Abstract
First-generation cephalosporins such as cefazolin (CEZ) have been widely used for mastitis treatment in dairy cattle. However, the use of antibiotics results in the presence of antibiotic residues in milk, which is used for human consumption. Nisin A, a bacteriocin produced by Lactococcus lactis, has been used as a broad-spectrum food preservative for over 50 years. Therefore, a combination of CEZ and nisin A might provide an extended activity spectrum against mastitis pathogens and reduce the antibiotic dose for mastitis treatment. This study aimed to evaluate the combined effect of CEZ and nisin A against mastitis pathogens using the checkerboard and time-kill assays. In the checkerboard assay, the CEZ-nisin A combination exhibited a synergistic effect against Staphylococcus aureus (n=20/20) and Enterococcus faecalis (n=13/18), and meanwhile exhibited a mostly additive effect against Staphylococcus intermedius (n=12/20), Streptococcus agalactiae (n=10/10), