.)
(b). Total major urinary protein levels and social status
Dominant males had significantly higher total MUP levels during pair-housing than subordinate males (b = −2.60 ± 0.95, n = 201, p = 0.010; electronic supplementary material, figure S5). Dominant males had significantly higher levels of MUPs at PD2 (Wilcoxon's signed-rank test, V = 406, p = 0.003) and PD15 (V = 403, p = 0.004) compared with subordinate pair-housed males. No significant difference was detected at PD7 (p = 0.237). The total MUP levels of all animals regardless of pair-housing social status dropped significantly from PD15 to GD1 when placed into the novel vivarium with novel conspecifics (electronic supplementary material, figure S6; b = −3.54 ± 0.47, n = 137, p < 0.001). Protein concentration was not associated with pair-housing social status (p = 0.533). MUP concentrations on PD15 and GD1 were not significantly different from each (p = 0.840). Each individual's total MUP levels during pair-housing at PD7 (b = −0.24 ± 0.07, n = 67, p = 0.001) and PD15 (b = −0.19 ± 0.06, n = 67, p = 0.004) were significantly associated with a higher final dominance rank in social hierarchies (electronic supplementary material, figure S7). Total MUP levels at PD2 were marginally associated with final rank (b = −0.15 ± 0.08, n = 67, p = 0.070). Protein concentration at each PD was not related to final social rank in a hierarchy (all p > 0.27).
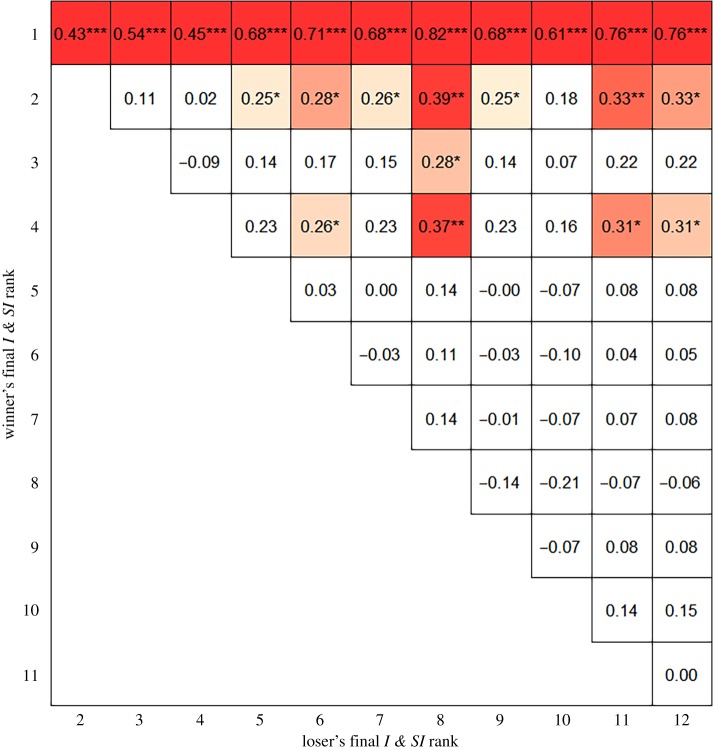
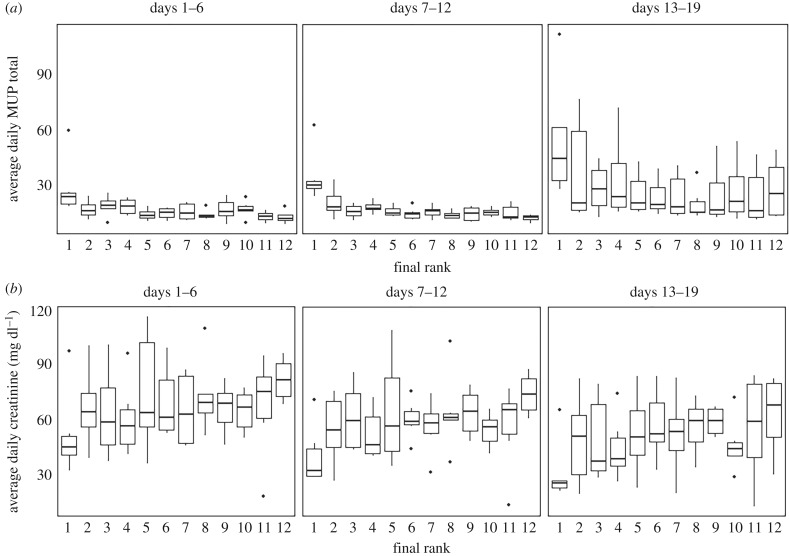
In social hierarchies, we found a significant effect of rank with more dominant males having higher total MUP levels than subordinate males (figures 2 and 3a; b = −0.048 ± 0.012, n = 925, p < 0.001). There was also a significant effect of day (figure 3a; b = 0.032 ± 0.01, n = 925, p = 0.005) with MUP levels increasing over days. Alpha males (rank 1) had significantly more MUPs than animals of all other ranks (figure 2; all p < 0.001). Subdominant males of ranks 2, 3 and 4 all had similar levels of MUPs to each other being generally higher overall than animals ranked 5–12 who also had similar total MUP levels to each other. We also calculated the day on which each alpha male had consistently the highest MUP levels in their hierarchy. This occurred on GD1 in two cohorts (B&C), GD3 in cohort F, GD4 in cohorts A&D and GD8 in cohort E (electronic supplementary material, table S1). The time taken for alpha males to have the consistently highest MUPs was not significantly correlated with the time taken for alpha males to behaviourally emerge. There was no effect of day or rank on urinary protein concentration (all p > 0.05).
