Abstract
Recombinant virus-like particle-based vaccines are composed of viral structural proteins and mimic authentic native viruses but are devoid of viral genetic materials. They are the active components in highly safe and effective vaccines for the prevention of infectious diseases. Several expression systems have been used for virus-like particle production, ranging from Escherichia coli to mammalian cell lines. The prokaryotic expression system, especially Escherichia coli, is the preferred expression host for producing vaccines for global use. Hecolin, the first licensed virus-like particle vaccine derived from Escherichia coli, has been demonstrated to possess good safety and high efficacy. In this review, we focus on Escherichia coli-derived virus-like particle based vaccines and vaccine candidates that are used for prevention (immunization against microbial pathogens) or disease treatment (directed against cancer or non-infectious diseases). The native-like spatial or higher-order structure is essential for the function of virus-like particles. Thus, the tool box for analyzing the key physicochemical, biochemical and functional attributes of purified virus-like particles will also be discussed. In summary, the Escherichia coli expression system has great potentials for producing a range of proteins with self-assembling properties to be used as vaccine antigens given the proper epitopes were preserved when compared to those in the native pathogens or disease-related target molecules.
Introduction
Vaccination is the most efficient way to control and prevent infectious diseases. Currently, the majority of licensed vaccines produced by traditional technologies are either live-attenuated or inactivated, although both may present safety issues (such as reversion to virulence and residual virulence).1 In the 1970s, scientists discovered that a single key protein from a virus could be a vaccine antigen.2, 3 Almost a decade later, the first genetically engineered vaccine using recombinant gene expression technology was produced in the prokaryotic microbe.4 With the advent of modern molecular biology, recombinant subunit vaccines have flourished in human vaccinology. Virus-like particles (VLPs) are composed of the virion-building proteins of a virus and spontaneously self-assemble into particles without incorporating the infective viral genome.5 Thus, VLP are extremely promising vaccine candidates due to their native-like and non-infective properties. VLPs can induce both innate and adaptive immune responses and have shown to be highly immunogenic in animals and humans.5, 6 The approved VLP-based vaccines have been produced in yeast,7, 8 insect, bacteria Escherichia coli (E. coli),9, 10 plant, and mammalian cells,11, 12 ranging from prokaryotic to eukaryotic expression systems.
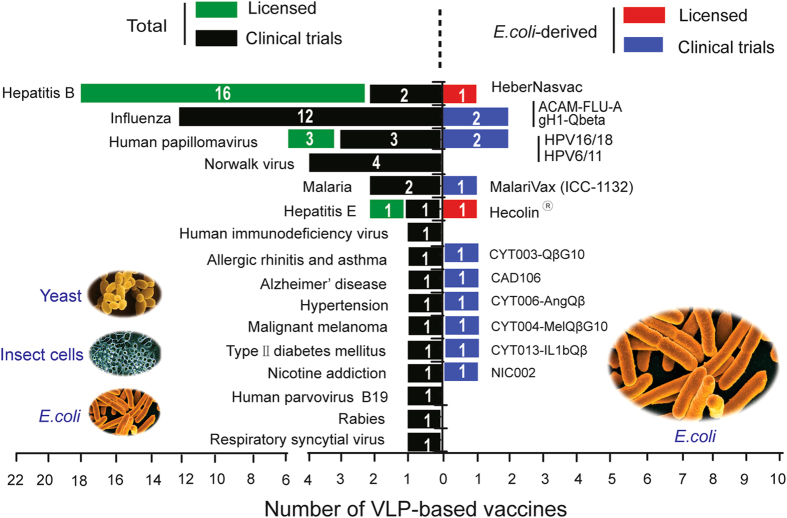
Bacteria, especially E. coli, have been widely used for producing recombinant proteins. The first recombinant pharmaceutical approved for the treatment of diabetes, recombinant human insulin (Humulin-US/Humuline-EU), was obtained from E. coli.13 Numerous recombinant protein-based products derived from E. coli have been approved for therapeutic use, including cytokines, hormones, growth factors, serine proteases, and fusion proteins.14 The food and drug administration and European medicines agency have approved 151 protein-based recombinant drugs, 45 (29.8%) of which are produced using products derived from E. coli.15 From 2010 to July 2014, almost 33% of the approved recombinant biopharmaceutical in the United States and EU were obtained from Chinese hamster ovary cells, while 29% and 16.5% were obtained from E. coli and yeast, respectively.16 Thus, E. coli is still a widely used host for the production of protein-based biopharmaceuticals. Of the 174 different types of VLPs successfully produced, approximately 28% were produced in bacterial systems, 20% in yeast systems and 28% in insect systems.17 As of 2015, over 50 VLP-based vaccines or vaccine candidates, derived from different expression hosts, have been licensed or are under clinical development (Fig. 1, Supplementary Table 1S). Hecolin was the first VLP-based vaccine against hepatitis E virus (HEV) obtained from E. coli and was licensed by the FDA of China, or CFDA, in 2011.18, 19 Currently, scientists in academia and industry are actively seeking ways to produce more cost-effective VLP-based vaccines, particularly low-cost vaccines for distribution in developing countries.
Fig. 1.
The number of VLP-based vaccines or vaccine candidates that were approved and in clinical studies from 1986 to 2015. Total numbers of VLP vaccines or candidates derived from different expression systems (including E. coli) are plotted on the left. The numbers of commercialized VLP vaccines or those being tested in clinical trials derived from E. coli are plotted on the right.
VLP-based vaccines are pre-eminent candidates for vaccination because of their high immunogenicity and good safety performance.5, 20 VLP-based vaccines derived from E. coli are more cost-effective than those derived from insect cells or yeasts during industrial production. However, only one VLP-based vaccine, Hecolin, fully derived from E. coli has been approved for use in humans.20 Currently, a number of recombinant specific E. coli strains have been developed with the intention of achieving high-yield and high-quality protein production. Proper folding that allows the formation of specific structures is essential for the function of VLPs in clinical use. Various in vitro analytical methods, in conjunction with in vivo evaluation, have been established to monitor the presence of native-like epitopes on VLPs obtained from E. coli, thereby ensuring the efficacy of VLP-based vaccines. This review highlights examples of E. coli-derived VLP-based vaccines and vaccine candidates. Additionally, the functional assessment of VLPs and the challenges associated with recombinant VLP proteins produced in E. coli will also be discussed.
Why use E. coli-derived VLPs for vaccine development?
As a robust protein expression host, E. coli has many advantages, such as inexpensive culturing, high expression levels, easy scale-up and short turnaround time.21, 22 It is a preferred expression system for protein production if the protein can be correctly folded. In vaccine development, high product cost would restrict utilization, especially in developing countries.23 Existing, emerging and re-emerging infectious diseases pose a threat to human life and productivity in both the developed and the developing world. There is thus an urgent need for new vaccine manufacturing platforms that are able to rapidly and cheaply produce vaccine antigens. Platform technology based on E. coli, which can synthesize viral capsomeres at gram-per-litre levels, was developed by Middelberg et al. The high yield of capsid proteins or structural proteins (the basic unit of VLPs) could significantly reduce the time and the cost of vaccine production.24 A microbe-based platform has the potential to quickly provide affordable, safe, and efficacious vaccines in developing countries.
E. coli-derived VLP vaccines and vaccine candidates
E. coli is the preferred recombinant expression host due to its ease of use and the low cost associated with cultivation. Several E. coli-derived VLP vaccines or vaccine candidates have entered clinical trials in recent years (Table 1). Hecolin, a p239 VLP-based vaccine, containing 368-606 aa of open reading frame 2 of a genotype 1 strain of HEV,25 was the first commercialized E. coli-derived vaccine for the prevention of HEV infection.19 Meanwhile, two E. coli-derived VLP-based vaccines (NCT01735006 and NCT02710851) against human papillomavirus (HPV) also has been developed in clinical trials. In addition, VLPs have been utilized as vaccine platforms to increase the immunogenicity of antigens. These chimeric VLP vaccines were both targeted against infectious and non-infectious diseases. The high-cost of the vaccines was the main limitation preventing worldwide implementation.26 Developing more affordable vaccines could partly address the inaccessibility and other hurdles faced by some commercial vaccines.
Table 1.
E. coli-derived VLP based vaccines or vaccine candidates
| Vaccine name | Company/Institution | VLP platform | Vaccine antigen | Clinical Trial/Approved | Reference or clinical trial identifier (NCT) * |
|---|---|---|---|---|---|
| Prophylactic vaccines | |||||
| HEV Hecolin | Xiamen Innovax Biotech Co., Ltd (Xiamen, China) | HEV | HEV capsid polypeptide | Licensed | 18, 19 |
| HPV HPV16/18 | Xiamen University, Xiamen Innovax Biotech Co., Ltd | HPV | HPV16/18 L1 major capsid protein | Phase III | NCT01735006 |
| HPV6/11 | Beijing Wantai Biological Pharmacy Enterprise Co., Ltd (Beijing, China) | HPV6/11 L1 major capsid protein | Phase II | NCT02710851 | |
| ACAM-FLU-Aa | Sanofi Pasteur | HBcAg | Influenza A M2e | Phase I | NCT00819013 |
| gH1-Qbetaa | A*STAR and Cytos Biotechnology | Bacteriophage Qβ | globular head domain (gH1) of haemagglutinin (HA) | Phase I | 61 |
| MalariVax (ICC-1132)a | Apovia | HBcAg | Plasmodium falciparum circumsporozoite protein | Phase I | NCT00587249 |
| Therapeutic vaccines | |||||
| HBV ABX203 (HeberNasvac)b | The Center for genetic Engineering and Biotechnology, Cuba | HBV | HBsAg/HBcAg | Licensed | 65, 66 |
| Allergic rhinitis and asthma CYT003-QβG10a | Cytos Biotechnology | Bacteriophage Qβ | G10 (CpG DNA) | Phase II | NCT00890734 |
| Malignant melanoma CYT004-MelQβG10a | Cytos Biotechnology | Bacteriophage Qβ | Melan-4, G10 DNA (CpG) | Phase II | NCT00651703 |
| Alzheimer’s disease CAD106a | Cytos Biotechnology | Bacteriophage Qβ | Aβ1-6 epitope | Phase II | NCT01097096 |
| Hypertension CYT006-AngQβa | Cytos Biotechnology | Bacteriophage Qβ | Angiotensin II | Phase II | NCT00500786 |
| Nicotine addiction NIC002a | Cytos Biotechnology | Bacteriophage Qβ | Nicotine hapten | Phase II | NCT01280968 |
| Type II diabetes mellitus CYT013-IL1bQβa | Cytos Biotechnology | Bacteriophage Qβ | IL-1β | Phase I | NCT00924105 |
*References or NCT numbers (registered at https://clinicaltrials.gov) are provided
a Chimeric VLP-based vaccines: VLPs as vaccine platforms display heterologous epitopes or antigens on their surface by the way of genetic fusion or chemical conjugation
b Hepatitis B virus surface antigen (HBsAg) and hepatitis B core antigen (HBcAg), were expressed in yeast (Pichia pastoris) and E. coli, respectively
HEV vaccine
The HEV, the causative agent of hepatitis E, is the sole member of the genus Hepevirus within the family Hepeviridae and transmits primarily in a fecal-oral manner.27, 28 HEV infection is a serious threat to public health, especially in developing countries. Mammalian HEV is classified into four major genotypes, but only one serotype.29 This opens up the opportunity for the development of a univalent, broad-spectrum HEV vaccine. HEV is a 34-nm, non-enveloped, positive-sense single-stranded RNA icosahedral virus with an approximately 7.2-kb genome containing three open reading frames (ORFs). These ORFs encode a number of different proteins for various biological functions, among which ORF2 (660 amino acid) encodes the sole capsid protein, pORF2.30
Hecolin, the first prophylactic hepatitis E vaccine, was licensed in 2011 and launched in 2012 in China.19 It is also the world’s first E. coli-derived VLP-based vaccine synthesized on a commercial scale.10 The neutralizing and immunodominant epitopes from HEV genotype 1 were present on the surface of p239 VLPs.9 The high efficacy of the HEV vaccine was demonstrated by a randomized, double-blind, placebo-controlled phase III clinical trial;31 a follow-up study subsequently demonstrated long-term efficacy of up to 4.5 years after the initial vaccination.32 Product consistency was demonstrated through comprehensive characterization of antigens in Hecolin. The comparable p239 VLPs characteristics of the antigen produced at different scales indicated that the antigen manufacturing process was robust and scalable.33 The vaccine contains 30-μg truncated capsid protein formulated with aluminum adjuvants.10 Zhang et al. have demonstrated the preservation of critical antigen epitopes absorbed on adjuvants and recovered antigens post-dissolution treatment using a set of biochemical, biophysical, and immunochemical methods (Fig. 2).34 In that study, the anti-HEV monoclonal antibody 8C11, which was applied in different immunochemical methods, was able to capture the native HEV virions.9 This result indicated that virion-like epitopes are present on the surface of E. coli-derived p239 VLPs. Multi-year stability is required for marketed vaccines. The real-time and long-term stability of Hecolin, stored at 4 °C for 24 months, was evaluated using a set of biophysical, biochemical, and immunochemical approaches. The results demonstrated overall high structural stability of p239 VLPs over 24 months.35
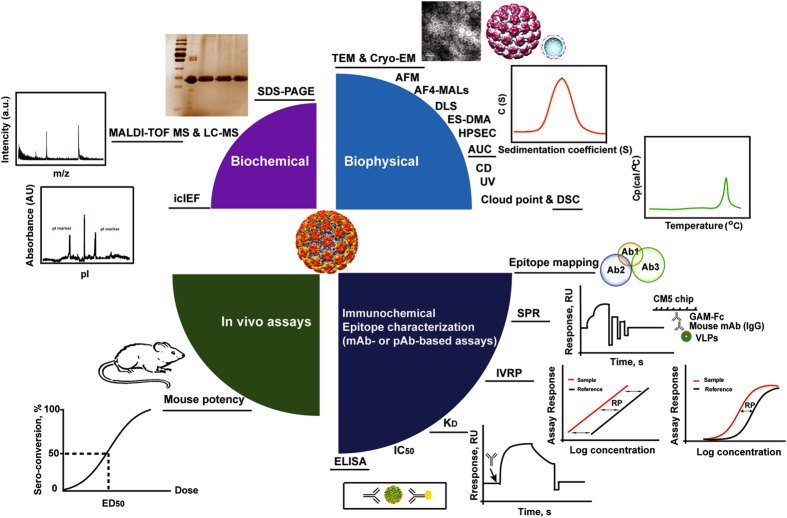
Fig. 2.
Analytical toolbox for the characterization of VLPs. A series of modern techniques make up a “toolbox” that has been extensively used for structural and functional characterization of VLPs. Biochemical: SDS-PAGE sodium dodecyl sulphate polyacrylamide gel electrophoresis, MALDI-TOF MS matrix-assisted laser desorption/ionization time of flight mass spectrometry,33 LC-MS liquid chromatography–mass spectrometry,33 icIEF imaged capillary isoelectric focusing has been widely used for protein characterization,33 Biophysical: the morphology of VLPs can be observed by TEM, Cry-EM, and AFM. TEM transmission electron microscopy, Cry-EM cry electron microscopy,85 AFM atomic force microscopy,114 AF4-MALS, DLS, ES-DMA, and HPSEC generally are used for the measurement the size of particles. AF4-MALS asymmetric flow field-flow fractionation coupled with multiple-angle light scattering,117, 118 DLS dynamic light scattering,119 ES-DMA electrospray differential mobility analysis,118 HPSEC high performance size exclusion chromatography,33 AUC analytical ultracentrifugation, CD Circular dichroism,33 UV ultraviolet spectroscopy,33 DSC differential scanning calorimetry, mAb or pAb-based assays are used to measure the concentration of functional epitopes in the vaccine samples. Epitope-mapping: comparable of epitope overlap of VLPs in different vaccine samples by mAbs; SPR surface plasmon resonance, IVRP in vitro relative potency, KD equilibrium dissociation constant,114 IC 50 half maximal inhibitory concentration, ELISA enzyme-linked immunosorbent assay, The mini-VLP in the figure is the structure mode of HPV59, which was adapted from Structure, Li et al.120
HPV vaccines
HPVs, non-enveloped double-stranded DNA viruses, are the causative agents of cervical cancer.36, 37 Papillomavirus virions consist of two structural proteins, L1 and L2; the major structural protein is L1, which is able to self-assemble into pentamers and subsequently into VLPs.38 Currently, three prophylactic HPV vaccines are based on VLPs, Gardasil-4 (a quadrivalent HPV16/18/6/11 vaccine produced in yeast), Gardasil-9 (a 9-valent 16/18/31/33/45/52/58/6/11 HPV vaccine produced in yeast), and Cervarix (a bivalent HPV 16/18 vaccine expressed via insect cells).39–41 Clinical trials have shown that all three vaccines consistently induced production of protective and neutralizing antibodies to prevent infection. These vaccines are generally well tolerated.42, 43 However, their high production and delivery costs are significant barriers to worldwide implementation.44 The globally licensed HPV vaccines, all produced in eukaryotic systems with high production cost,45 are thus excluded low-income regions, where cervical cancer results in higher mortality.46 Thus, there is a pressing need for more cost-effective vaccines.
Xiamen Innovax Biotech has used E. coli to produce a low-cost HPV vaccine based on L1 VLPs. L1 is the HPV major structural protein (the other minor capsid protein is L2).47 A bivalent HPV vaccine (Types 16, 18), based on these VLPs, has been developed and has been shown to be safe and highly immunogenic in preclinical studies. The data indicated that HPV16/18 VLPs were obtained from a prokaryotic expression system with desired immunogenicity.48 The results of a phase I safety trial showed that the E. coli expressed recombinant HPV 16/18 bivalent vaccine candidate is well tolerated in healthy women, with just few minor adverse events attributable to the vaccination were observed.48 The immunogenicity of vaccine was demonstrated in healthy young women in a phase II clinical trial.49 A large-scale phase III efficacy trial was initiated in November 2012 in China (NCT01735006). Additionally, another bivalent HPV vaccine candidate (Types 6, 11) obtained from E. coli is currently undergoing a phase II clinical trial (NCT02710851) (Table 1). The success of HPV L1 VLPs and HEV p239 obtained from E. coli indicates that this microbe-based vaccine technology may facilitate the development of cost-effective vaccines and bring benefits to people in developing countries.
Other prophylactic vaccines
VLPs have been used as a vaccine delivery platform to increase the immunogenicity of antigens.50 Several chimeric VLP vaccine candidates are listed in Table 1. In addition to the HEV vaccine and HPV vaccines mentioned above, a malaria vaccine and two influenza VLP-based vaccines expressed by the E. coli system were reported.
Malaria vaccine
Malaria, caused by the Plasmodium parasite, is a serious public health problem in the tropics.51 There is no highly effective vaccine for malaria.52 A chimeric VLP-based vaccine candidate, MalariVax (ICC-1132), consists of a hepatitis B virus core VLPs produced in E. coli, displaying malaria epitopes (the Plasmodium falciparum circumsporozoite) on their surface. The results of a phase I trial showed clinical efficiency against malaria parasites.53 No subsequent clinical data were published.
Influenza vaccines
Due to viral drifts and shifts, a particular influenza vaccine cannot provide long-term immunity.54 A microbial platform may rapidly provide a vaccine to combat seasonal influenza epidemics.55 The anti-influenza A M2e-HBc vaccine candidate, ACAM-FLU-A, was produced by E. coli. Recombinant hepatitis B core antigen (HBcAg), as a carrier VLP, is one of the main structural antigens of HBV.56 The M2 external domain is a relatively conserved epitope in both human and avian influenza A viruses that is present on the surface of HBcAg VLPs. The immunogenicity has been confirmed in a phase I clinical trial (NCT00819013).57–60 In addition, globular head domain (gH1)-Qbeta, a fully bacterially produced influenza vaccine, was obtained by chemically conjugating the gH1 of hemagglutinin (HA) from the pandemic A/California/07/2009(H1N1) influenza strain to the Qbeta VLPs. A phase I trial has demonstrated that gH1-Qbeta was able to induce high titer of anti-viral antibodies with a favorable safety profile.61
Therapeutic vaccines in clinical trials for human diseases
A combination vaccine used for chronic hepatitis B treatment, ABX203 (trade name HeberNasvac), is composed of hepatitis B virus surface (HBsAg) and core antigens (HBcAg), which are expressed in Pichia pastoris and E. coli, respectively.62 ABX203 has been shown to be effective and well tolerated in clinical trials.63–65 The Cuban regulatory authorities granted the Center for genetic Engineering and Biotechnology their first marketing authorization application for ABX203 in 2015.66 Additionally, a number of chimeric VLP vaccine candidates, chemically conjugated antigens to the RNA bacteriophage Qβ VLPs derived from E. coli, have been developed by Cytos Biotechnology AG (Switzerland) (Table 1). These chimeric VLP vaccine candidates are designed to targeted non-infectious diseases such as nicotine addiction, hypertension, cancer, diabetes, allergies, and Alzheimer’s disease. Results showed that the use of nicotine-Qβ VLPs, such as NIC002 (formerly CYT002-Nic002), have promoted long-term abstinence from smoking.67, 68 Similarly, a Qβ VLP conjugated with a modified Ang II peptide, CYT006-AngQβ, were developed as an anti-hypertensive vaccine.69 Additionally, CYT004-MelQβG10 (NCT00651703), CYT103-IL1bQβ (NCT00924105), and CYT003-QβG10 (NCT00890734), which are directed against malignant melanoma, Type II diabetes, allergic rhinitis, and asthma, respectively, are currently in various stages of clinical trials. CAD-106, in which Qβ VLP is covalently coupled to the Aβ1-6 peptide, is an immunotherapeutic vaccine for Alzheimer’s disease currently undergoing a Phase II trial.70–72
In addition, many E. coli-derived VLP-based vaccine candidates, against West Nile virus,73 foot-and-mouth disease virus74 and hepatitis C virus75 also have been developed in preclinical studies. The potency of these E. coli-derived VLP antigens has been demonstrated in different animal models. The efficacy and safety of a vaccine need to be demonstrated for licensing in human use.76, 77 Post licensure, the quality of vaccines during manufacturing and storage should be assessed to ensure their safety and efficacy throughout the life cycle management of vaccine commercialization. Structural and functional assessment of VLPs is the most critical antigen characterization assays for recombinant protein based vaccines.
Structural and functional assessment of VLPs
E. coli-derived HEV p239 VLPs and HPV VLPs consist only of the viral capsid protein without incorporating genetic materials but retain a conformation similar to that of the native virus.9, 78 Generation of functional antibodies is dependent on the correct antigen conformation and native-like epitopes being present on the surface of VLPs.79 VLPs containing virion-like epitopes can be acquired via antigen-presenting cells and then induce a protective humoral immune response.50, 80 Thus, recombinant VLPs must be correctly folded to ensure their function by inducing a protective humoral immune response. The spatial or higher-order structure of the vaccine antigen is the basis of the various biological functions of protein-based vaccines. Quantitative analysis of the functional epitopes on VLPs using monoclonal antibody-based assays can be an advantageous way to ensure vaccine safety and efficacy.81 Multifaceted analytical approaches, such as biochemical, biophysical, and immunochemical methods (Fig. 2), have been well established and are widely used for the evaluation of three different licensed recombinant VLP-based vaccines: hepatitis B vaccine, hepatitis E vaccine, and HPV vaccine.82
The identification of the primary structure indicated that the target protein composed of VLPs was successfully expressed. Biochemical characterization includes the primary amino acid sequence, molecular weight, isoelectric point, and purity of the VLPs.10 The secondary and tertiary structures of the VLPs can be measured by circular dichroism and ultraviolet spectroscopy.33 Mass spectrometry is an indispensable analytical technique used to determine the mass of proteins and their amino acid composition.33, 83, 84 This tool is useful for process monitoring and demonstrating final product consistency at single amino acid level. SDS-PAGE, the most common method that used to determine the purity, integrity and molecular weight of the purified antigen.25, 84 The morphological characteristics and the state of the VLP are amenable to imaging by transmission electron microscopy (TEM),85 analytical ultracentrifugation (AUC) or density gradient ultracentrifugation.86, 87 TEM methods can be used to determine the three-dimensional structure of VLPs and investigate their interaction with antibodies or their appearance when adsorbed to an adjuvant, in combination with modern computational tools, bioinformatics, homology modeling, docking, and MD simulation.20, 85, 88 Differential scanning calorimetry and cloud point have been widely applied for investigating the thermal stability and aggregation propensity of recombinant proteins.33, 89–91 These modern techniques make up a “toolbox” that has been extensively used for structural and functional characterization of VLP-based vaccines (Fig. 2).
As an immunogen, VLP-based vaccines generally stimulate a humoral and a mostly CD4 T cell-mediated immune response.80 VLPs were injected into individuals to develop protective immunity against infection. Neutralizing and immunodominant epitopes on antigen are the structural basis of an epitope to elicit functional and protective antibodies.79 The functional epitopes can be quantitated by their ability to bind to a panel of specific monoclonal antibodies.92–94 Monoclonal antibodies have been developed as specific probes to identify and characterize virion-like epitopes.34, 95, 96 Binding activity to a certain neutralizing epitope can serve as an excellent surrogate marker for in vivo immunogenicity or vaccine efficacy. Currently, various immunoassays have been applied for assessment of the antigenicity of HBV, HPV, and HEV VLPs using a panel of specific and functional monoclonal antibodies (Fig. 2). These methods include one-site binding and label-free SPR technology,33, 88, 97 solution competition ELISA (IC50),88, 98, 99 and sandwich ELISA.95, 100, 101 In vitro relative potency assays (IVRP assays) generally is a sandwich-type immunoassay that uses neutralizing monoclonal antibodies to measure the concentration of functional epitopes in the vaccine sample. The IVRP assay has been shown to have a good correlation with mouse potency in Gardasil-4.101 Thus, mouse potency can be replaced by IVRP for release and stability testing, as well as monitoring of the production process.
Discussion
VLPs have been widely used in vaccinology. The next generation of VLP-based vaccine candidates must be creative in form and function to satisfy diverse needs.50 Several viral structures produced in E. coli, such as HBcAg, Qβ, AP250, murine polyomavirus and HPV, have been used for vaccine platforms.22, 59, 69, 102, 103 Vaccinologists can now add heterologous epitopes or antigens to these VLPs from different origins achieved by genetically fusing or chemical conjugation.104 Middelberg et al. have developed in vitro cell-free assembly of modular VLPs based on murine polyomavirus capsid proteins expressed in E. coli as vaccine carriers to enhance immune responses, especially to weakly or non-immunogenic antigens.22 These modular VLPs have potential for use as a vaccine platform to increase the efficacy and stability and to allow for more versatile display of antigens. Re-engineering or grafting epitopes in chimeric VLPs may widen the coverage spectrum compared to monovalent vaccines.50 Several chimeric VLP vaccine candidates have been developed by chemically conjugating foreign antigens to RNA bacteriophage Qβ VLPs obtained from E. coli. These chimeric VLP vaccines are currently in clinical development (Table 1). The yield of Qβ VLP production in E. coli is higher than that in yeast.105 RNA bacteriophage VLPs naturally encapsidated ssRNA in E. coli, such that it could influence the immune bias when used in mouse immunizations, with a shift from IgG1 to IgG2a compared to VLPs without RNA, indicating that the Th1-biased immune response has occurred.106–108 The Th1-type immune response is essential for the control of intracellular pathogens and could be an ideal platform for future prophylactic (malaria, HIV, Herpes viruses) and therapeutic vaccine applications (cancer and chronic hepatitis).109 VLPs derived from a given viral protein or as displaying bionanoparticles of foreign epitopes could enhance the immunogenicity of the B-cell epitopes on the particle surface or modulate the Th1- and/or Th2-immune response due to the the nature of the B-cell or T-cell epitopes built in via recombinant technology as part of the protein-based particles.
The commercial VLP-based vaccines have been constructed through eukaryotic or prokaryotic systems. A brief comparison among different expression systems with respect to their applications in producing recombinant VLPs has been summarized in Table 2. As a manufacturing platform, E. coli faced several obstacles, which may limit its application in protein-based biopharmaceuticals. Their limitation factors included: (1) lack of ability to produce the correct disulfide bonds, (2) fail to produce recombinant proteins with mammalian-like post-translational modifications, (3) the problems of protein solubility, and (4) the presence of endotoxins (lipopolysaccharide, LPS).17 Post-translational modifications play an important role in protein folding, processing, and stability, as well as final biological activity and even the immunogenicity of the protein.110 Disulfide bond formation, glycosylation, phosphorylation and proteolysis processing play a crucial role in biological activity of some recombinant proteins.15 E. coli cannot synthesize useful HBsAg particles, probably because of unfavorable environmental conditions, such as pH and redox potential, or lipid compositions within the bacterium.111 Studies have also shown that HBcAg phosphorylation is essential for viral replication and capsid formation.112 Therefore, the expressed proteins may be insoluble, unstable or inactive without post-translational modifications. E. coli stands out as the expression system for the production of small recombinant proteins without post-translational modifications.14, 15 However, Spiess et al. developed an approach for the efficient generation of nonimmunogenic, stable bispecific antibodies with a natural IgG architecture by co-culture of bacteria (E. coli) expressing two distinct half-antibodies.113 This technology provides a rapid generation of biospecific antibodies with natural architecture from any two existing antibodies for academic research and industrial development.
Table 2.
A brief comparison among different systems with respect to their applications in producing recombinant VLPs
| Property | E. coli | Yeast | Baculovirus-insect cells | Mammalian cells |
|---|---|---|---|---|
| Production cost | + | ++ | +++ | ++++ |
| VLP production levels | ++++ | +++ | ++ | + |
| VLP complexity20 | + | ++ | ++++ | ++ |
| Post-translational modifications(PTMs)* | ||||
| Disulfide bond | Unfavorable redox potential for disulfide bond formation | Yes | Yes | Yes |
| O-glycosylation | No | Yes | Yes | Yes |
| N-glycosylation | No | Yes | The inability to synthesize mammalian-type N-glycans | Yes |
| Phosphorylation | No | Yes | Yes | Yes |
| Acylation | No | Yes | Yes | Yes |
| γ-Carboxylation | No | No | No | Yes |
| Applications** | Simple polypeptides and proteins (Hecolin) | Mammalian-like or secreted proteins (Gardasil-4 and Gardasil-9) | Mammalian-like or secreted proteins (Cervarix) | Mammalian proteins (GenHevac B) |
*Post-translational modifications (PTMs) are similar or identical to those occurring in mammalian cells
**The application examples of VLP-based vaccines derived from different expression systems were summarized in Fig. 1 and Supplementary Table 1S. Hecolin (HEV vaccine): manufactured by Xiamen Innovax Biotech Co., Ltd. Gardasil-4 and Gardasil-9 (HPV vaccines): manufactured by Merck. Cervarix (HPV vaccine): manufactured by GSK. GenHevac B (HBV vaccine): manufactured by Pasteur-Merieux Aventis
Currently, numerous mutant E. coli strains have been developed to improve the different protein expression (representative examples are shown in Table 3). Origami™ 2(DE3) is mutated in glutathione reductase and thioredoxin reductase to promote target protein disulfide bond formation. Several strategies were applied to solve the insolubility of proteins by adjusting culture conditions (such as low temperature), using fusion protein systems and in vitro denaturing/re-assembly of insoluble inclusion body.17 The key for VLPs to elicit both humoral and CD4 T cell-mediated immune responses is that the VLPs retain a conformation similar to that of native viruses in molecular scaffolds. Zhao et al. have demonstrated that disassembly-reassembly (D/R) of HPV VLPs produced more virion-like antibody reactivity.114 The D/R treatment with defined and controlled physicochemical conditions have been explored for better folding of structural proteins, such as the formation of correct disulfide bond.87 These additional bioprocessing steps with well-controlled conditions will be essential to obtain more desirable VLPs with in-particle assembly and particle-to-particle homogeneity. Most gram-negative bacteria contain endotoxins, LPSs, which can induce a pyrogenic response.115 Thus, for safe use in humans, LPSs must be removed from the recombinant proteins expressed in E. coli to maintain the levels of endotoxins below a certain threshold. However, the removal of LPSs increases the complexity and the cost of protein purification processes.115 Recently, Mamat et al. constructed endotoxin-free E. coli strains by multi-step mutagenesis, KPM335, which provided an endotoxin-free environment and can be a versatile expression system for protein production.115, 116 Thus, although E. coli has some limitations, it could be a potential expression host for rapid, scalable and economical VLP-based vaccine production.
