Abstract
Remote ischemic preconditioning (RIPC) by repeated brief cycles of limb ischemia/reperfusion may reduce myocardial ischemia/reperfusion injury and improve patients‘ prognosis after elective coronary artery bypass graft (CABG) surgery. The signal transducer and activator of transcription (STAT)5 activation in left ventricular myocardium is associated with RIPC´s cardioprotection. Cytokines and growth hormones typically activate STATs and could therefore act as humoral transfer factors of RIPC´s cardioprotection. We here determined arterial plasma concentrations of 25 different cytokines, growth hormones, and other factors which have previously been associated with cardioprotection, before (baseline)/after RIPC or placebo (n = 23/23), respectively, and before/after ischemic cardioplegic arrest in CABG patients. RIPC-induced protection was reflected by a 35% reduction of serum troponin I release. With the exception of interleukin-1α, none of the humoral factors changed in their concentrations after RIPC or placebo, respectively. Interleukin-1α, when normalized to baseline, increased after RIPC (280 ± 56%) but not with placebo (97 ± 15%). The interleukin-1α concentration remained increased until after ischemic cardioplegic arrest and was also higher than with placebo in absolute concentrations (25 ± 6 versus 16 ± 3 pg/mL). Only interleukin-1α possibly fulfills the criteria which would be expected from a substance to be released in response to RIPC and to protect the myocardium during ischemic cardioplegic arrest.
Introduction
Remote ischemic conditioning (RIC) by brief episodes of ischemia/reperfusion in parenchymal organs or limbs before (remote ischemic preconditioning; RIPC) or during (remote ischemic perconditioning) sustained myocardial ischemia and subsequent reperfusion is a non-invasive strategy to protect the myocardium from irreversible ischemia/reperfusion injury. The protection by RIC has been demonstrated in many experimental studies and confirmed in patients undergoing elective interventional1 or surgical coronary revascularization2–5 and in patients with reperfused acute myocardial infarction6–10. The efficacy of RIC was established by a reduction in cardiac biomarker release1–5,9 or by cardiac imaging6–8,10 and resulted in improved short-term4,7 and long-term clinical outcome1,3,11. However, two large-scaled randomized trials in patients undergoing cardiac surgery, ERICCA12 and RIPHeart13, were neutral and did not confirm reduced biomarker release and improved clinical outcome with RIPC. Potential reasons for the lack of protection by RIPC in both trials relate to the use of propofol anesthesia14,15 and the inclusion of patients undergoing isolated or additional valve surgery which causes traumatic rather than ischemia/reperfusion myocardial injury and may have diluted the protection by RIPC15,16. For a more successful use of RIC in patients, a better understanding of the signal transfer from the stimulus site to the heart and of RIC´s intracellular signal transduction is mandatory.
In different animal models and in healthy volunteers, a neuronal and a humoral signal transfer as well as a neurohumoral interaction in signal transfer have been proposed17,18. A humoral signal transfer has been evidenced by the transfer of cardioprotection via plasma19–22 or plasma-derived dialysate/filtrate23–25 from one individual to another individual’s heart, even across species. In respective experiments, several amino acids26–29, cytokines/chemokines30–33, neuropeptides34,35 as well as other substances, such as adenosine36,37, apolipoprotein-A1 (Apo-A1)38,39, circulating RNase-140, glucagon like peptide-1 (GLP-1)41, microRNA-14442 and nitrite24 have been identified and proposed as potential humoral transfer factors of RIC. Apo-A1, cytokines, circulating RNase-1, microRNA-144 and nitrite have been reported in healthy volunteers in association with the RIC procedure24,33,38,40,42. In patients undergoing cardiac surgery, only some of the potential humoral transfer factors (amino acids, circulating RNase-1, cytokines/chemokines) have been associated with the RIC procedure26,30,31,40, but only in two studies there was also a reduction of myocardial injury by RIC30,31, and one of these studies was in infants30.
Within the myocardium, the putative humoral factors activate intracellular signaling pathways, which ultimately transmit the cardioprotective signal to end-effectors, notably the mitochondria22,43,44. Conceptually, the intracellular signaling pathways have been categorized as the nitric oxide synthase/protein kinase G pathway, the reperfusion injury salvage kinase pathway, and the survival activating factor enhancement pathway18,45,46. In left ventricular biopsies of patients undergoing coronary artery bypass graft (CABG) surgery, only the phosphorylation of signal transducer and activator of transcription (STAT)5 of the survival activating factor enhancement pathway47 was associated with cardioprotection by RIPC48,49. STAT is typically activated by members of the cytokine and the growth hormone family44,50,51. Therefore, cytokines and growth hormones could potentially serve as humoral transfer factors of RIPC in patients.
We have now quantified the arterial concentration of a number of humoral factors, which may potentially activate STAT and the survival activating factor enhancement pathway, in a cohort of consecutive patients undergoing CABG surgery under isoflurane anesthesia before and after RIPC/placebo, respectively, and before and after ischemic cardioplegic arrest: chemokines/cytokines, i.e. erythropoietin (EPO)52, interleukin-(IL-)1α53, IL-1β54, IL-255, IL-656, IL-857, IL-1058, IL-1555, IL-1759, IL-3360, stromal cell-derived factor-1α (SDF-1α)61, tumor necrosis factor-α (TNF-α)62 and growth hormones, i.e. growth hormone (GH)63,64, growth differentiation factor-11 (GDF-11)65, growth hormone releasing hormone (GHRH)66, growth hormone-releasing peptide (GHRP)67. In addition, we determined a few other factors which have been reported before in association with cardioprotection and/or STAT activation, i.e. Apo-A138,39, GLP-141, HIF-1α68,69, leptin70,71, pentraxin-372, prolactin73, RNase-140, survivin74,75 and thymosin-β476,77.
Results
Cardioprotection by RIPC
Demographics and intraoperative characteristics were not different between patients with RIPC and placebo, respectively (Table 1). The preoperative serum troponin I (TnI) concentration did not differ between patients with RIPC and placebo, respectively. The TnI concentration area under the curve (AUC) over 72 h was decreased by RIPC, indicating cardioprotection (190 ± 16 versus 543 ± 145 ng/mL × 72 h, p = 0.015; Fig. 1). In this small cohort of consecutive patients, the RIPC-related decrease in TnI release was more pronounced than that in the larger cohort reported before3.
Table 1.
Patient demographics and intraoperative characteristics of patients.
| RIPC (n = 23) | placebo (n = 23) | p-value | |
|---|---|---|---|
| demographics | |||
| age [years] | 66.4 ± 1.5 | 67.7 ± 2.0 | 0.479 |
| sex [male] | 23 | 19 | 0.109 |
| body weight [kg] | 87.2 ± 2.7 | 84.6 ± 2.6 | 0.499 |
| risk factors and co-morbidities | |||
| diabetes mellitus | 11 | 6 | 0.221 |
| hypertension | 20 | 22 | 0.608 |
| hyperlipidemia | 9 | 8 | 1.000 |
| peripheral vessel disease | 2 | 4 | 0.666 |
| COPD | 4 | 2 | 0.666 |
| renal disease [creatinine > 200 μmol/L] | 1 | 3 | 0.608 |
| cardiac status | |||
| angina CCS III–IV | 1 | 2 | 1.000 |
| previous myocardial infarction | 2 | 5 | 0.414 |
| left ventricular ejection fraction [%] | 50.5 ± 2.1 | 51.5 ± 2.3 | 0.747 |
| medication | |||
| aspirin | 23 | 19 | 0.109 |
| clopidogrel | 4 | 2 | 0.666 |
| β-blockers | 20 | 16 | 0.284 |
| statins | 17 | 17 | 1.000 |
| ACE inhibitors or ARBs | 8 | 10 | 0.763 |
| risk scores | |||
| additive EuroSCORE | 3.9 ± 0.5 | 5.0 ± 0.6 | 0.174 |
| logistic EuroSCORE [%] | 3.4 ± 0.5 | 5.1 ± 1.0 | 0.109 |
| EuroSCORE II [%] | 1.8 ± 0.2 | 2.8 ± 0.4 | 0.058 |
| intraoperative characteristics | |||
| time from end of RIPC/placebo to ischemic cardioplegic arrest [min] | 64.6 ± 8.0 | 49.8 ± 10.0 | 0.280 |
| time from end of RIPC/placebo to reperfusion [min] | 130.2 ± 8.1 | 118.8 ± 7.1 | 0.304 |
| aortic cross-clamp duration [min] | 70.0 ± 4.8 | 65.4 ± 3.7 | 0.454 |
| cardioplegia [mL] | 1528 ± 46 | 1546 ± 49 | 0.798 |
| reperfusion time [min] | 34.8 ± 3.1 | 38.9 ± 3.6 | 0.393 |
| number of bypass grafts | 3.7 ± 0.2 | 3.6 ± 0.2 | 0.752 |
| transit time graft flow [mL/min] | 87.7 ± 12.3 | 66.6 ± 9.9 | 0.204 |
Data are mean ± standard error of the mean or number. Patient demographics and intraoperative characteristics were compared using unpaired Student’s t-test (continuous data) and 2-tailed Fisher’s exact test (categorical data). Chronic obstructive pulmonary disease (COPD), Canadian cardiovascular society score (CCS), angiotensin-converting enzyme (ACE), angiotensin-II-receptor blockers (ARBs), European system for cardiac operative risk evaluation (EuroSCORE), remote ischemic preconditioning (RIPC). Reperfusion time: time from release of aortic cross-clamp to end of cardiopulmonary bypass.