Figure 2.
β coefficients for pairwise comparison for rank comparisons from GLMM for differences in total MUP levels. Positive values indicate ranks in rows having higher MUP levels than individuals in columns. β coefficients in coloured squares are statistically significant and darker colour indicates lower p-value (*p < 0.05; *p < 0.01; ***p < 0.001). Standard errors of all β coefficients were ranged between 0.09 and 0.10. (Online version in colour.)
Figure 3.
Average daily levels of (a) total MUPs and (b) creatinine by social rank over three weeks of group housing. Data are boxplots showing median (horizontal bars), IQR (boxes) and 95% CI (whiskers).
(c). Urinary creatinine levels and social status
Dominant and subordinate males housed in pairs did not differ in creatinine levels (electronic supplementary material, figure S8). All individuals increased their creatinine levels from PD15 to GD1/2, indicating that they decreased their urination as they were put in a novel group-housing environment.
In social hierarchies, we found that creatinine levels significantly decreased over days (figure 3b, b = −1.10 ± 0.22 mg dl−1, n = 925, p < 0.001). We also found that more dominant animals had significantly lower creatinine levels than subordinate males (b = 1.17 ± 0.54 mg dl−1, p = 0.030). Based on our behavioural observations, we further tested the creatinine level differences among three social status groups: alpha (rank 1, the highest positive David's score), subdominant (other males with positive David's score) and subordinate groups (males with negative David's score) (see electronic supplementary material, table S2). Alpha males had significantly lower creatinine levels compared with both subdominants (b = 16.91 ± 7.10 mg dl−1, n = 925, p = 0.026) and subordinates (b = 19.03 ± 6.83 mg dl−1, n = 925, p = 0.016). Creatinine levels of subdominants did not differ from those of subordinates (p = 0.583). Initial or final body weight was not significantly related to creatinine levels. There was no significant effect of the degree of received aggression on creatinine levels (electronic supplementary material, figure S9; b = −0.14 ± 0.15, p = 0.371).
(d). Urinary MUP20 levels and social status
In pair housing, dominant males had significantly higher MUP20 levels than subordinates (electronic supplementary material, figure S11). All individuals significantly increased MUP20 production from PD2 to PD15 (b = 0.91 ± 0.08, n = 127, p < 0.001). MUP20 concentration was not different between dominant and subordinate males (p = 0.107), although MUP20 concentration increased from PD2 to PD15 for all animals (electronic supplementary material, table S4; b = 0.50 ± 0.06, n = 128, p < 0.001). We also found a significant interaction effect between day and social status for both MUP20 production (b = −0.50 ± 0.11, n = 127, p < 0.001) and concentration (b = −0.25 ± 0.09, p = 0.009) during pair housing, indicating that dominant individuals increased their MUP20 production levels and concentration more than subordinates from PD2 to PD15. When animals transitioned from pair housing (PD15) to group housing (GD1/2), there was no change in total MUP20 levels (p = 0.303), but MUP20 concentration in urine did significantly increase (b = 0.15 ± 0.05, n = 135, p = 0.002). Final social rank in social hierarchies was not predicted by either of MUP20 production or MUP20 concentration measured from urine collected during pair-housing period (PD2 and PD15) (all p > 0.21).
In social hierarchies, there was a significant effect of final social rank on MUP20 production, indicating that more dominant males express MUP20 levels during group housing (b = −0.04 ± 0.02, n = 198, p = 0.039). Using social status group and day as predictors, MUP20 production levels of alpha males were significantly higher than those of subdominants (figure 4; b = −0.86 ± 0.16, n = 198, p < 0.001) and subordinates (b = −0.96 ± 0.14, n = 198, p < 0.001), but subdominants were not significantly different from subordinates in MUP20 production levels (p = 0.56). All individuals in social hierarchies increased their MUP20 production throughout group-housing period. Animals on GD15/17 had significantly higher MUP20 levels than those on GD7/9 (b = 0.74 ± 0.08, n = 198, p < 0.001) and GD1/2 (b = 1.59 ± 0.16, n = 198, p < 0.001), and significantly higher levels on GD7/9 compared with those on GD1/2 (b = 0.85 ± 0.17, n = 198, p < 0.001). Alpha males increased MUP20 production from GD1/2 to GD15 significantly more compared with both subdominants (interaction effects of day and social status: b = −0.35 ± 0.16, n = 198, p = 0.047) and subordinates (b = −0.50 ± 0.13, n = 198, p = 0.0006). Congruently, there was no difference in MUP20 production on GD1/2 between the alpha group and the other groups (all p > 0.72). MUP20 concentration also increased during the first week of group housing (electronic supplementary material, table S4; GD1/2 to GD7/9: b = 0.35 ± 0.07, n = 202, p < 0.001; GD1/2 to GD15/17: b = 0.39 ± 0.07, n = 202, p < 0.001) but not thereafter (GD7/9 to GD15/17: p = 0.491). MUP20 concentration was not associated with final social rank (p = 0.893).