Table 3.
Representative recombinant E. coli strains for protein expression
| Strains | Description | Applications | Company/Institution |
|---|---|---|---|
| BL21 | Deficient in both Lon and OmpT proteases | General purpose expression host | Novagen |
| C2528 (Lemo21(DE3) Competent E. coli ) | Deficient in Lon and OmpT proteases, T7 expression strain, resistant to phage T1 (fhuA2), tunable expression by lysozyme (lysY) | Expression membrane proteins, toxic proteins and proteins prone to insoluble expression | New England Biolabs |
| BL21 Star™(DE3) pLysS | RNaseE (rne131) mutant | Ideal for high-level expression of non-toxic but potentially growth-inhibiting recombinant proteins | Invitrogen |
| Origami™ 2(DE3) | Mutations in glutathione reductase (gor) and thioredoxin reductase (trxB) | Disulfide-bonded protein expression | Novagen |
| C2529 (NiCo21(DE3) Competent E. coli ) | Mutation at GlmS, target proteins carrying an intein-chitin binding domain (intein-CBD) tag/ Poly-histidine tag | Improved purity of target proteins isolated by immobilized chitin affinity chromatography/ immobilized metal affinity chromatography | New England Biolabs |
| KPM335 | Mutations in ΔkdsD, ΔgutQ, ΔlpxL, ΔlpxM, ΔpagP, ΔlpxP, ΔeptA, msbA52 and frr181 | Deficient in synthesis of LPS, endotoxin free strains for proteins expression | Research Corporation Technologies |
Conclusions
In summary, several promising E. coli-derived VLP-based vaccines or vaccine candidates, directed against both infectious and non-infectious diseases, have been currently commercialized or are being developed in the clinical testing stage. The success of E. coli-derived VLPs (Hecolin) as recombinant vaccine antigens suggested that using microbial synthesis has the potentials to facilitate the production of low-cost vaccines for global use. VLPs mimicking viral capsids or chimeric VLPs by grafting epitopes of interests to a well-behaved VLP display vector are platforms for future vaccines via structure-based modular design. Different analytical methods for antigen characterization provide important supports for recombinant VLP-based vaccines to ensure their efficacy and safety, and most importantly the preservation of native-like epitopes during manufacturing, storage and transportation of the vaccines. Further improvements on the E. coli platform could be achieved by genetically modify the expression host for achieving certain specific goals, such as protein expression with post-translational modifications. Better understanding of protein production and self-assembly would facilitate scale up and better process control at commercial production scale. As a result, rapid and inexpensive VLP-based vaccine production could be realized for global accessibility.
Electronic supplementary material
Acknowledgements
Financial support for this work was provided by National Natural Science Foundation of China (81471934 & 31670935 & 31670939), Major Tech. Project funds of Fujian Province (2014Y2004).
Authors Contributions
Q.Z. conceptualized the review and X.H., X.W., J.Z., N.X., and Q.Z. wrote the manuscript. All authors improved the manuscript before it was submitted by the corresponding author.
Competing interests
The authors declare no competing interest.
Footnotes
Electronic supplementary material
Supplementary Information accompanies the paper on the npj Vaccines website (doi:10.1038/s41541-017-0006-8).
References
- 1.Zepp F. Principles of vaccine design-Lessons from nature. Vaccine. 2010;28:C14–C24 . doi: 10.1016/j.vaccine.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 2.Laporte J, Grosclaude J, Wantyghem J, Bernard S, Rouze P. Neutralization of the infective power of the foot-and-mouth disease virus in cell culture by using serums from pigs immunized with a purified viral protein. Comptes rendus hebdomadaires des seances de l’Academie des sciences. Serie D: Sciences naturelles. 1973;276:3399–3401. [PubMed] [Google Scholar]
- 3.Bachrach HL, Moore DM, McKercher PD, Polatnick J. Immune and antibody responses to an isolated capsid protein of foot-and-mouth disease virus. J. immunol. 1975;115:1636–1641. [PubMed] [Google Scholar]
- 4.Kleid DG, et al. Cloned viral protein vaccine for foot-and-mouth disease: responses in cattle and swine. Science. 1981;214:1125–1129. doi: 10.1126/science.6272395. [DOI] [PubMed] [Google Scholar]
- 5.Roldao A, Mellado MC, Castilho LR, Carrondo MJ, Alves PM. Virus-like particles in vaccine development. Expert Rev. Vaccines. 2010;9:1149–1176. doi: 10.1586/erv.10.115. [DOI] [PubMed] [Google Scholar]
- 6.Crisci E, Barcena J, Montoya M. Virus-like particles: the new frontier of vaccines for animal viral infections. Vet. Immunol. Immunopathol. 2012;148:211–225. doi: 10.1016/j.vetimm.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Venters C, Graham W, Cassidy W. Recombivax-HB: perspectives past, present and future. Expert Rev. Vaccines. 2004;3:119–129. doi: 10.1586/14760584.3.2.119. [DOI] [PubMed] [Google Scholar]
- 8.Andre FE, Safary A. Summary of clinical findings on Engerix-B, a genetically engineered yeast derived hepatitis B vaccine. Postgrad. Med. J. 1987;63:169–177. doi: 10.1136/pgmj.63.737.169. [DOI] [PubMed] [Google Scholar]
- 9.Wei M, et al. Bacteria expressed hepatitis E virus capsid proteins maintain virion-like epitopes. Vaccine. 2014;32:2859–2865. doi: 10.1016/j.vaccine.2014.02.025. [DOI] [PubMed] [Google Scholar]
- 10.Li SW, et al. The development of a recombinant hepatitis E vaccine HEV 239. Hum. vaccin immunother. 2015;11:908–914. doi: 10.1080/21645515.2015.1008870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kapusta J, et al. A plant-derived edible vaccine against hepatitis B virus. FASEB J.:Off. Publ. Fed. Am. Soc. Exp. Biol. 1999;13:1796–1799. doi: 10.1096/fasebj.13.13.1796. [DOI] [PubMed] [Google Scholar]
- 12.Hepatitis B vaccine recombinant (bio-technology general) BioHepB, Sci-B-Vac. Drugs R&D. 1999;2:199–200. doi: 10.2165/00126839-199902030-00010. [DOI] [PubMed] [Google Scholar]
- 13.Altman, L. K. A new insulin given approval for use in U.S. The New York Times (1982).