Figure 1.
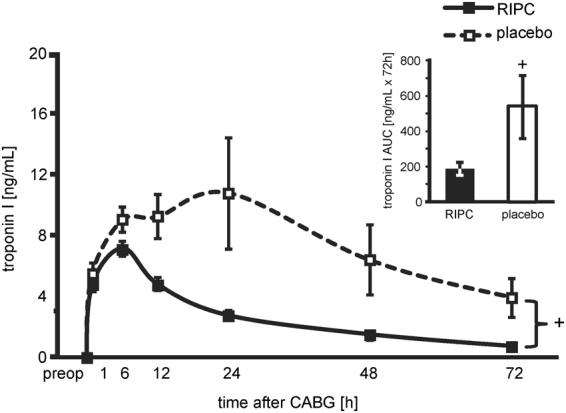
Serum concentration of troponin I. The serum concentration of troponin I at baseline before (preop) and over 72 h after coronary artery bypass graft (CABG) surgery in patients undergoing remote ischemic preconditioning (RIPC; n = 23, black symbols/bars) or placebo (n = 23, white symbols/bars). Decreased troponin I concentrations confirmed protection by RIPC. Insert: area under the curve (AUC) for serum troponin I concentrations over 72 h. +p < 0.05 versus RIPC using 2-way ANOVA for repeated measures or unpaired Student’s t-test (AUC).
Concentration of humoral factors
The concentrations of the analyzed humoral factors were not significantly different between RIPC and placebo at baseline, with the exception of prolactin, which was lower with RIPC than with placebo (Table 2). To normalize for interindividual differences, the concentrations of all factors were also normalized to their baseline.
Table 2.
Concentration of humoral factors.
| parameter | protocol | original data | parameter | protocol | data normalized to baseline | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| baseline | after placebo/RIPC | before ischemic cardioplegic arrest | after ischemic cardioplegic arrest | after placebo/RIPC | before ischemic cardioplegic arrest | after ischemic cardioplegic arrest | ||||
| Apo-A1 [ng/mL] | RIPC | 408 ± 36 | 365 ± 42 | 364 ± 36 | 251 ± 32*,# | Apo-A1 [%] | RIPC | 106 ± 23 | 108 ± 18 | 68 ± 9*,# |
| placebo | 351 ± 35 | 337 ± 57 | 292 ± 35 | 164 ± 21*,# | placebo | 115 ± 23 | 94 ± 12 | 55 ± 10*,# | ||
| EPO [pg/mL] | RIPC | 22 ± 2 | 22 ± 2 | 20 ± 2 | 22 ± 3 | EPO [%] | RIPC | 134 ± 35 | 105 ± 26 | 103 ± 19 |
| placebo | 28±3 | 26 ± 3 | 22 ± 3 | 21 ± 3 | placebo | 100 ± 15 | 84 ± 11 | 77 ± 9 | ||
| GDF-11 [fg/mL] | RIPC | 6836 ± 1544 | 5678 ± 1031 | 6104 ± 1174 | 15244 ± 2244*,# | GDF-11 [%] | RIPC | 99 ± 17 | 106 ± 17 | 275 ± 54*,# |
| placebo | 5314 ± 633 | 5960 ± 771 | 6329 ± 852 | 9764 ± 1303*,#,+ | placebo | 126 ± 19 | 142 ± 22 | 219 ± 34*,# | ||
| GHRH [fg/mL] | RIPC | 1694 ± 56 | 1636 ± 52 | 1656 ± 48 | 1621 ± 59 | GHRH [%] | RIPC | 99 ± 4 | 100 ± 4 | 98 ± 4 |
| placebo | 1538 ± 55 | 1664 ± 59 | 1699 ± 54 | 1636 ± 48 | placebo | 109 ± 2+ | 113 ± 4*,+ | 109 ± 4*,+ | ||
| GHRP [fg/mL] | RIPC | 998 ± 189 | 1008 ± 208 | 1220 ± 236 | 1096 ± 187 | GHRP [%] | RIPC | 275 ± 180 | 338 ± 187 | 123 ± 18 |
| placebo | 1211 ± 263 | 1147 ± 240 | 1120 ± 236 | 1117 ± 145 | placebo | 120 ± 16 | 137 ± 18 | 225 ± 37 | ||
| GLP-1 [pg/mL] | RIPC | 1.5 ± 0.2 | 1.4 ± 0.2 | 1.5 ± 0.2 | 2.2 ± 0.2*,# | GLP-1 [%] | RIPC | 93 ± 3 | 103 ± 7 | 173 ± 17*,# |
| placebo | 1.5 ± 0.2 | 1.5 ± 0.2 | 1.6 ± 0.2 | 2.4 ± 0.2*,# | placebo | 107 ± 8 | 122 ± 16 | 201 ± 29*,# | ||
| GH [pg/mL] | RIPC | 830 ± 146 | 323 ± 85* | 221 ± 44* | 882 ± 158# | GH [%] | RIPC | 60 ± 19 | 153 ± 99 | 500 ± 327* |
| placebo | 510 ± 125 | 401 ± 124 | 320 ± 93 | 1059 ± 270*,# | placebo | 127 ± 33 | 305 ± 116 | 820 ± 221*,# | ||
| HIF-1α [fg/mL] | RIPC | 18 ± 2 | 16 ± 3 | 17 ± 2 | 17 ± 2 | HIF-1α [%] | RIPC | 101 ± 20 | 102 ± 12 | 101 ± 10 |
| placebo | 19 ± 3 | 21 ± 5 | 20 ± 5 | 22 ± 6 | placebo | 133 ± 17 | 130 ± 18 | 159 ± 33 | ||
| IL-1α [pg/mL] | RIPC | 12 ± 2 | 20 ± 2 | 16 ± 2 | 25 ± 6*,# | IL-1α [%] | RIPC | 280 ± 56* | 235 ± 96* | 298 ± 71* |
| placebo | 18 ± 3 | 18 ± 2 | 15 ± 3 | 16 ± 3+ | placebo | 97 ± 15+ | 97 ± 16+ | 135 ± 40+ | ||
| IL-1β [fg/mL] | RIPC | 746 ± 220 | 734 ± 210 | 881 ± 223 | 1630 ± 316*,# | IL-1β [%] | RIPC | 220 ± 94 | 251 ± 75 | 517 ± 173*,# |
| placebo | 631 ± 97 | 752 ± 100 | 739 ± 103 | 1367 ± 222*,# | placebo | 169 ± 28 | 178 ± 46 | 337 ± 66* | ||
| IL-2 [fg/mL] | RIPC | 4936 ± 440 | 6205 ± 735 | 5391 ± 601 | 5908 ± 685 | IL-2 [%] | RIPC | 143 ± 27 | 160 ± 44 | 189 ± 53* |
| placebo | 7040 ± 1326 | 7291 ± 1000 | 5114 ± 458 | 7351 ± 1098 | placebo | 132 ± 21 | 103 ± 14 | 211 ± 59*,# | ||
| IL-6 [fg/mL] | RIPC | 4108 ± 715 | 4133 ± 664 | 5498 ± 947 | 14633 ± 941*,# | IL-6 [%] | RIPC | 105 ± 6 | 166 ± 23 | 588 ± 92*,# |
| placebo | 6239 ± 882 | 6169 ± 890 | 6251 ± 789 | 16572 ± 1200*,# | placebo | 99 ± 2 | 129 ± 16 | 439 ± 85*,#,+ | ||
| IL-8 [pg/mL] | RIPC | 14 ± 1 | 14 ± 1 | 18 ± 3 | 59 ± 12*,# | IL-8 [%] | RIPC | 100 ± 2 | 125 ± 22 | 441 ± 65*,# |
| placebo | 16 ± 2 | 15 ± 2 | 18 ± 3 | 49 ± 9*,#,+ | placebo | 100 ± 4 | 122 ± 20 | 367 ± 42*,# | ||