Figure 4.
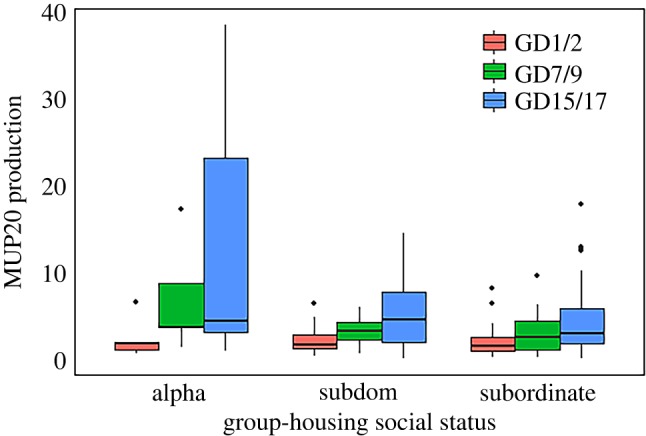
Daily levels of MUP20 by social status over three weeks of group housing. Data are boxplots showing median (horizontal bars), IQR (boxes) and 95% CI (whiskers). (Online version in colour.)
(e). Social status and hepatic mRNA levels of Ar, Mup3 and Mup20
Alpha males had a significantly higher level of hepatic Mup3 expression than subdominant (b = −0.32 ± 0.14, n = 43, p = 0.023) and subordinate (b = −0.44 ± 0.14, p = 0.002) males (electronic supplementary material, figure S12). Alpha males differed in Mup20 expression marginally compared with subdominant (b = −0.57 ± 0.26, n = 44, p = 0.053) and significantly compared with subordinate males (b = −0.65 ± 0.25, p = 0.044). There were no differences between subdominants and subordinates in the relative expression of either gene (all p > 0.21). There were no significant differences between animals of different social statuses in Ar mRNA expression (all p > 0.82).
4. Discussion
In this study, we show that in pair-housed animals, dominant males produce higher levels of MUPs and MUP20 in urine than subordinate males and that in social hierarchies, alpha males produce far higher levels of total MUPs and MUP20 than all other animals, with subdominant males also excreting slightly more MUPs than subordinate mice. Furthermore, alpha males have significantly lower creatinine levels in urine, indicating that they excrete a higher volume of urine per day than other males. We also find that alpha males express a higher level of Mup3 and Mup20 mRNA in the liver, indicating a higher overall production of these proteins.
Our finding that dominant males have higher levels of total MUPs and MUP20 than subordinate males in pair-housed dyads is consistent with previous studies conducted with inbred and wild-derived M. musculus [34–36]. Guo et al. [34] found higher levels of MUPs and MUP20 in the urine of dominant-inbred C57BL/6 males housed in isolation and given 10 min of social interaction with the same individual for 21 consecutive days. This difference in MUPs was observed on day 21 but not on day 1. Janotova & Stopka [36] found higher levels of MUPs in dominant wild-derived mice compared with subordinate males housed together for 1 day following 10 days of social isolation, suggesting that differences in MUP levels can emerge rapidly following resolution of social status. Similarly, we found that differences in total MUP levels between dominant and subordinate mice were apparent as early as PD2—the earliest time-point we assessed.
The social context of a social hierarchy is dramatically different from that of dyads. In a dyad, animals that are dominant will rarely exhibit subordinate behaviours and animals that are subordinate will rarely exhibit aggressive behaviour. Conversely, in a social hierarchy with multiple relationships, animals have to flexibly switch between aggressive and subordinate behaviours with only the alpha male rarely showing any subordinate behaviours. Over 21 days of living in a social group, we found that total MUP and MUP20 production were dramatically higher in alpha males compared with all other animals. Subdominant animals (ranks 2–4) who exhibit more aggressive behaviour than subordinate behaviour on average but still receive significant aggression from animals ranked higher than themselves had much lower MUP levels than alpha males but significantly higher levels than subordinate animals (ranks 5–12). These findings extend those of Nelson et al. [35] who found higher levels of MUPs and MUP20 in the urine of dominant male versus a subordinate individual in groups of four wild-derived mice after 3 days of group housing. Interestingly, the levels of MUPs we observed for alpha males are higher than those previously reported for animals housed in pairs or small groups [34,35], but equivalent to those found in breeding males with territories [37].