- 14.Kyriakopoulos S, Kontoravdi C. Analysis of the landscape of biologically-derived pharmaceuticals in Europe: dominant production systems, molecule types on the rise and approval trends. Eur. J. Pharm. Sci. 2013;48:428–441. doi: 10.1016/j.ejps.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 15.Sanchez-Garcia L, et al. Recombinant pharmaceuticals from microbial cells: a 2015 update. Microb. Cell. Fact. 2016;15:33. doi: 10.1186/s12934-016-0437-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walsh G. Biopharmaceutical benchmarks 2014. Nat. Biotechnol. 2014;32:992–1000. doi: 10.1038/nbt.3040. [DOI] [PubMed] [Google Scholar]
- 17.Zeltins A. Construction and characterization of virus-like particles: a review. Mol. Biotechnol. 2013;53:92–107. doi: 10.1007/s12033-012-9598-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wedemeyer H, Pischke S. Hepatitis: Hepatitis E vaccination[mdash]is HEV 239 the breakthrough? Nat. Rev. Gastroenterol. Hepatol. 2011;8:8–10. doi: 10.1038/nrgastro.2010.207. [DOI] [PubMed] [Google Scholar]
- 19.Proffitt A. First HEV vaccine approved. Nat. Biotech. 2012;30:300–300. doi: 10.1038/nbt0412-300a. [DOI] [Google Scholar]
- 20.Lua LH, et al. Bioengineering virus-like particles as vaccines. Biotechnol. Bioeng. 2014;111:425–440. doi: 10.1002/bit.25159. [DOI] [PubMed] [Google Scholar]
- 21.Choi JH, Keum KC, Lee SY. Production of recombinant proteins by high cell density culture of Escherichia coli. Chem. Eng. Sci. 2006;61:876–885. doi: 10.1016/j.ces.2005.03.031. [DOI] [Google Scholar]
- 22.Middelberg AP, et al. A microbial platform for rapid and low-cost virus-like particle and capsomere vaccines. Vaccine. 2011;29:7154–7162. doi: 10.1016/j.vaccine.2011.05.075. [DOI] [PubMed] [Google Scholar]
- 23.Andrus JK, Sherris J, Fitzsimmons JW, Kane MA, Aguado MT. Introduction of human papillomavirus vaccines into developing countries - international strategies for funding and procurement. Vaccine. 2008;26:K87–92. doi: 10.1016/j.vaccine.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 24.Liew MW, Rajendran A, Middelberg AP. Microbial production of virus-like particle vaccine protein at gram-per-litre levels. J. Biotechnol. 2010;150:224–231. doi: 10.1016/j.jbiotec.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 25.Yang C, et al. Hepatitis E virus capsid protein assembles in 4M urea in the presence of salts. Protein sci. 2013;22:314–326. doi: 10.1002/pro.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hill AB, Kilgore C, McGlynn M, Jones CH. Improving global vaccine accessibility. Curr. Opin. Biotechnol. 2016;42:67–73. doi: 10.1016/j.copbio.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 27.Balayan MS, et al. Evidence for a virus in non-A, non-B hepatitis transmitted via the fecal-oral route. Intervirology. 1983;20:23–31. doi: 10.1159/000149370. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J, et al. Hepatitis E virus: neutralizing sites, diagnosis, and protective immunity. Rev. Med. Virol. 2012;22:339–349. doi: 10.1002/rmv.1719. [DOI] [PubMed] [Google Scholar]
- 29.Kumar S, Subhadra S, Singh B, Panda BK. Hepatitis E virus: the current scenario. Int. J. Infect. Dis. 2013;17:e228–233. doi: 10.1016/j.ijid.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 30.Tam AW, et al. Hepatitis E virus (HEV): molecular cloning and sequencing of the full-length viral genome. Virology. 1991;185:120–131. doi: 10.1016/0042-6822(91)90760-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu FC, et al. Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: a large-scale, randomised, double-blind placebo-controlled, phase 3 trial. Lancet. 2010;376:895–902. doi: 10.1016/S0140-6736(10)61030-6. [DOI] [PubMed] [Google Scholar]
- 32.Zhang J, et al. Long-term efficacy of a hepatitis E vaccine. N. Engl. J. Med. 2015;372:914–922. doi: 10.1056/NEJMoa1406011. [DOI] [PubMed] [Google Scholar]
- 33.Zhang X, et al. Robust manufacturing and comprehensive characterization of recombinant hepatitis E virus-like particles in Hecolin. Vaccine. 2014;32:4039–4050. doi: 10.1016/j.vaccine.2014.05.064. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, et al. Comparable quality attributes of hepatitis E vaccine antigen with and without adjuvant adsorption-dissolution treatment. Hum. Vaccin. Immunother. 2015;11:1129–1139. doi: 10.1080/21645515.2015.1009343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang X, et al. Real-time stability of a hepatitis E vaccine (Hecolin) demonstrated with potency assays and multifaceted physicochemical methods. Vaccine. 2016;34:5871–5877. doi: 10.1016/j.vaccine.2016.10.045. [DOI] [PubMed] [Google Scholar]
- 36.Kremsdorf D, Jablonska S, Favre M, Orth G. Human papillomaviruses associated with epidermodysplasia verruciformis. II. Molecular cloning and biochemical characterization of human papillomavirus 3a, 8, 10, and 12 genomes . J. Virol. 1983;48:340–351. doi: 10.1128/jvi.48.2.340-351.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Syrjanen K, Syrjanen S, Lamberg M, Pyrhonen S, Nuutinen J. Morphological and immunohistochemical evidence suggesting human papillomavirus (HPV) involvement in oral squamous cell carcinogenesis. Int. J. Oral Surg. 1983;12:418–424. doi: 10.1016/S0300-9785(83)80033-7. [DOI] [PubMed] [Google Scholar]
- 38.Kirnbauer R, Booy F, Cheng N, Lowy DR, Schiller JT. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc Natl Acad Sci USA. 1992;89:12180–12184. doi: 10.1073/pnas.89.24.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McLemore MR. Gardasil: Introducing the new human papillomavirus vaccine. Clin. J. Oncol. Nurs. 2006;10:559–560. doi: 10.1188/06.CJON.559-560. [DOI] [PubMed] [Google Scholar]
- 40.Monie A, Hung CF, Roden R, Wu TC. Cervarix: a vaccine for the prevention of HPV 16, 18-associated cervical cancer. Biologics. 2008;2:97–105. [PMC free article] [PubMed] [Google Scholar]
- 41.Printz C. FDA approves Gardasil 9 for more types of HPV. Cancer. 2015;121:1156–1157. doi: 10.1002/cncr.29374. [DOI] [PubMed] [Google Scholar]
- 42.Schiller JT, Castellsague X, Garland SM. A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine. 2012;30:F123–F138. doi: 10.1016/j.vaccine.2012.04.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vesikari T, et al. A randomized, double-blind, phase III study of the immunogenicity and safety of a 9-valent human papillomavirus L1 virus-like particle vaccine (V503) versus gardasil(R) in 9-15-year-old girls. Pediatr. Infect. Dis. J. 2015;34:992–998. doi: 10.1097/INF.0000000000000773. [DOI] [PubMed] [Google Scholar]
- 44.Schiller JT, Müller M. Next generation prophylactic human papillomavirus vaccines. Lancet Oncol. 2015;16:e217–e225. doi: 10.1016/S1470-2045(14)71179-9. [DOI] [PubMed] [Google Scholar]
- 45.Clendinen, C., Zhang, Y., Warburton, R. N. & Light, D. W. Manufacturing costs of HPV vaccines for developing countries. Vaccine34, 5984–5989 (2016). [DOI] [PubMed]
- 46.de Sanjose S, et al. Human papillomavirus (HPV) and related cancers in the Global Alliance for Vaccines and Immunization (GAVI) countries. A WHO/ICO HPV Information Centre Report. Vaccine. 2012;30:D1-83. doi: 10.1016/j.vaccine.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 47.Ribeiro-Muller L, Muller M. Prophylactic papillomavirus vaccines. Clin. Dermatol. 2014;32:235–247. doi: 10.1016/j.clindermatol.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 48.Hu YM, et al. Safety of an Escherichia coli-expressed bivalent human papillomavirus (types 16 and 18) L1 virus-like particle vaccine: an open-label phase I clinical trial. Hum. Vaccin. Immunother. 2014;10:469–475. doi: 10.4161/hv.26846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu T, et al. Immunogenicity and safety of an E. coli-produced bivalent human papillomavirus (type 16 and 18) vaccine: A randomized controlled phase 2 clinical trial. Vaccine. 2015;33:3940–3946. doi: 10.1016/j.vaccine.2015.06.052. [DOI] [PubMed] [Google Scholar]
- 50.Frietze KM, Peabody DS, Chackerian B. Engineering virus-like particles as vaccine platforms. Curr. Opin. Virol. 2016;18:44–49. doi: 10.1016/j.coviro.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Caraballo H, King K. Emergency department management of mosquito-borne illness: malaria, dengue, and West Nile virus. Emerg. Med. Pract. 2014;16:1–23. [PubMed] [Google Scholar]
- 52.Geels MJ, et al. European Vaccine Initiative: lessons from developing malaria vaccines. Expert. Rev. Vaccines. 2011;10:1697–1708. doi: 10.1586/erv.11.158. [DOI] [PubMed] [Google Scholar]