| IL-10 [fg/mL] | RIPC | 3449 ± 826 | 3943 ± 1034 | 11320 ± 5458 | 56674 ± 1921*,# | IL-10 [%] | RIPC | 107 ± 7 | 324 ± 129 | 2227 ± 792*,# |
| placebo | 2875 ± 419 | 3096 ± 487 | 3827 ± 884 | 57356 ± 1216*,# | placebo | 109 ± 11 | 146 ± 23 | 3120 ± 1007*,# | ||
| IL-15 [fg/mL] | RIPC | 4380 ± 280 | 4312 ± 324 | 3933 ± 258 | 4832 ± 300# | IL-15 [%] | RIPC | 102 ± 6 | 93 ± 6 | 117 ± 9# |
| placebo | 5253 ± 690 | 5633 ± 566 | 5218 ± 682 | 6234 ± 613*,# | placebo | 120 ± 14 | 108 ± 14 | 134 ± 19*,# | ||
| IL-17 [pg/mL] | RIPC | 20 ± 2 | 28 ± 2 | 32 ± 4* | 35 ± 5* | IL-17 [%] | RIPC | 174 ± 24 | 212 ± 51* | 266 ± 69* |
| placebo | 28 ± 3 | 29 ± 4 | 32 ± 7 | 36 ± 9 | placebo | 120 ± 18 | 103 ± 18+ | 143 ± 32+ | ||
| IL-33 [fg/mL] | RIPC | 3997 ± 544 | 5178 ± 525 | 5983 ± 665 | 19054 ± 1826*,# | IL-33 [%] | RIPC | 146 ± 15 | 178 ± 23 | 615 ± 92*,# |
| placebo | 3679 ± 530 | 5454 ± 675 | 7059 ± 1587* | 21732 ± 1888*,# | placebo | 153 ± 13 | 190 ± 40 | 633 ± 72*,# | ||
| leptin [pg/mL] | RIPC | 56 ± 9 | 49 ± 9 | 43 ± 8 | 40 ± 1 | leptin [%] | RIPC | 88 ± 3* | 74 ± 3* | 67 ± 3*,# |
| placebo | 73 ± 23 | 65 ± 23 | 50 ± 11* | 48 ± 8* | placebo | 86 ± 3* | 77 ± 3* | 72 ± 4* | ||
| pentraxin-3 [pg/mL] | RIPC | 804 ± 137 | 808 ± 129 | 1002 ± 1211 | 3453 ± 281*,# | pentraxin-3 [%] | RIPC | 106 ± 4 | 160 ± 16 | 697 ± 104*,# |
| placebo | 1061 ± 204 | 982 ± 160 | 1240 ± 162 | 4745 ± 831*,#,+ | placebo | 99 ± 5 | 159 ± 23 | 928 ± 239*,# | ||
| prolactin [ng/mL] | RIPC | 34 ± 3 | 44 ± 4 | 52 ± 5* | 58 ± 7* | prolactin [%] | RIPC | 158 ± 31 | 208 ± 56* | 233 ± 51* |
| placebo | 48 ± 4+ | 60 ± 6+ | 61 ± 7 | 52 ± 7 | placebo | 138 ± 30 | 143 ± 35 | 119 ± 29+ | ||
| RNase-1 [pg/mL] | RIPC | 663 ± 150 | 464 ± 94 | 728 ± 100 | 1744 ± 120*,# | RNase-1 [%] | RIPC | 93 ± 10 | 200 ± 37 | 405 ± 69*,# |
| placebo | 477 ± 37 | 460 ± 82 | 795 ± 94* | 1627 ± 241*,# | placebo | 131 ± 20 | 244 ± 33* | 519 ± 96*,# | ||
| SDF-1α [pg/mL] | RIPC | 2270 ± 94 | 2197 ± 99 | 2766 ± 126* | 2846 ± 98* | SDF-1α [%] | RIPC | 97 ± 1 | 123 ± 4* | 127 ± 3* |
| placebo | 2382 ± 102 | 2327 ± 97 | 2881 ± 105* | 2922 ± 120* | placebo | 98 ± 2 | 124 ± 5* | 126 ± 5* | ||
| surviving [pg/mL] | RIPC | 36 ± 9 | 46 ± 7 | 34 ± 3 | 58 ± 1 | surviving [%] | RIPC | 227 ± 51 | 152 ± 25 | 313 ± 57 |
| placebo | 45 ± 7 | 56 ± 9 | 51 ± 8 | 90 ± 8*,#,+ | placebo | 151 ± 28 | 193 ± 56 | 472 ± 277*,# | ||
| thymosin-β4 [ng/mL] | RIPC | 349 ± 29 | 322 ± 25 | 285 ± 22 | 280 ± 31 | thymosin-β4 [%] | RIPC | 96 ± 4 | 90 ± 7 | 99 ± 15 |
| placebo | 364 ± 46 | 371 ± 39 | 362 ± 42 | 316 ± 27 | placebo | 110 ± 9 | 109 ± 8 | 105 ± 11 | ||
| TNF-α [fg/mL] | RIPC | 2973 ± 744 | 3107 ± 737 | 3259 ± 800 | 4301 ± 744*,# | TNF-α [%] | RIPC | 108 ± 3 | 116 ± 6 | 198 ± 42*,# |
| placebo | 2892 ± 580 | 3105 ± 566 | 2772 ± 265 | 3827 ± 450*,# | placebo | 111 ± 3 | 116 ± 7 | 166 ± 19*,# | ||
Data are mean ± standard error of the mean. Concentrations of all humoral factors were analyzed by 2-way (group, time) ANOVA for repeated measures followed by Fisher’s post hoc tests. *p < 0.05 versus baseline, #p < 0.05 versus before ischemic cardioplegic arrest, +p < 0.05 versus RIPC. Apolipoprotein A1 (Apo-A1), erythropoietin (EPO), growth differentiation factor-11 (GDF-11), growth hormone (GH), growth hormone-releasing peptide (GHRP), glucagon like peptide-1 (GLP-1), hypoxia inducible factor 1α (HIF-1α), interleukin (IL), remote ischemic preconditioning (RIPC), ribonuclease A (RNase-1), stromal cell derived factor-1 α (SDF-1α), tumor necrosis factor-α (TNF-α).
The concentrations (normalized and not normalized to baseline) of Apo-A1, EPO, GHRP, GLP-1, GH, HIF-1α, IL-1β, IL-2, IL-10, IL-15, IL-33, leptin, RNase-1, SDF-1α, thymosin-β4 and TNF-α did not differ between RIPC and placebo at all analyzed time points (Table 2). The concentration of Apo-A1 decreased, whereas the concentrations of GLP-1, GH, IL-1β, IL-10, IL-15, IL-33, RNase-1, SDF-1α and TNF-α increased after ischemic cardioplegic arrest over that at baseline and before ischemic cardioplegic arrest (Table 2).
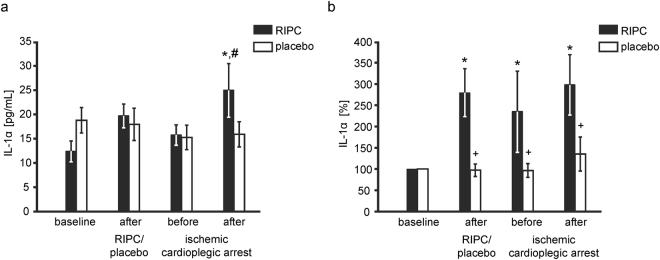
The IL-1α concentration, when normalized to baseline, increased after the RIPC procedure and remained increased until after ischemic cardioplegic arrest, whereas it was unchanged with placebo. In absolute concentrations, interleukin-1α increased after ischemic cardioplegic arrest over that at baseline and before ischemic cardioplegic arrest with RIPC, whereas it did not change over time with placebo (Table 2 and Fig. 2).