Consistent with these data, we found that alpha males had higher levels of Mup20 mRNA levels and Mup3 mRNA levels in the liver than the subordinate group. However, there were only marginally significant differences in Mup20 expression between the alpha and the subdominant groups. It is possible that these marginal differences in mRNA levels are manifested in significant differences in urinary protein levels as the proteins are accumulated in the bladder over time. This further strengthens our finding, suggesting that the elevated total MUP and MUP20 levels are not a by-product of lowered creatinine levels measured in urine. Mup gene expression in the liver is upregulated by both growth hormones and testosterone with the final protein products being transported to urine for excretion [44–47]. Indeed, castrated males have significantly lower Mup expression in the liver and lower levels of MUPs in urine [34,48]. Interestingly, we did not find any differences between animals of different social rank and Ar mRNA expression similar to Guo et al. [34]. This does not necessarily preclude the possibility that differences in endogenous testosterone may facilitate the upregulation of Mup genes in alpha males, but we believe that this is unlikely given the inconsistent relationships between endogenous testosterone and social rank observed in mice and rats [13,14,49]. It is possible that the release of growth hormone-releasing hormone (GHRH) is increased in the anterior pituitary while an individual establishes its alpha status in a group. GHRH is inhibited by another neuropeptide somatostatin, and the decrease in somatostatin has been shown to correspond with the social ascent of an individual in a group [50] and increased aggressive behaviour [51]. However, it is not clear yet which brain regions are responsible to activate the somatostatin neurons in response to the perceived social information.
An important outstanding question is whether observed differences in MUP levels are the result of differences in social status or are pre-existing to social relationship resolution. We argue that individual differences in MUP levels are likely a proxy for individual differences in fitness that modulate an animal's ability to rise up a social hierarchy, but that this not the ultimate determinant of social rank. Consistent with this hypothesis, levels of total MUPs at the end of pair housing were associated with individuals reaching higher social ranks in the hierarchy overall, but individuals who became alpha males were not necessarily those who had the absolute highest pair-housing MUP levels. As producing and excreting protein in urine costs vast metabolic resources [18,25,37,52–55], we argue that those animals in pair housing that are able to produce higher amounts of MUPs are likely those with a higher metabolic capacity and consequently those better able to ascend a hierarchy. It is well established that those animals that reach the top position in a hierarchy are not necessarily those that have the absolute highest individual fitness, but are those individuals that are beneficiaries of the unique social dynamics such as winner and loser effects within each social hierarchy [56,57]. Indeed, we observed that social status in pair housing affected initial behaviour in the vivarium (dominant pair-housed animals engaged in more aggressive behaviour in the first 2 days), but was not related to final social rank—consistent with previous work by Williamson et al. [9] and Buwalda et al. [49] showing that social behaviour prior to group housing predicts only initial behaviour and not final rank in the social hierarchy.
Once alpha males have established their position in social hierarchies, they show a number of behavioural and physiological transitions. In particular, alpha males increase their aggression towards other mice and engage in elevated patrolling behaviour [10]. Alpha males also increase their scent marking [5,6]. Our finding of lower levels of creatinine in the urine of alpha males compared with others males is possibly a by-product of alpha males urinating more frequently to mark their territory. Importantly, this dramatic reduction in urinary creatinine is not related to differences in body weight between animals. Notably, we only found the effect of social status on urinary creatinine levels in groups but not in pairs, which may be related to the increased space and number of animals and potentially increased number of urine marks that need to be countermarked over by alpha males living in large groups. Previously, it was reported that more subordinate wild mice have a higher concentration of creatinine than dominant mice when pair-housed for 24 h consistent with these animals producing a lower daily urine volume [58]. Conversely, Nelson et al. [35] did not see a difference in urinary creatinine levels between dominant and non-dominant males. Our results suggest that mice living in large complex social environments are able to change daily production of urine flexibly in response to a given social environment.
We found two major notable dynamic changes in MUP levels across the experiment. First, immediately after being placed into group housing following pair housing, all animals showed a dramatic decline in total MUP production on the first day of being housed with previously unknown individuals. This is related to an increase in creatinine levels detected in the urine of all animals, indicating a decrease in the total amount of urine produced. It is likely that being placed into a new physical and social environment and establishing a new social hierarchy are stressful to every individual in the group. It has previously been shown that social stress leads to increased expression of corticotrophin-releasing factor (CRF) in Barrington's nucleus (BN), leading to inhibition of urination in socially defeated rats and mice [8,59]. However, it is yet to be concluded whether the higher CRF level in BN corresponds to changes in the stress response mediated by the hypothalamic–pituitary–adrenal axis. Hou et al. [8] showed that GABAergic projection from the medial preoptic nucleus to BN is involved in urinary inhibition. Furthermore, many brain regions of the social decision-making network [60] such as the periaqueductal grey and bed nucleus of the stria terminalis also project directly to BN. Secondly, we observed an increase in total MUP production and a decrease in creatinine for all animals with increasing time being spent together in stable pairs or social groups. This suggests that although there are differences in the degree to which animals inhibit their urination and MUP production due to social status, individuals increase their urination volume as the social hierarchy is stabilized likely because overall levels of social stress are reduced in stable social groups [61].