- 53.Nardin EH, et al. Phase I testing of a malaria vaccine composed of hepatitis B virus core particles expressing Plasmodium falciparum circumsporozoite epitopes. Infect. Immun. 2004;72:6519–6527. doi: 10.1128/IAI.72.11.6519-6527.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ameghi A, et al. Protective immunity against homologous and heterologous influenza virus lethal challenge by immunization with new recombinant chimeric HA2-M2e fusion protein in BALB/C mice. Viral. Immunol. 2016;29:228–234. doi: 10.1089/vim.2015.0050. [DOI] [PubMed] [Google Scholar]
- 55.Wibowo N, et al. Protective efficacy of a bacterially produced modular capsomere presenting M2e from influenza: Extending the potential of broadly cross-protecting epitopes. Vaccine. 2014;32:3651–3655. doi: 10.1016/j.vaccine.2014.04.062. [DOI] [PubMed] [Google Scholar]
- 56.Pumpens P, Grens E. The true story and advantages of the famous Hepatitis B virus core particles: Outlook 2016. Mol. Biol. (Mosk). 2016;50:558–576. doi: 10.1134/S0026893316040099. [DOI] [PubMed] [Google Scholar]
- 57.De Filette M, et al. Improved design and intranasal delivery of an M2e-based human influenza A vaccine. Vaccine. 2006;24:6597–6601. doi: 10.1016/j.vaccine.2006.05.082. [DOI] [PubMed] [Google Scholar]
- 58.De Filette M, et al. The universal influenza vaccine M2e-HBc administered intranasally in combination with the adjuvant CTA1-DD provides complete protection. Vaccine. 2006;24:544–551. doi: 10.1016/j.vaccine.2005.08.061. [DOI] [PubMed] [Google Scholar]
- 59.De Filette M, et al. Universal influenza A M2e-HBc vaccine protects against disease even in the presence of pre-existing anti-HBc antibodies. Vaccine. 2008;26:6503–6507. doi: 10.1016/j.vaccine.2008.09.038. [DOI] [PubMed] [Google Scholar]
- 60.Fiers W, et al. M2e-based universal influenza A vaccine. Vaccine. 2009;27:6280–6283. doi: 10.1016/j.vaccine.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 61.Low JG, et al. Safety and immunogenicity of a virus-like particle pandemic influenza A (H1N1) 2009 vaccine: results from a double-blinded, randomized Phase I clinical trial in healthy Asian volunteers. Vaccine. 2014;32:5041–5048. doi: 10.1016/j.vaccine.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 62.Aguilar JC, et al. Development of a nasal vaccine for chronic hepatitis B infection that uses the ability of hepatitis B core antigen to stimulate a strong Th1 response against hepatitis B surface antigen. Immunol. Cell. Biol. 2004;82:539–546. doi: 10.1111/j.0818-9641.2004.01278.x. [DOI] [PubMed] [Google Scholar]
- 63.Betancourt AA, et al. Phase I clinical trial in healthy adults of a nasal vaccine candidate containing recombinant hepatitis B surface and core antigens. Int. J. Infect. Dis. 2007;11:394–401. doi: 10.1016/j.ijid.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 64.Lobaina Y, et al. The effect of the parenteral route of administration on the immune response to simultaneous nasal and parenteral immunizations using a new HBV therapeutic vaccine candidate. Viral. Immunol. 2010;23:521–529. doi: 10.1089/vim.2010.0024. [DOI] [PubMed] [Google Scholar]
- 65.Lobaina Y, et al. Demonstration of safety, immunogenicity and evidences of efficacy of the therapeutic vaccine candidate HeberNasvac and characterization of chronic hepatitis B patient populations. Biotecnología Aplicada. 2015;32:3511–3513. [Google Scholar]
- 66.ABX203 (HeberNasvac) granted cuban marketing authorization to treat chronic Hepatitis B. Available at: http://www.abivax.com/images/pdf/151208_ABX203_Cuban_Authorization.pdf (2015).
- 67.Maurer P, et al. A therapeutic vaccine for nicotine dependence: preclinical efficacy, and Phase I safety and immunogenicity. Eur. J. Immunol. 2005;35:2031–2040. doi: 10.1002/eji.200526285. [DOI] [PubMed] [Google Scholar]
- 68.Cornuz J, et al. A vaccine against nicotine for smoking cessation: a randomized controlled trial. PLoS ONE. 2008;3:e2547. doi: 10.1371/journal.pone.0002547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ambuhl PM, et al. A vaccine for hypertension based on virus-like particles: preclinical efficacy and phase I safety and immunogenicity. J. Hypertens. 2007;25:63–72. doi: 10.1097/HJH.0b013e32800ff5d6. [DOI] [PubMed] [Google Scholar]
- 70.Farlow MR, et al. Long-term treatment with active Abeta immunotherapy with CAD106 in mild Alzheimer’s disease. Alzheimer’s Res. Ther. 2015;7:23. doi: 10.1186/s13195-015-0108-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Godyn J, Jonczyk J, Panek D, Malawska B. Therapeutic strategies for Alzheimer’s disease in clinical trials. Pharmacolog. Rep. 2016;68:127–138. doi: 10.1016/j.pharep.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 72.Winblad B, et al. Safety, tolerability, and antibody response of active Abeta immunotherapy with CAD106 in patients with Alzheimer’s disease: randomised, double-blind, placebo-controlled, first-in-human study. Lancet Neurol. 2012;11:597–604. doi: 10.1016/S1474-4422(12)70140-0. [DOI] [PubMed] [Google Scholar]
- 73.Spohn G, et al. A VLP-based vaccine targeting domain III of the West Nile virus E protein protects from lethal infection in mice. Virol. J. 2010;7:146. doi: 10.1186/1743-422X-7-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee CD, Yan YP, Liang SM, Wang TF. Production of FMDV virus-like particles by a SUMO fusion protein approach in Escherichia coli. J. Biomed. Sci. 2009;16:69. doi: 10.1186/1423-0127-16-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lorenzo LJ, et al. Assembly of truncated HCV core antigen into virus-like particles in Escherichia coli. Biochem. Biophys. Res. Commun. 2001;281:962–965. doi: 10.1006/bbrc.2001.4449. [DOI] [PubMed] [Google Scholar]
- 76.WHO guidelines on nonclinical evaluation of vaccines. Available at: http://www.who.int/biologicals/publications/trs/areas/vaccines/nonclinical_evaluation/ANNEX%201Nonclinical.P31-63.pdf?ua=1 (2015).
- 77.Guidelines on clinical evaluation of vaccines: regulatory expectations. Available at: http://www.who.int/biologicals/Clinical_guidelines_27_January_2016.pdf?ua=1 (2016).
- 78.Zhao H, et al. Correlation between ELISA and pseudovirion-based neutralisation assay for detecting antibodies against human papillomavirus acquired by natural infection or by vaccination. Hum. Vaccin. Immunother. 2014;10:740–746. doi: 10.4161/hv.27619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang X, et al. Lessons learned from successful human vaccines: Delineating key epitopes by dissecting the capsid proteins. Hum. Vaccin. Immunother. 2015;11:1277–1292. doi: 10.1080/21645515.2015.1016675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shirbaghaee Z. Different applications of virus-like particles in biology and medicine: Vaccination and delivery systems. Biopolymers. 2016;105:113–132. doi: 10.1002/bip.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang Z, Christensen N, Schiller JT, Dillner J. A monoclonal antibody against intact human papillomavirus type 16 capsids blocks the serological reactivity of most human sera. J. Gen. Virol. 1997;78:2209–2215. doi: 10.1099/0022-1317-78-9-2209. [DOI] [PubMed] [Google Scholar]
- 82.Zhao Q, Li S, Yu H, Xia N, Modis Y. Virus-like particle-based human vaccines: quality assessment based on structural and functional properties. Trends Biotechnol. 2013;31:654–663. doi: 10.1016/j.tibtech.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 83.Deschuyteneer M, et al. Molecular and structural characterization of the L1 virus-like particles that are used as vaccine antigens in Cervarix, the AS04-adjuvanted HPV-16 and -18 cervical cancer vaccine. Hum. Vaccin. 2010;6:407–419. doi: 10.4161/hv.6.5.11023. [DOI] [PubMed] [Google Scholar]
- 84.Hickey JM, et al. Challenges and opportunities of using liquid chromatography and mass spectrometry methods to develop complex vaccine antigens as pharmaceutical dosage forms. J. Chromatogr. B. Analyt. Technol. Biomed. Life. Sci. 2016;1032:23–38. doi: 10.1016/j.jchromb.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 85.Zhao Q, et al. Characterization of virus-like particles in GARDASIL by cryo transmission electron microscopy. Hum. Vaccin. Immunother. 2013;10:734–739. doi: 10.4161/hv.27316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sitrin, R. D., Zhao, Q., Potter, C. S., Carragher, B. & Washabaugh, M. W. Recombinant virus-like particle protein vaccines. 81–112 (2015).