Figure 2.
Plasma concentration of interleukin-1α. The plasma concentration of interleukin-1α (IL-1α) before (baseline) and after remote ischemic preconditioning (RIPC; n = 23, black bars) or the placebo protocol (n = 23, white bars) and before and after ischemic cardioplegic arrest, respectively, in patients undergoing coronary artery bypass graft surgery. The plasma concentration of IL-1α was increased after ischemic cardioplegic arrest with RIPC and was greater with RIPC than with placebo (a). After normalization to baseline, the IL-1α plasma concentration was greater with RIPC than with placebo throughout the remaining protocol (b). *p < 0.05 versus baseline, #p < 0.05 versus before ischemic cardioplegic arrest, +p < 0.05 versus RIPC using 2-way ANOVA for repeated measures, followed by Fisher’s post hoc tests.
The concentrations of GDF-11 and IL-8 increased after ischemic cardioplegic arrest and were greater with RIPC than with placebo, but after normalization to baseline these changes were no longer significant (Table 2). The concentrations of pentraxin-3 and survivin increased after ischemic cardioplegic arrest and were lower with RIPC than with placebo, but again after normalization to baseline these changes were no longer significant (Table 2).
Exclusively after normalization to baseline, the GHRH concentration was lower with RIPC than with placebo throughout the remaining protocol. The normalized concentrations of IL-6 and prolactin were greater with RIPC than with placebo after ischemic cardioplegic arrest. The normalized concentration of IL-17 was greater with RIPC than with placebo before and after ischemic cardioplegic arrest (Table 2).
Discussion
Except for IL-1α, none of the analyzed humoral factors in our study appeared to fulfill the criteria for a transfer factor of cardioprotection by RIC (increase in the factor’s concentration after the RIC procedure and before myocardial ischemia as well as association with reduced myocardial ischemia/reperfusion injury), and we thus add another mostly negative study to the so far elusive search for RIC´s transfer factor17. Our study was unique in that it was conducted in patients undergoing CABG surgery, where the RIPC procedure indeed induced cardioprotection. However, none of the humoral factors differed in absolute concentration between RIPC and placebo before ischemic cardioplegic arrest. The concentrations of some factors (GDF-11, GHRH, IL-1α, IL-6, IL-8 and IL-17) were greater with RIPC than with placebo after ischemic cardioplegic arrest. For these factors, however, it is unclear whether this difference is truly related to myocardial ischemia/reperfusion injury and protection from it. Cardiopulmonary bypass inflicts a systemic inflammatory injury to the entire body and induces damage to various parenchymal organs78. RIC, in turn, is also a systemic response and provides protection to a number of parenchymal organs79,80. Therefore, the observed differences in the concentrations of the above humoral factors may originate from other organs than the heart.
The IL-1α concentration, when normalized to baseline, was increased after the RIPC procedure and it remained increased until after ischemic cardioplegic arrest whereas it was not changed throughout the placebo protocol. In absolute concentrations, IL-1α was also greater with RIPC than with placebo after ischemic cardioplegic arrest. IL-1α is a member of the IL-1 cytokine family and involved in inflammatory processes. IL-1α is released from macrophages, monocytes, endothelial and epithelial cells81,82 but also from cardiomyocytes83 in response to cell injury. In mice with myocardial infarction, IL-1α was released into the systemic circulation, whereas IL-1α in the myocardial tissue did not change83. In isolated perfused rat hearts, IL-1α blockade after reperfusion reduced infarct size84, suggesting that intracellular IL-1α contributes to ischemia/reperfusion injury. However, exogenous IL-1α preconditioning85 and pretreatment86 in isolated perfused rat hearts improved ventricular systolic pressure and reduced infarct size, suggesting that circulating, extracellular IL-1α induces cardioprotection. A causal role of IL-1α as humoral mediator and trigger for intracellular signaling in RIC remains to be established. Whereas IL-1β is known to activate STATs54, the exact role of IL-1α in STAT activation is not clear so far. IL-1α could indirectly activate STATs by induction of IL-653. Except for IL-1α, which has not been associated with RIC before, we could not confirm any of the previously reported humoral factors to be associated with cardioprotection by RIC.
There are limitations of our current study: 1) Given our small patient cohort and the high number of analyzed humoral factors, the risk of a type I error with respect to IL-1α is high, in particular since its increase after the RIPC procedure was only evident with normalized data. Our exploratory study is hypothesis generating, so replication in a larger cohort of patients is mandatory. 2) We used plasma samples from a consecutive patient cohort with co-morbidities and co-medications, some of which may potentially interfere with the protection by ischemic conditioning maneuvers87–89, but also with the release of humoral factors. Patients undergoing RIPC were younger and had lower preoperative risk scores than those undergoing the placebo procedure, and these differences may have contributed to the more pronounced decrease in TnI release than that in the larger cohort reported before3. 3) We analyzed the plasma concentrations only at four defined time points, i.e. before/5 min after the RIPC/placebo protocol and before/10 min after ischemic cardioplegic arrest, not considering for the potentially different kinetics of each humoral factor. In particular, the time from the end of the RIPC/placebo procedure to ischemic cardioplegic arrest was a bit longer in patients with RIPC than with placebo, and we may have missed a transient increase or decrease in humoral factors with RIPC.
Methods
Ethics Statement
The study conforms to the principles of the Declaration of Helsinki. With approval by the local ethics committee (Institutional Review Board, University of Duisburg-Essen, no. 08–3683) and patients’ written informed consent, arterial blood samples were harvested from a small cohort of consecutive patients (n = 23 RIPC/23 placebo) who underwent elective isolated first-time CABG surgery3. These patients were enrolled between February 2012 and April 2013 and within the framework of a larger, randomized, prospective, double-blind, placebo-controlled trial (ClinicalTrials.gov NCT01406678, date of registration: December 1, 2009). The inclusion and exclusion criteria for the trial as well as its results have been reported3.
Study procedure
Anesthesia was induced with sufentanil (1 µg/kg), etomidate (0.3 mg/kg) and rocuronium (0.6 mg/kg) and maintained with isoflurane (0.6–1.0% end-tidal). The RIPC protocol consisted of 3 cycles of 5 min left upper arm ischemia/5 min reperfusion, and data were compared to placebo (cuff left deflated for 30 min). CABG was performed using median sternotomy, mild systemic hypothermia (>32 °C) and antegrade cold crystalloid Bretschneider (Köhler Chemie GmbH, Bensheim, Germany) cardioplegia, with additional topical cooling and single aortic cross-clamping for all distal anastomoses3.
Arterial blood samples and plasma preparation
Arterial blood samples were taken before (baseline) and 5 min after the end of the RIPC/placebo procedure as well as before and 10 min after the ischemic cardioplegic arrest. These time points were chosen to detect changes induced by the RIPC protocol per se and the interaction of RIPC with ischemic cardioplegic arrest. At each time point, 25 mL arterial blood was withdrawn and sampled in vials containing lithium-heparin (Sarstedt, Nümbrecht, Germany). The arterial blood was then immediately centrifuged at 4 °C with 800 g for 15 min, plasma was separated, stored at −80 °C for later use and again centrifuged for 10 min at 4500 g before use. Additionally, 5 mL of arterial blood was withdrawn in separate vials (Sarstedt, Nümbrecht, Germany) to analyze the serum concentration of prolactin.
Serum troponin I
Venous blood samples were withdrawn from each patient on the day before surgery and postoperatively at 1, 6, 12, 24, 48, and 72 h. Serum TnI concentration was measured using a specific two-side immunoassay with the DimensionR RxL MaxR integrated system (Dimension Flex, Dade Behring GmbH, Marburg, Germany) in the central laboratory of the University Duisburg-Essen Medical School. The detection range of TnI was 0.04 to 40 µg/L, the upper limit of normal 0.1 µg/L. The AUC for serum TnI concentration was calculated according to the trapezoidal rule. Missing values were replaced by linear inter- and extrapolation3.