A number of communicative functions have been ascribed to MUPs deposited in urine [54]. There is strong evidence that MUPs and MUP20, in particular, in male urine attract females and that females assess the quality of male scent marks in making mating decisions [30,62–64]. Subordinate males avoid scent marks of more dominant males [65,66] and indeed avoid urinating near to dominant male scent marks [7,67]. This behavioural strategy appears to reduce the likelihood of receiving aggression as castrated males daubed with MUP3 or MUP20 onto their fur become the targets of attacks from socially isolated intact males [33]. Conversely, other dominant males from neighbouring territories do not avoid scent marks, but will countermark over the scent marks of other males including those of neighbouring territories. A major role of MUPs is therefore to act as boundary markers between alpha male territories [5]. The much higher production of MUPs including MUP3 and MUP20 and lower creatinine levels by alpha males in our study is consistent with these dominant individuals scent marking and countermarking throughout their environment, while subordinate males are downregulating their MUP production to avoid invoking further aggression from dominant alpha males. Subdominant males may be investing moderately in MUP production to be ready for any potential takeover of the hierarchy should the alpha male lose status [13]. Finally, it does not appear from this study that absolute differences in the levels of MUPs are used by mice living in a hierarchy to distinguish relative rank, though it is possible that mice may use unique MUP profiles to individually recognize familiar group members to facilitate socially contextually appropriate behaviour [29,33,68–71].
In summary, it is clear from this study that socially dominant male mice living in a social hierarchy produce and excrete far higher levels of MUPs including MUP20 and produce a higher volume of urine than more socially subordinate animals. Future studies will address the neurobiological mechanisms through which changes in social status and social context can lead to such dynamic changes in MUP production in the liver and MUP excretion in urine.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Dr Hoau Yan Wang for support in SDS–PAGE analysis, Cait Williamson for reading the manuscript, Morgan Firestein for help with PCR and Curley Lab students for help with behavioural observations.
Ethics
All experiments were conducted with approval from the Columbia University Institutional Animal Care and Use Committee (IACUC Protocol: AC-AAAP5405).
Data accessibility
All raw data and code used in this paper are publically available at Github (https://github.com/jalapic/mups).
Authors' contributions
W.L. and J.P.C. designed the study, analysed data and wrote the manuscript. W.L. collected data from behavioural observations, Bradford assay, creatinine assay and PCR. W.L. and A.K. conducted SDS-PAGE. All authors contributed to the final version of the manuscript.
Competing interests
We declare no competing interests.
Funding
This work was supported by the Department of Psychology, Columbia University (J.P.C.) and the Samsung Scholarship Foundation (W.L.).
References
- 1.Berry R. 1970. The natural history of the house mouse. Field Stud. 3, 219–262. [Google Scholar]
- 2.Crowcroft P. 1973. Mice all over. Chicago, IL: Chicago Zoological Society. [Google Scholar]
- 3.Reimer JD, Petras ML. 1967. Breeding structure of the house mouse, Mus musculus, in a population cage. J. Mammal. 48, 88–99. ( 10.2307/1378173) [DOI] [PubMed] [Google Scholar]
- 4.Hurst JL. 1990. Urine marking in populations of wild house mice Mus domesticus rutty. I. Communication between males. Anim. Behav. 40, 209–222. ( 10.1016/S0003-3472(05)80916-9) [DOI] [Google Scholar]
- 5.Hurst JL, Beynon RJ. 2004. Scent wars: the chemobiology of competitive signalling in mice. BioEssays 26, 1288–1298. ( 10.1002/bies.20147) [DOI] [PubMed] [Google Scholar]
- 6.Rich TJ, Hurst JL. 1998. Scent marks as reliable signals of the competitive ability of mates. Anim. Behav. 56, 727–735. ( 10.1006/anbe.1998.0803) [DOI] [PubMed] [Google Scholar]
- 7.Desjardins C, Maruniak JA, Bronson FH. 1973. Social rank in house mice: differentiation revealed by ultraviolet visualization of urinary marking patterns. Science 182, 939–941. ( 10.1126/science.182.4115.939) [DOI] [PubMed] [Google Scholar]
- 8.Hou XH, et al. 2016. Central control circuit for context-dependent micturition. Cell 167, 73–86. ( 10.1016/j.cell.2016.08.073) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williamson CM, Franks B, Curley JP. 2016. Mouse social network dynamics and community structure are associated with plasticity-related brain gene expression. Front. Behav. Neurosci. 10, 152 ( 10.3389/fnbeh.2016.00152) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williamson CM, Lee W, Curley JP. 2016. Temporal dynamics of social hierarchy formation and maintenance in male mice. Anim. Behav. 115, 259–272. ( 10.1016/j.anbehav.2016.03.004) [DOI] [Google Scholar]