- 87.Mach H, et al. Disassembly and reassembly of yeast-derived recombinant human papillomavirus virus-like particles (HPV VLPs) J. Pharm. Sci. 2006;95:2195–2206. doi: 10.1002/jps.20696. [DOI] [PubMed] [Google Scholar]
- 88.Mulder AM, et al. Toolbox for non-intrusive structural and functional analysis of recombinant VLP based vaccines: a case study with hepatitis B vaccine. PLoS. ONE. 2012;7:e33235. doi: 10.1371/journal.pone.0033235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhao Q, et al. Disassembly and reassembly improves morphology and thermal stability of human papillomavirus type 16 virus-like particles. Nanomedicine. 2012;8:1182–1189. doi: 10.1016/j.nano.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 90.Shank-Retzlaff ML, et al. Evaluation of the thermal stability of Gardasil. Hum. Vaccin. 2006;2:147–154. doi: 10.4161/hv.2.4.2989. [DOI] [PubMed] [Google Scholar]
- 91.Jain NK, et al. Formulation and stabilization of recombinant protein based virus-like particle vaccines. Adv. Drug. Deliv. Rev. 2015;93:42–55. doi: 10.1016/j.addr.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 92.Li M, et al. Quantitative and epitope-specific antigenicity analysis of the human papillomavirus 6 capsid protein in aqueous solution or when adsorbed on particulate adjuvants. Vaccine. 2016;24:874–885. doi: 10.1016/j.vaccine.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 93.Christensen ND, et al. Surface conformational and linear epitopes on HPV-16 and HPV-18 L1 virus-like particles as defined by monoclonal antibodies. Virology. 1996;223:174–184. doi: 10.1006/viro.1996.0466. [DOI] [PubMed] [Google Scholar]
- 94.Brown MJ, Seitz H, Towne V, Muller M, Finnefrock AC. Development of neutralizing monoclonal antibodies for oncogenic human papillomavirus types 31, 33, 45, 52, and 58. Clin. Vaccine Immunol.: CVI. 2014;21:587–593. doi: 10.1128/CVI.00773-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhu Y, et al. Toward the development of monoclonal antibody-based assays to probe virion-like epitopes in hepatitis B vaccine antigen. Hum. Vaccin. Immunother. 2014;10:1013–1023. doi: 10.4161/hv.27753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Riddell MA, Li F, Anderson DA. Identification of immunodominant and conformational epitopes in the capsid protein of hepatitis E virus by using monoclonal antibodies. J. Virol. 2000;74:8011–8017. doi: 10.1128/JVI.74.17.8011-8017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhao Q, et al. Real time monitoring of antigenicity development of HBsAg virus-like particles (VLPs) during heat- and redox-treatment. Biochem. Biophys. Res. Commun. 2011;408:447–453. doi: 10.1016/j.bbrc.2011.04.048. [DOI] [PubMed] [Google Scholar]
- 98.Cao L, Wang X, Fang M, Xia N, Zhao Q. Detection of subtle differences in analogous viral capsid proteins by allowing unrestricted specific interaction in solution competition ELISA. J. Virol. Methods. 2016;236:1–4. doi: 10.1016/j.jviromet.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 99.Weng Z, Zhao Q. Utilizing ELISA to monitor protein-protein interaction. Methods Mol. Biol. 2015;1278:341–352. doi: 10.1007/978-1-4939-2425-7_21. [DOI] [PubMed] [Google Scholar]
- 100.Mostafa, M. M., Al-Ghobashy, M. A., Fathalla, F. A. & Salem, M. Y. Optimization and validation of ELISA immunoassay for the evaluation of in-vitro relative potency of a four-component human papillomavirus vaccine products. Biologicals: journal of the International Association of Biological Standardization. 44, 596–599 (2016). [DOI] [PubMed]
- 101.Shank-Retzlaff M, et al. Correlation between mouse potency and in vitro relative potency for human papillomavirus type 16 virus-like particles and Gardasil vaccine samples. Hum. Vaccin. 2005;1:191–197. doi: 10.4161/hv.1.5.2126. [DOI] [PubMed] [Google Scholar]
- 102.Brune KD, et al. Plug-and-display: decoration of virus-like particles via isopeptide bonds for modular immunization. Sci. Rep. 2016;6:19234. doi: 10.1038/srep19234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Thrane S, et al. A novel virus-like particle based vaccine platform displaying the placental malaria antigen VAR2CSA. PLoS. ONE. 2015;10:e0143071. doi: 10.1371/journal.pone.0143071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pitoiset F, Vazquez T, Bellier B. Enveloped virus-like particle platforms: vaccines of the future? Exp. Rev. Vaccin. 2015;14:913–915. doi: 10.1586/14760584.2015.1046440. [DOI] [PubMed] [Google Scholar]
- 105.Freivalds J, et al. Assembly of bacteriophage Qbeta virus-like particles in yeast Saccharomyces cerevisiae and Pichia pastoris. J. Biotechnol. 2006;123:297–303. doi: 10.1016/j.jbiotec.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 106.Pickett GG, Peabody DS. Encapsidation of heterologous RNAs by bacteriophage MS2 coat protein. Nucleic. Acids. Res. 1993;21:4621–4626. doi: 10.1093/nar/21.19.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jegerlehner A, et al. TLR9 signaling in B cells determines class switch recombination to IgG2a. J. immunol. 2007;178:2415–2420. doi: 10.4049/jimmunol.178.4.2415. [DOI] [PubMed] [Google Scholar]
- 108.Akache, B. et al. Anti-IgE Qb-VLP conjugate vaccine self-adjuvants through activation of TLR7. Vaccines4, 1 (2016). [DOI] [PMC free article] [PubMed]
- 109.Pasquale AD, Preiss S, Silva FT, Garcon N. Vaccine adjuvants: from 1920 to 2015 and Beyond. . Vaccines. 2015;3:320–343. doi: 10.3390/vaccines3020320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Walsh G, Jefferis R. Post-translational modifications in the context of therapeutic proteins. Nat. Biotechnol. 2006;24:1241–1252. doi: 10.1038/nbt1252. [DOI] [PubMed] [Google Scholar]
- 111.Michel ML, Tiollais P. Hepatitis B vaccines: protective efficacy and therapeutic potential. Pathol. Biol. 2010;58:288–295. doi: 10.1016/j.patbio.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 112.Freivalds J, et al. Highly efficient production of phosphorylated hepatitis B core particles in yeast Pichia pastoris. Protein Expr. Purif. 2011;75:218–224. doi: 10.1016/j.pep.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 113.Spiess C, et al. Bispecific antibodies with natural architecture produced by co-culture of bacteria expressing two distinct half-antibodies. Nat. Biotechnol. 2013;31:753–758. doi: 10.1038/nbt.2621. [DOI] [PubMed] [Google Scholar]
- 114.Zhao Q, et al. Disassembly and reassembly of human papillomavirus virus-like particles produces more virion-like antibody reactivity. Virol. J. 2012;9:52. doi: 10.1186/1743-422X-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mamat U, et al. Detoxifying Escherichia coli for endotoxin-free production of recombinant proteins. Microb. Cell. Fact. 2015;14:57. doi: 10.1186/s12934-015-0241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rueda F, et al. Structural and functional features of self-assembling protein nanoparticles produced in endotoxin-free Escherichia coli. Microb. Cell. Fact. 2016;15:59. doi: 10.1186/s12934-016-0457-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chuan YP, Fan YY, Lua L, Middelberg AP. Quantitative analysis of virus-like particle size and distribution by field-flow fractionation. Biotechnol. Bioeng. 2008;99:1425–1433. doi: 10.1002/bit.21710. [DOI] [PubMed] [Google Scholar]
- 118.Pease LF, 3rd, et al. Quantitative characterization of virus-like particles by asymmetrical flow field flow fractionation, electrospray differential mobility analysis, and transmission electron microscopy. Biotechnol. Bioeng. 2009;102:845–855. doi: 10.1002/bit.22085. [DOI] [PubMed] [Google Scholar]
- 119.Hu L, et al. Biophysical characterization and conformational stability of Ebola and Marburg virus-like particles. J. Pharm. Sci. 2011;100:5156–5173. doi: 10.1002/jps.22724. [DOI] [PubMed] [Google Scholar]
- 120.Li, Z., et al. The C-terminal arm of the human papillomavirus major capsid protein is immunogenic and involved in virus-host interaction. Structure 24, 874–885 (2016). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.