Plasma concentrations of humoral factors
The plasma concentrations of humoral factors were determined using enzyme immunoassays. Standards and samples were added to microplates, which were precoated with the specific antibody against the respective protein.
For the detection of Apo-A190, EPO91, GDF-1192, RNase-193 (ELISA Cloud-Immunoassay, Houston, USA) and HIF-1α94 (RayBio, Georgia, USA) avidin-conjugated horseradish peroxidase was supplemented. For the detection of GLP-195 (Abcam, Cambridge, UK) an antibody cocktail consisting of a capture and a detector antibody was supplemented. For the detection of GH96, IL-1α97, IL-298, IL-1599, IL-1799, IL-33100, leptin101, pentraxin-3102, SDF-1α103 and survivin104 (R&D systems, Abingdon, UK) an enzyme-linked polyclonal horseradish peroxidase-conjugated antibody was supplemented. For the detection of GHRH105 and GHRP106 (ELISA Cloud-Immunoassay, Houston, USA) biotin-conjugated antibodies against the respective protein were added to the microplate, and the antibodies on the plate and the biotin-labeled antibodies then competed for each other. An avidin-conjugated horseradish peroxidase-conjugated secondary antibody was supplemented. For the detection of thymosin-β4107 (Immundiagnostik, Bensheim, Germany) an antibody against thymosin-β4 was added to the microplate, which was precoated with the immobilized antigen to thymosin-β4. The antigen of the sample and the immobilized antigen then competed for each other. A horseradish peroxidase-conjugated secondary antibody was supplemented.
After adding the respective substrate, the enzyme-substrate reaction resulted in a blue product. The color intensity was proportional to the concentration of the protein. The reaction was stopped, and the color changed to yellow. The color intensity was measured at 450 nm using a spectrophotometer (Microplate Reader 680, BIORAD, München, Germany).
For the detection of IL-1β97, IL-6108, IL-8108, IL-1098 and TNF-α108 (R&D systems, Abingdon, UK) an enzyme-linked polyclonal antibody and a substrate solution were supplemented. After adding an amplifier enzyme the enzyme-substrate reaction resulted in a violet product. The color intensity was proportional to the enzyme activity, which was related to the concentration of bound proteins. The reaction was stopped, and the color intensity was measured at 490 nm using a spectrophotometer (Microplate Reader 680, BIORAD, München, Germany).
The prolactin concentration was measured in the central laboratory of the University Duisburg-Essen Medical School. The detection range of prolactin assay was 0.3 μg/L to 200 μg/L. The serum concentration of prolactin was measured using a two-side sandwich chemiluminescence immunoassay with an acridinium ester-conjugated antibody against prolactin and a secondary antibody covalently coupled to paramagnetic particles (ADVIAR Centaur XP, Siemens, Tarrytown, USA)109.
The concentrations of the respective proteins were quantified by comparison to a standard curve.
Statistics
Data are expressed as mean ± standard error of the mean (SEM). Statistics were performed using SigmaStat software (SigmaStat 2.03, SPSS Inc., Chicago, IL, USA). Patient demographics and intraoperative characteristics were compared using unpaired Student’s t-test (continuous data) and 2-tailed Fisher’s exact test (categorical data). Serum TnI of patients was analyzed by 2-way (group, time) ANOVA for repeated measures. The AUC for the serum TnI over 72 h was compared between RIPC and placebo by unpaired Student’s t-test. Plasma concentrations of all humoral factors were analyzed by 2-way (group, time) ANOVA for repeated measures. When a significant difference was detected, ANOVA was followed by Fisher’s post hoc tests. Differences were considered significant at the level of p < 0.05.
Acknowledgements
G.H. was supported by the German Research Foundation (He 1320/18–3; SFB 1116 B08), the Hans und Gertie Fischer Foundation, the Dr. Deichmann Foundation and the EU (COST Action CA16225). P.K. was supported by the German Research Foundation (SFB 1116 B08).
Author Contributions
N.G. and P.K. conceived and designed the experiments. N.G. and P.K. performed the experiments. M.T., E.K., U.F., J.P. and H.J. performed the clinical study. N.G. and P.K. analyzed the data. P.K. contributed reagents/materials/analysis tools. N.G. and P.K. wrote the paper. GH designed the original study and finalized the paper. All authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Davies WR, et al. Remote ischemic preconditioning improves outcome at 6 years after elective percutaneous coronary intervention: The CRISP stent trial long-term follow-up. Circ Cardiovasc Interv. 2013;6:246–251. doi: 10.1161/CIRCINTERVENTIONS.112.000184. [DOI] [PubMed] [Google Scholar]
- 2.Hausenloy DJ, et al. Effect of remote ischaemic preconditioning on myocardial injury in patients undergoing coronary artery bypass graft surgery: a randomized controlled trial. Lancet. 2007;370:575–579. doi: 10.1016/S0140-6736(07)61296-3. [DOI] [PubMed] [Google Scholar]
- 3.Thielmann M, et al. Cardioprotective and prognostic effects of remote ischaemic preconditioning in patients undergoing coronary artery bypass surgery: a single-centre randomised, double-blind, controlled trial. Lancet. 2013;382:597–604. doi: 10.1016/S0140-6736(13)61450-6. [DOI] [PubMed] [Google Scholar]
- 4.Candilio L, et al. Effect of remote ischaemic preconditioning on clinical outcomes in patients undergoing cardiac bypass surgery: a randomised controlled clinical trial. Heart. 2015;10:185–192. doi: 10.1136/heartjnl-2014-306178. [DOI] [PubMed] [Google Scholar]
- 5.Nouraei SM, Baradari AG, Jazayeri A. Does remote ischaemic preconditioning protect kidney and cardiomyocytes after coronary revascularization? A double blind controlled clinical trial. Med Arch. 2016;70:373–378. doi: 10.5455/medarh.2016.70.373-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bøtker HE, et al. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet. 2010;375:727–734. doi: 10.1016/S0140-6736(09)62001-8. [DOI] [PubMed] [Google Scholar]
- 7.Eitel I, et al. Cardioprotection by combined intrahospital remote ischaemic perconditioning and postconditioning in ST-elevation myocardial infarction: the randomized LIPSIA CONDITIONING trial. Eur Heart J. 2015;36:3049–3057. doi: 10.1093/eurheartj/ehv463. [DOI] [PubMed] [Google Scholar]
- 8.White SK, et al. Remote ischemic conditioning reduces myocardial infarct size and edema in patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol Cardiovasc Interv. 2015;8:178–188. doi: 10.1016/j.jcin.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 9.Yellon DM, et al. Remote ischemic conditioning reduces myocardial infarct size in STEMI patients treated by thrombolysis. J Am Coll Cardiol. 2015;65:2764–2765. doi: 10.1016/j.jacc.2015.02.082. [DOI] [PubMed] [Google Scholar]
- 10.Liu Z, Zhao L, Hong D, Gao J. Remote ischaemic preconditioning reduces myocardial ischaemic reperfusion injury in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Acta Cardiol. 2016;71:596–603. doi: 10.1080/AC.71.5.3167504. [DOI] [PubMed] [Google Scholar]
- 11.Sloth AD, et al. Improved long-term clinical outcomes in patients with ST-elevation myocardial infarction undergoing remote ischaemic conditioning as an adjunct to primary percutaneous coronary intervention. Eur Heart J. 2014;35:168–175. doi: 10.1093/eurheartj/eht369. [DOI] [PubMed] [Google Scholar]
- 12.Hausenloy DJ, et al. Remote ischemic preconditioning and outcomes of cardiac surgery. N Engl J Med. 2015;373:1408–1417. doi: 10.1056/NEJMoa1413534. [DOI] [PubMed] [Google Scholar]