- 11.So N, Franks B, Lim S, Curley JP. 2015. A social network approach reveals associations between mouse social dominance and brain gene expression. PLoS ONE 10, e0134509 ( 10.1371/journal.pone.0134509) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curley JP. 2016. Temporal pairwise-correlation analysis provides empirical support for attention hierarchies in mice. Biol. Lett. 12, 20160192 ( 10.1098/rsbl.2016.0192) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williamson CM, Romeo R, Curley JP. 2017. Dynamic changes in social dominance and mPOA GnRH mRNA expression in male mice following social opportunity. Horm. Behav . 87, 80–88. ( 10.1016/j.yhbeh.2016.11.001) [DOI] [PubMed] [Google Scholar]
- 14.Williamson CM, Lee W, Romeo RD, Curley JP. 2017. Social context-dependent relationships between mouse dominance rank and plasma hormone levels. Physiol. Behav. 171, 110–119. ( 10.1016/j.physbeh.2016.12.038) [DOI] [PubMed] [Google Scholar]
- 15.Hiadlovská Z, Mikula O, Macholán M, Hamplová P, Vošlajerová Bímová B, Daniszová K. 2015. Shaking the myth: body mass, aggression, steroid hormones, and social dominance in wild house mouse. Gen. Comp. Endocrinol. 223, 16–26. ( 10.1016/j.ygcen.2015.09.033) [DOI] [PubMed] [Google Scholar]
- 16.Nevison CM, Hurst JL, Barnard CJ. 1999. Strain-specific effects of cage enrichment in male laboratory mice (Mus musculus). Anim. Welf. 8, 361–379. [Google Scholar]
- 17.Nevison CM, Barnard CJ, Beynon RJ, Hurst JL. 2000. The consequences of inbreeding for recognizing competitors. Proc. R. Soc. Lond. B 267, 687–694. ( 10.1098/rspb.2000.1057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garratt M, McArdle F, Stockley P, Vasilaki A, Beynon RJ, Jackson MJ, Hurst JL. 2012. Tissue-dependent changes in oxidative damage with male reproductive effort in house mice. Funct. Ecol. 26, 423–433. ( 10.1111/j.1365-2435.2011.01952.x) [DOI] [Google Scholar]
- 19.Harvey S, Jemiolo B, Novotny M. 1989. Pattern of volatile compounds in dominant and subordinate male mouse urine. J. Chem. Ecol. 15, 2061–2072. ( 10.1007/BF01207438) [DOI] [PubMed] [Google Scholar]
- 20.Novotny M, Harvey S, Jemiolo B. 1990. Chemistry of male dominance in the house mouse, Mus domesticus. Experientia 46, 109–113. ( 10.1007/BF01955433) [DOI] [PubMed] [Google Scholar]
- 21.Apps PJ, Rasa A, Viljoen HW. 1988. Quantitative chromatographic profiling of odours associated with dominance in male laboratory mice. Aggress. Behav. 14, 451–461. ( 10.1002/1098-2337(1988)14:6%3C451::AID-AB2480140606%3E3.0.CO;2-2) [DOI] [Google Scholar]
- 22.Fang Q, Zhang Y-H, Shi Y-L, Zhang J-H, Zhang J-X. 2016. Individuality and transgenerational inheritance of social dominance and sex pheromones in isogenic male mice. J. Exp. Zoolog. B Mol. Dev. Evol. 326, 225–236. ( 10.1002/jez.b.22681) [DOI] [PubMed] [Google Scholar]
- 23.Shahan K, Denaro M, Gilmartin M, Shi Y, Derman E. 1987. Expression of six mouse major urinary protein genes in the mammary, parotid, sublingual, submaxillary, and lachrymal glands and in the liver. Mol. Cell. Biol. 7, 1947–1954. ( 10.1128/MCB.7.5.1947) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chamero P, Marton TF, Logan DW, Flanagan K, Cruz JR, Saghatelian A, Cravatt BF, Stowers L. 2007. Identification of protein pheromones that promote aggressive behaviour. Nature 450, 899–902. ( 10.1038/nature05997) [DOI] [PubMed] [Google Scholar]
- 25.Stopka P, Janotova K, Heyrovsky D. 2007. The advertisement role of major urinary proteins in mice. Physiol. Behav. 91, 667–670. ( 10.1016/j.physbeh.2007.03.030) [DOI] [PubMed] [Google Scholar]
- 26.Kwak J, Grigsby CC, Rizki MM, Preti G, Köksal M, Josue J, Yamazaki K, Beauchamp GK. 2012. Differential binding between volatile ligands and major urinary proteins due to genetic variation in mice. Physiol. Behav. 107, 112–120. ( 10.1016/j.physbeh.2012.06.008) [DOI] [PubMed] [Google Scholar]
- 27.Novotny MV, Ma W, Wiesler D, Zídek L. 1999. Positive identification of the puberty-accelerating pheromone of the house mouse: the volatile ligands associating with the major urinary protein. Proc. R. Soc. Lond. B 266, 2017–2022. ( 10.1098/rspb.1999.0880) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robertson DH, Beynon RJ, Evershed RP. 1993. Extraction, characterization, and binding analysis of two pheromonally active ligands associated with major urinary protein of house mouse (Mus musculus). J. Chem. Ecol. 19, 1405–1416. ( 10.1007/BF00984885) [DOI] [PubMed] [Google Scholar]
- 29.Armstrong SD, Robertson DHL, Cheetham SA, Hurst JL, Beynon RJ. 2005. Structural and functional differences in isoforms of mouse major urinary proteins: a male-specific protein that preferentially binds a male pheromone. Biochem. J. 391, 343–350. ( 10.1042/BJ20050404) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roberts SA, Simpson DM, Armstrong SD, Davidson AJ, Robertson DH, McLean L, Beynon RJ, Hurst JL. 2010. Darcin: a male pheromone that stimulates female memory and sexual attraction to an individual male's odour. BMC Biol. 8, 75 ( 10.1186/1741-7007-8-75) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roberts SA, Davidson AJ, McLean L, Beynon RJ, Hurst JL. 2012. Pheromonal induction of spatial learning in mice. Science 338, 1462–1465. ( 10.1126/science.1225638) [DOI] [PubMed] [Google Scholar]
- 32.Martín-Sánchez A, McLean L, Beynon RJ, Hurst JL, Ayala G, Lanuza E, Martínez-Garcia F. 2015. From sexual attraction to maternal aggression: when pheromones change their behavioural significance. Horm. Behav. 68, 65–76. ( 10.1016/j.yhbeh.2014.08.007) [DOI] [PubMed] [Google Scholar]
- 33.Kaur AW, et al. 2014. Murine pheromone proteins constitute a context-dependent combinatorial code governing multiple social behaviors. Cell 157, 676–688. ( 10.1016/j.cell.2014.02.025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo H, Fang Q, Huo Y, Zhang Y, Zhang J. 2015. Social dominance-related major urinary proteins and the regulatory mechanism in mice. Integr. Zool. 10, 543–554. ( 10.1111/1749-4877.12165) [DOI] [PubMed] [Google Scholar]
- 35.Nelson A, Cunningham C, Ruff J, Potts W. 2015. Protein pheromone expression levels predict and respond to the formation of social dominance networks. J. Evol. Biol. 28, 1213–1224. ( 10.1111/jeb.12643) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Janotova K, Stopka P. 2011. The level of major urinary proteins is socially regulated in wild Mus musculus musculus. J. Chem. Ecol. 37, 647–656. ( 10.1007/s10886-011-9966-8) [DOI] [PubMed] [Google Scholar]
- 37.Garratt M, Vasilaki A, Stockley P, McArdle F, Jackson M, Hurst JL. 2011. Is oxidative stress a physiological cost of reproduction? An experimental test in house mice. Proc. R. Soc. B 278, 1098–1106. ( 10.1098/rspb.2010.1818) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.R Core Team. 2016. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 39.Bates D, Maechler M, Bolker B, Walker S, Christensen RHB, Singmann H, Dai B, Grothendieck G, Green P.2016. lme4: linear mixed-effects models using ‘Eigen’ and S4. See https://cran.r-project.org/web/packages/lme4/index.html .
- 40.Kuznetsova A, Brockhoff PB, Christensen RHB.2016. lmerTest: tests in linear mixed effects models. See https://cran.r-project.org/web/packages/lmerTest/index.html .
- 41.Skaug H, Fournier D, Magnusson A, Nielsen A. 2016. glmmADMB: generalized Linear mixed models using ‘AD Model Builder’. R package version 0.6. 5. r143.
- 42.Ripley B, Venables B, Bates DM.1998. KH (partial port ca. 1998) AG (partial port ca. Firth D. 2016 MASS: support functions and datasets for Venables and Ripley’s MASS. See https://cran.r-project.org/web/packages/MASS/index.html .
- 43.Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 57, 289–300. [Google Scholar]
- 44.Noaín D, et al. 2013. Central dopamine D2 receptors regulate growth-hormone-dependent body growth and pheromone signaling to conspecific males. J. Neurosci. 33, 5834–5842. ( 10.1523/JNEUROSCI.5673-12.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sagazio A, Shohreh R, Salvatori R. 2011. Effects of GH deficiency and GH replacement on inter-male aggressiveness in mice. Growth Horm. IGF Res. 21, 76–80. ( 10.1016/j.ghir.2011.01.002) [DOI] [PubMed] [Google Scholar]
- 46.Norstedt G, Palmiter R. 1984. Secretory rhythm of growth hormone regulates sexual differentiation of mouse liver. Cell 36, 805–812. ( 10.1016/0092-8674(84)90030-8) [DOI] [PubMed] [Google Scholar]
- 47.Flower DR. 1996. The lipocalin protein family: structure and function. Biochem. J. 318, 1–14. ( 10.1042/bj3180001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hastie ND, Held WA, Toole JJ. 1979. Multiple genes coding for the androgen-regulated major urinary proteins of the mouse. Cell 17, 449–457. ( 10.1016/0092-8674(79)90171-5) [DOI] [PubMed] [Google Scholar]
- 49.Buwalda B, Koolhaas JM, de Boer SF. 2017. Trait aggressiveness does not predict social dominance of rats in the visible burrow system. Physiol. Behav. 178, 134–143. ( 10.1016/j.physbeh.2017.01.008) [DOI] [PubMed] [Google Scholar]
- 50.Hofmann HA, Fernald RD. 2000. Social status controls somatostatin neuron size and growth. J. Neurosci. 20, 4740–4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trainor BC, Hofmann HA. 2006. Somatostatin regulates aggressive behavior in an African cichlid fish. Endocrinology 147, 5119–5125. ( 10.1210/en.2006-0511) [DOI] [PubMed] [Google Scholar]