- 13.Meybohm P, et al. A multicenter trial of remote ischemic preconditioning for heart surgery. N Engl J Med. 2015;373:1397–1407. doi: 10.1056/NEJMoa1413579. [DOI] [PubMed] [Google Scholar]
- 14.Kottenberg E, et al. Protection by remote ischaemic preconditioning during coronary artery bypass grafting with isoflurane but not with propofol anesthesia - a clinical trial. Acta Anaesthesiol Scand. 2012;56:30–38. doi: 10.1111/j.1399-6576.2011.02585.x. [DOI] [PubMed] [Google Scholar]
- 15.Heusch G, Gersh BJ. ERICCA and RIPHeart: two nails in the coffin for cardioprotection by remote ischemic conditioning? Probably not! Eur Heart J. 2016;37:200–202. doi: 10.1093/eurheartj/ehv606. [DOI] [PubMed] [Google Scholar]
- 16.Heusch G, Rassaf T. Time to give op on cardioprotection? A critical appraisal of clinical studies on ischemic pre-, post-, and remote conditioning. Circ Res. 2016;119:676–695. doi: 10.1161/CIRCRESAHA.116.308736. [DOI] [PubMed] [Google Scholar]
- 17.Heusch G. Remote ischemic conditioning: the enigmatic transfer of protection. Cardiovasc Res. 2017;113:1–2. doi: 10.1093/cvr/cvw240. [DOI] [PubMed] [Google Scholar]
- 18.Kleinbongard P, Skyschally A, Heusch G. Cardioprotection by remote ischemic conditioning and its signal transduction. Pflugers Arch. 2017;469:159–181. doi: 10.1007/s00424-016-1922-6. [DOI] [PubMed] [Google Scholar]
- 19.Dickson EW, et al. Rabbit heart can be “preconditioned” via transfer of coronary effluent. Am J Physiol. 1999;277:H2451–H2457. doi: 10.1152/ajpheart.1999.277.6.H2451. [DOI] [PubMed] [Google Scholar]
- 20.Shimizu M, et al. Transient limb ischaemia remotely preconditions through a humoral mechanism acting directly on the myocardium: evidence suggesting cross-species protection. Clin Sci (Lond). 2009;117:191–200. doi: 10.1042/CS20080523. [DOI] [PubMed] [Google Scholar]
- 21.Skyschally A, et al. Across-species transfer of protection by remote ischemic preconditioning with species-specific myocardial signal transduction by reperfusion injury salvage kinase and survival activating factor enhancement pathways. Circ Res. 2015;117:279–288. doi: 10.1161/CIRCRESAHA.117.306878. [DOI] [PubMed] [Google Scholar]
- 22.Gedik N, et al. Cardiomyocyte mitochondria as targets of humoral factors released by remote ischemic preconditioning. Arch Med Sci. 2016;13:448–458. doi: 10.5114/aoms.2016.61789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jensen RV, Stottrup NB, Kristiansen SB, Bøtker HE. Release of a humoral circulating cardioprotective factor by remote ischemic preconditioning is dependent on preserved neural pathways in diabetic patients. Basic Res Cardiol. 2012;107:285. doi: 10.1007/s00395-012-0285-1. [DOI] [PubMed] [Google Scholar]
- 24.Rassaf T, et al. Circulating nitrite contributes to cardioprotection by remote ischemic preconditioning. Circ Res. 2014;114:1601–1610. doi: 10.1161/CIRCRESAHA.114.303822. [DOI] [PubMed] [Google Scholar]
- 25.Hildebrandt HA, et al. Kinetics and signal activation properties of circulating factor(s) from healthy volunteers undergoing remote ischemic pre-conditioning. JACC Basic Transl Sci. 2016;1:3–13. doi: 10.1016/j.jacbts.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chao de la Barca, J. M. et al. Metabolic signature of remote ischemic preconditioning involving a cocktail of amino acids and biogenic amines. J Am Heart Assoc. 5 (2016). [DOI] [PMC free article] [PubMed]
- 27.Olenchock BA, et al. EGLN1 inhibition and rerouting of alpha-ketoglutarate suffice for remote ischemic protection. Cell. 2016;164:884–895. doi: 10.1016/j.cell.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jose Alburquerque-Bejar J, et al. Remote ischemic conditioning provides humoural cross-species cardioprotection through glycine receptor activation. Cardiovasc Res. 2017;113:52–60. doi: 10.1093/cvr/cvw242. [DOI] [PubMed] [Google Scholar]
- 29.Kouassi Nzoughet J, et al. A nontargeted UHPLC-HRMS metabolomics pipeline for metabolite identification: Application to cardiac remote ischemic preconditioning. Anal Chem. 2017;89:2138–2146. doi: 10.1021/acs.analchem.6b04912. [DOI] [PubMed] [Google Scholar]
- 30.Zhou W, et al. Limb ischemic preconditioning reduces heart and lung injury after an open heart operation in infants. Pediatr Cardiol. 2010;31:22–29. doi: 10.1007/s00246-009-9536-9. [DOI] [PubMed] [Google Scholar]
- 31.Albrecht M, et al. Remote ischemic preconditioning regulates HIF-1alpha levels, apoptosis and inflammation in heart tissue of cardiosurgical patients: a pilot experimental study. Basic Res Cardiol. 2013;108:314. doi: 10.1007/s00395-012-0314-0. [DOI] [PubMed] [Google Scholar]
- 32.Davidson SM, et al. Remote ischaemic preconditioning involves signalling through the SDF-1alpha/CXCR4 signalling axis. Basic Res Cardiol. 2013;108:377. doi: 10.1007/s00395-013-0377-6. [DOI] [PubMed] [Google Scholar]
- 33.Oba T, et al. Renal nerve-mediated erythropoietin release confers cardioprotection during remote ischemic preconditioning. Circ J. 2015;79:1557–1567. doi: 10.1253/circj.CJ-14-1171. [DOI] [PubMed] [Google Scholar]
- 34.Zhang SZ, et al. Kappa-opioid receptors mediate cardioprotection by remote preconditioning. Anesthesiology. 2006;105:550–556. doi: 10.1097/00000542-200609000-00019. [DOI] [PubMed] [Google Scholar]
- 35.Gao Y, Song J, Chen H, Cao C, Lee C. TRPV1 activation is involved in the cardioprotection of remote limb ischemic postconditioning in ischemia-reperfusion injury rats. Biochem Biophys Res Commun. 2015;463:1034–1039. doi: 10.1016/j.bbrc.2015.06.054. [DOI] [PubMed] [Google Scholar]
- 36.Liem DA, Verdouw PD, Ploeg H, Kazim S, Duncker D. Sites of action of adenosine in interorgan preconditioning of the heart. Am J Physiol Heart Circ Physiol. 2002;283:H29–H37. doi: 10.1152/ajpheart.01031.2001. [DOI] [PubMed] [Google Scholar]
- 37.Leung CH, et al. Remote cardioprotection by transfer of coronary effluent from ischemic preconditioned rabbit heart preserves mitochondrial integrity and function via adenosine receptor activation. Cardiovasc Drugs Ther. 2014;28:7–17. doi: 10.1007/s10557-013-6489-2. [DOI] [PubMed] [Google Scholar]
- 38.Hepponstall M, et al. Remote ischemic preconditioning (RIPC) modifies plasma proteome in humans. PLoS One. 2012;7:e48284. doi: 10.1371/journal.pone.0048284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hibert P, et al. Apolipoprotein A-I is a potential mediator of remote ischemic preconditioning. PLoS One. 2013;8:e77211. doi: 10.1371/journal.pone.0077211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cabrera-Fuentes, H. A. et al. RNase1 as a potential mediator of remote ischaemic preconditioning for cardioprotection. Eur J Cardiothorac Surg. 48, 732–737; discussion 737 (2015). [DOI] [PubMed]
- 41.Basalay M, et al. Glucagon-like peptide-1 (GLP-1) mediates cardioprotection by remote ischaemic conditioning. Cardiovasc Res. 2016;112:669–676. doi: 10.1093/cvr/cvw216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li J, et al. MicroRNA-144 is a circulating effector of remote ischemic preconditioning. Basic Res Cardiol. 2014;109:423. doi: 10.1007/s00395-014-0423-z. [DOI] [PubMed] [Google Scholar]
- 43.Downey JM, Krieg T, Cohen MV. Mapping preconditioning’s signaling pathways: an engineering approach. Ann N Y Acad Sci. 2008;1123:187–196. doi: 10.1196/annals.1420.022. [DOI] [PubMed] [Google Scholar]
- 44.Heusch G. Molecular basis of cardioprotection: signal transduction in ischemic pre-, post-, and remote conditioning. Circ Res. 2015;116:674–699. doi: 10.1161/CIRCRESAHA.116.305348. [DOI] [PubMed] [Google Scholar]
- 45.Heusch G, Boengler K, Schulz R. Cardioprotection: nitric oxide, protein kinases, and mitochondria. Circulation. 2008;118:1915–1919. doi: 10.1161/CIRCULATIONAHA.108.805242. [DOI] [PubMed] [Google Scholar]