- 52.Gosling LM, Roberts SC. 2001. Scent-marking by male mammals: cheat-proof signals to competitors and mates. Adv. Study Behav. 30, 169–217. [Google Scholar]
- 53.Gosling LM, Roberts SC, Thornton EA, Andrew MJ. 2000. Life history costs of olfactory status signalling in mice. Behav. Ecol. Sociobiol. 48, 328–332. ( 10.1007/s002650000242) [DOI] [Google Scholar]
- 54.Beynon RJ, Hurst JL. 2004. Urinary proteins and the modulation of chemical scents in mice and rats. Peptides 25, 1553–1563. ( 10.1016/j.peptides.2003.12.025) [DOI] [PubMed] [Google Scholar]
- 55.Penn D, Potts WK. 1998. Chemical signals and parasite-mediated sexual selection. Trends Ecol. Evol. 13, 391–396. ( 10.1016/S0169-5347(98)01473-6) [DOI] [PubMed] [Google Scholar]
- 56.Chase ID. 1982. Dynamics of hierarchy formation: the sequential development of dominance relationships. Behaviour 80, 218–239. ( 10.1163/156853982X00364) [DOI] [Google Scholar]
- 57.Chase ID, Tovey C, Spangler-Martin D, Manfredonia M. 2002. Individual differences versus social dynamics in the formation of animal dominance hierarchies. Proc. Natl Acad. Sci. USA 99, 5744–5749. ( 10.1073/pnas.082104199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Drickamer LC. 1995. Rates of urine excretion by house mouse (Mus domesticus): differences by age, sex, social status, and reproductive condition. J. Chem. Ecol. 21, 1481–1493. ( 10.1007/BF02035147) [DOI] [PubMed] [Google Scholar]
- 59.Wood SK, Baez MA, Bhatnagar S, Valentino RJ. 2009. Social stress-induced bladder dysfunction: potential role of corticotropin-releasing factor. Am. J. Physiol. Regul. Integr. Comp. Physiol. 296, R1671–R1678. ( 10.1152/ajpregu.91013.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.O'Connell LA, Hofmann HA. 2012. Evolution of a vertebrate social decision-making network. Science 336, 1154–1157. ( 10.1126/science.1218889) [DOI] [PubMed] [Google Scholar]
- 61.DeVries AC, Glasper ER, Detillion CE. 2003. Social modulation of stress responses. Physiol. Behav. 79, 399–407. ( 10.1016/S0031-9384(03)00152-5) [DOI] [PubMed] [Google Scholar]
- 62.Jones RB, Nowell NW. 1974. A comparison of the aversive and female attractant properties of urine from dominant and subordinate male mice. Anim. Learn. Behav. 2, 141–144. ( 10.3758/BF03199141) [DOI] [PubMed] [Google Scholar]
- 63.Drickamer LC. 1992. Oestrous female house mice discriminate dominant from subordinate males and sons of dominant from sons of subordinate males by odour cues. Anim. Behav. 43, 868–870. ( 10.1016/S0003-3472(05)80212-X) [DOI] [Google Scholar]
- 64.Garratt M, Stockley P, Armstrong SD, Beynon RJ, Hurst JL. 2011. The scent of senescence: sexual signalling and female preference in house mice. J. Evol. Biol. 24, 2398–2409. ( 10.1111/j.1420-9101.2011.02367.x) [DOI] [PubMed] [Google Scholar]
- 65.Jones RB, Nowell NW. 1989. Aversive potency of urine from dominant and subordinate male laboratory mice (Mus musculus): resolution of a conflict. Aggress. Behav. 15, 291–296. ( 10.1002/ab.2480150404) [DOI] [Google Scholar]
- 66.Hurst JL, Robertson DH, Tolladay U, Beynon RJ. 1998. Proteins in urine scent marks of male house mice extend the longevity of olfactory signals. Anim. Behav. 55, 1289–1297. ( 10.1006/anbe.1997.0650) [DOI] [PubMed] [Google Scholar]
- 67.Hurst JL, Fang J, Barnard C. 1994. The role of substrate odours in maintaining social tolerance between male house mice, Mus musculus domesticus: relatedness, incidental kinship effects and the establishment of social status. Anim. Behav. 48, 157–167. ( 10.1006/anbe.1994.1222) [DOI] [Google Scholar]
- 68.Hurst JL, Payne CE, Nevison CM, Marie AD, Humphries RE, Robertson DHL, Cavaggioni A, Beynon RJ. 2001. Individual recognition in mice mediated by major urinary proteins. Nature 414, 631–634. ( 10.1038/414631a) [DOI] [PubMed] [Google Scholar]
- 69.Beynon RJ, Armstrong SD, Gómez-Baena G, Lee V, Simpson D, Unsworth J, Hurst JL. 2014. The complexity of protein semiochemistry in mammals. Biochem. Soc. Trans. 42, 837–845. ( 10.1042/BST20140133) [DOI] [PubMed] [Google Scholar]
- 70.Cheetham SA, Thom MD, Jury F, Ollier WER, Beynon RJ, Hurst JL. 2007. The genetic basis of individual-recognition signals in the mouse. Curr. Biol. 17, 1771–1777. ( 10.1016/j.cub.2007.10.007) [DOI] [PubMed] [Google Scholar]
- 71.Cheetham SA, Smith AL, Armstrong SD, Beynon RJ, Hurst JL. 2009. Limited variation in the major urinary proteins of laboratory mice. Physiol. Behav. 96, 253–261. ( 10.1016/j.physbeh.2008.10.005) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All raw data and code used in this paper are publically available at Github (https://github.com/jalapic/mups).