- 46.Kleinbongard P, Heusch G. Extracellular signalling molecules in the ischaemic/reperfused heart - druggable and translatable for cardioprotection? Br J Pharmacol. 2015;172:2010–2025. doi: 10.1111/bph.12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lecour S. Activation of the protective survivor activating factor enhancement (SAFE) pathway against reperfusion injury: Does it go beyond the RISK path? J Mol Cell Cardiol. 2009;47:32–40. doi: 10.1016/j.yjmcc.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 48.Heusch G, et al. STAT5 activation and cardioprotection by remote ischemic preconditioning in humans. Circ Res. 2012;110:111–115. doi: 10.1161/CIRCRESAHA.111.259556. [DOI] [PubMed] [Google Scholar]
- 49.Gedik N, et al. No evidence for activated autophagy in left ventricular myocardium at early reperfusion with protection by remote ischemic preconditioning in patients undergoing coronary artery bypass grafting. PLoS One. 2014;9:e96567. doi: 10.1371/journal.pone.0096567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boengler K, Hilfiker-Kleiner D, Drexler H, Heusch G, Schulz R. The myocardial JAK/STAT pathway: from protection to failure. Pharmacol Ther. 2008;120:172–185. doi: 10.1016/j.pharmthera.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 51.Haghikia A, Stapel B, Hoch M, Hilfiker-Kleiner D. STAT3 and cardiac remodeling. Heart Fail Rev. 2011;16:35–47. doi: 10.1007/s10741-010-9170-x. [DOI] [PubMed] [Google Scholar]
- 52.Piuhola J, et al. Direct cardiac actions of erythropoietin (EPO): effects on cardiac contractility, BNP secretion and ischaemia/reperfusion injury. Clin Sci (Lond). 2008;114:293–304. doi: 10.1042/CS20070229. [DOI] [PubMed] [Google Scholar]
- 53.Libert C, Brouckaert P, Shaw A, Fiers W. Induction of interleukin 6 by human and murine recombinant interleukin 1 in mice. Eur J Immunol. 1990;20:691–694. doi: 10.1002/eji.1830200333. [DOI] [PubMed] [Google Scholar]
- 54.Morton NM, de Groot RP, Cawthorne MA, Emilsson V. Interleukin-1beta activates a short STAT-3 isoform in clonal insulin-secreting cells. FEBS Lett. 1999;442:57–60. doi: 10.1016/S0014-5793(98)01623-8. [DOI] [PubMed] [Google Scholar]
- 55.Lin JX, et al. The role of shared receptor motifs and common Stat proteins in the generation of cytokine pleiotropy and redundancy by IL-2, IL-4, IL-7, IL-13, and IL-15. Immunity. 1995;2:331–339. doi: 10.1016/1074-7613(95)90141-8. [DOI] [PubMed] [Google Scholar]
- 56.Dawn B, et al. Il-6 plays an obligatory role in late preconditioning via JAK-STAT signaling and upregulation of iNOS and COX-2. Cardiovasc Res. 2004;64:61–71. doi: 10.1016/j.cardiores.2004.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gharavi NM, et al. Role of the Jak/STAT pathway in the regulation of interleukin-8 transcription by oxidized phospholipids in vitro and in atherosclerosis in vivo. J Biol Chem. 2007;282:31460–31468. doi: 10.1074/jbc.M704267200. [DOI] [PubMed] [Google Scholar]
- 58.Manukyan MC, et al. Interleukin-10 protects the ischemic heart from reperfusion injury via the STAT3 pathway. Surgery. 2011;150:231–239. doi: 10.1016/j.surg.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 59.Su SA, et al. Interleukin-17A mediates cardiomyocyte apoptosis through Stat3-iNOS pathway. Biochim Biophys Acta. 2016;1863:2784–2794. doi: 10.1016/j.bbamcr.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 60.Ruisong M, et al. The protective role of interleukin-33 in myocardial ischemia and reperfusion is associated with decreased HMGB1 expression and up-regulation of the p38 MAPK signaling pathway. PLoS One. 2015;10:e0143064. doi: 10.1371/journal.pone.0143064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang C, et al. SDF-1/CXCR4 mediates acute protection of cardiac function through myocardial STAT3 signaling following global ischemia/reperfusion injury. Am J Physiol Heart Circ Physiol. 2011;301:H1496–H1505. doi: 10.1152/ajpheart.00365.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lacerda L, Somers S, Opie LH, Lecour S. Ischemic postconditioning protects against reperfusion injury via the SAFE pathway. Cardiovasc Res. 2009;84:201–208. doi: 10.1093/cvr/cvp274. [DOI] [PubMed] [Google Scholar]
- 63.Ram PA, Park SH, Choi HK, Waxman DJ. Growth hormone activation of Stat 1, Stat 3, and Stat 5 in rat liver. Differential kinetics of hormone desensitization and growth hormone stimulation of both tyrosine phosphorylation and serine/threonine phosphorylation. J Biol Chem. 1996;271:5929–5940. doi: 10.1074/jbc.271.10.5929. [DOI] [PubMed] [Google Scholar]
- 64.Waters MJ, Brooks AJ. JAK2 activation by growth hormone and other cytokines. Biochem J. 2015;466:1–11. doi: 10.1042/BJ20141293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Loffredo FS, et al. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell. 2013;153:828–839. doi: 10.1016/j.cell.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Penna C, et al. GH-releasing hormone induces cardioprotection in isolated male rat heart via activation of RISK and SAFE pathways. Endocrinology. 2013;154:1624–1635. doi: 10.1210/en.2012-2064. [DOI] [PubMed] [Google Scholar]
- 67.Chung H, Li E, Kim Y, Kim S, Park S. Multiple signaling pathways mediate ghrelin-induced proliferation of hippocampal neural stem cells. J Endocrinol. 2013;218:49–59. doi: 10.1530/JOE-13-0045. [DOI] [PubMed] [Google Scholar]
- 68.Cai Z, Luo W, Zhan H, Semenza GL. Hypoxia-inducible factor 1 is required for remote ischemic preconditioning of the heart. Proc Natl Acad Sci USA. 2013;110:17462–17467. doi: 10.1073/pnas.1317158110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Heusch G. HIF-1alpha and paradoxical phenomena in cardioprotection. Cardiovasc Res. 2012;96:214–215. doi: 10.1093/cvr/cvs145. [DOI] [PubMed] [Google Scholar]
- 70.Heusch G. Obesity–a risk factor or a RISK factor for myocardial infarction? Br.J Pharmacol. 2006;149:1–3. doi: 10.1038/sj.bjp.0706833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smith CC, et al. Leptin-induced cardioprotection involves JAK/STAT signaling that may be linked to the mitochondrial permeability transition pore. Am J Physiol Heart Circ Physiol. 2010;299:H1265–H1270. doi: 10.1152/ajpheart.00092.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Salio M, et al. Cardioprotective function of the long pentraxin PTX3 in acute myocardial infarction. Circulation. 2008;117:1055–1064. doi: 10.1161/CIRCULATIONAHA.107.749234. [DOI] [PubMed] [Google Scholar]
- 73.Hsieh DJ, et al. Prolactin protects cardiomyocytes against intermittent hypoxia-induced cell damage by the modulation of signaling pathways related to cardiac hypertrophy and proliferation. Int J Cardiol. 2015;181:255–266. doi: 10.1016/j.ijcard.2014.11.154. [DOI] [PubMed] [Google Scholar]
- 74.Levkau B, et al. Survivin determines cardiac function by controlling total cardiomyocyte number. Circulation. 2008;117:1583–1593. doi: 10.1161/CIRCULATIONAHA.107.734160. [DOI] [PubMed] [Google Scholar]
- 75.Si R, et al. Survivin: a novel player in insulin cardioprotection against myocardial ischemia/reperfusion injury. J Mol Cell Cardiol. 2011;50:16–24. doi: 10.1016/j.yjmcc.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 76.Hinkel R, et al. Thymosin beta4 is an essential paracrine factor of embryonic endothelial progenitor cell-mediated cardioprotection. Circulation. 2008;117:2232–2240. doi: 10.1161/CIRCULATIONAHA.107.758904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bao W, et al. Cardioprotection by systemic dosing of thymosin beta four following ischemic myocardial injury. Front Pharmacol. 2013;4:149. doi: 10.3389/fphar.2013.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lok Z, Chen Y, Smith JA. Understanding noncardiac complications following coronary artery bypass graft surgery. Clin Pract. 2014;11:193–206. doi: 10.2217/cpr.14.1. [DOI] [Google Scholar]
- 79.Candilio L, Malik A, Hausenloy DJ. Protection of organs other than the heart by remote ischemic conditioning. J Cardiovasc Med (Hagerstown.). 2013;14:193–205. doi: 10.2459/JCM.0b013e328359dd7b. [DOI] [PubMed] [Google Scholar]
- 80.Zarbock A, et al. Effect of remote ischemic preconditioning on kidney injury among high-risk patients undergoing cardiac surgery: a randomized clinical trial. JAMA. 2015;313:2133–2141. doi: 10.1001/jama.2015.4189. [DOI] [PubMed] [Google Scholar]
- 81.Rider P, Carmi Y, Voronov E, Apte RN. Interleukin-1alpha. Semin Immunol. 2013;25:430–438. doi: 10.1016/j.smim.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 82.Borthwick LA. The IL-1 cytokine family and its role in inflammation and fibrosis in the lung. Semin Immunopathol. 2016;38:517–534. doi: 10.1007/s00281-016-0559-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lugrin J, et al. Cutting edge: IL-1alpha is a crucial danger signal triggering acute myocardial inflammation during myocardial infarction. J Immunol. 2015;194:499–503. doi: 10.4049/jimmunol.1401948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mauro AG, et al. Reduction of myocardial ischemia-reperfusion injury by inhibiting interleukin-1 alpha. J Cardiovasc Pharmacol. 2017;69:156–160. doi: 10.1097/FJC.0000000000000452. [DOI] [PubMed] [Google Scholar]
- 85.Maulik N, et al. Interleukin-1a preconditioning reduces myocardial ischemia reperfusion injury. Circulation. 1993;88:387–394. [PubMed] [Google Scholar]
- 86.Nogae C, et al. Interleukin 1 alpha-induced expression of manganous superoxide dismutase reduces myocardial reperfusion injury in the rat. J Mol Cell Cardiol. 1995;27:2091–2099. doi: 10.1016/S0022-2828(95)91155-3. [DOI] [PubMed] [Google Scholar]
- 87.Ferdinandy P, Hausenloy DJ, Heusch G, Baxter GF, Schulz R. Interaction of risk factors, comorbidities and comedications with ischemia/reperfusion injury and cardioprotection by preconditioning, postconditioning, and remote conditioning. Pharmacol Rev. 2014;66:1142–1174. doi: 10.1124/pr.113.008300. [DOI] [PubMed] [Google Scholar]
- 88.Varga ZV, et al. Functional genomics of cardioprotection by ischemic conditioning and the influence of comorbid conditions: implications in target identification. Curr Drug Targets. 2015;16:904–911. doi: 10.2174/1389450116666150427154203. [DOI] [PubMed] [Google Scholar]
- 89.Andreadou I, et al. Effect of hypercholesterolaemia on myocardial function, ischaemia-reperfusion injury and cardioprotection by preconditioning, postconditioning and remote conditioning. Br J Pharmacol. 2017;174:1555–1569. doi: 10.1111/bph.13704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Guo X, et al. The association of a distinct plasma proteomic profile with the cervical high-grade squamous intraepithelial lesion of Uyghur women: a 2D liquid-phase chromatography/mass spectrometry study. Biomarkers. 2012;17:352–361. doi: 10.3109/1354750X.2012.673133. [DOI] [PubMed] [Google Scholar]
- 91.Ciniselli CM, et al. Plasma hepcidin in early-stage breast cancer patients: no relationship with interleukin-6, erythropoietin and erythroferrone. Expert Rev Proteomics. 2015;12:695–701. doi: 10.1586/14789450.2015.1099436. [DOI] [PubMed] [Google Scholar]
- 92.Han Y, et al. GDF11 is increased in patients with myelodysplastic syndrome. Int J Clin Exp Pathol. 2016;9:6031–6038. [Google Scholar]
- 93.Martin L, et al. The human host defense ribonucleases 1, 3 and 7 are elevated in patients with sepsis after major surgery: A pilot study. Int J Mol Sci. 2016;17:294. doi: 10.3390/ijms17030294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li G, et al. The relationship between serum hypoxia-inducible factor 1alpha and coronary artery calcification in asymptomatic type 2 diabetic patients. Cardiovasc Diabetol. 2014;13:52. doi: 10.1186/1475-2840-13-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang H, et al. Uncoupling protein 2 negatively regulates glucose-induced glucagon-like peptide 1 secretion. J Mol Endocrinol. 2012;48:151–158. doi: 10.1530/JME-11-0114. [DOI] [PubMed] [Google Scholar]
- 96.Betts JA, Beelen M, Stokes KA, Saris WH, van Loon LJ. Endocrine responses during overnight recovery from exercise: impact of nutrition and relationships with muscle protein synthesis. Int J Sport Nutr Exerc Metab. 2011;21:398–409. doi: 10.1123/ijsnem.21.5.398. [DOI] [PubMed] [Google Scholar]
- 97.Vambutas A, et al. Early efficacy trial of anakinra in corticosteroid-resistant autoimmune inner ear disease. J Clin Invest. 2014;124:4115–4122. doi: 10.1172/JCI76503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sadeghi M, et al. Short communication: decreasing soluble CD30 and increasing IFN-gamma plasma levels are indicators of effective highly active antiretroviral therapy. AIDS Res Hum Retroviruses. 2007;23:886–890. doi: 10.1089/aid.2006.0228. [DOI] [PubMed] [Google Scholar]
- 99.Zheng MM, et al. The systemic cytokine environment is permanently altered in multiple myeloma. PLoS One. 2013;8:e58504. doi: 10.1371/journal.pone.0058504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shen J, et al. Carotid plaque and bone density and microarchitecture in psoriatic arthritis: the correlation with soluble ST2. Sci Rep. 2016;6:32116. doi: 10.1038/srep32116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu Y, et al. A dietary supplement containing cinnamon, chromium and carnosine decreases fasting plasma glucose and increases lean mass in overweight or obese pre-diabetic subjects: A randomized, placebo-controlled trial. PLoS One. 2015;10:e0138646. doi: 10.1371/journal.pone.0138646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chu SH, et al. Chemerin and adiponectin contribute reciprocally to metabolic syndrome. PLoS One. 2012;7:e34710. doi: 10.1371/journal.pone.0034710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dei Cas A, et al. Vildagliptin, but not glibenclamide, increases circulating endothelial progenitor cell number: a 12-month randomized controlled trial in patients with type 2 diabetes. Cardiovasc Diabetol. 2017;16:27. doi: 10.1186/s12933-017-0503-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Khan S, et al. Plasma-derived exosomal survivin, a plausible biomarker for early detection of prostate cancer. PLoS One. 2012;7:e46737. doi: 10.1371/journal.pone.0046737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tarasiuk A, Berdugo-Boura N, Troib A, Segev Y. Role of growth hormone-releasing hormone in sleep and growth impairments induced by upper airway obstruction in rats. Eur Respir J. 2011;38:870–877. doi: 10.1183/09031936.00197610. [DOI] [PubMed] [Google Scholar]
- 106.Li BB, et al. Expression of ghrelin in human salivary glands and its levels in saliva and serum in Chinese obese children and adolescents. Arch Oral Biol. 2011;56:389–394. doi: 10.1016/j.archoralbio.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 107.Smart N, Dube KN, Riley PR. Identification of thymosin beta4 as an effector of Hand1-mediated vascular development. Nat Commun. 2010;1:46. doi: 10.1038/ncomms1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Teichert T, et al. Association between traffic-related air pollution, subclinical inflammation and impaired glucose metabolism: results from the SALIA study. PLoS One. 2013;8:e83042. doi: 10.1371/journal.pone.0083042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schuring AN, Kelsch R, Pierscinski G, Nofer JR. Establishing reference intervals for sex hormones on the analytical platforms Advia Centaur and Immulite 2000XP. Ann Lab Med. 2016;36:55–59. doi: 10.3343/alm.2016.36.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]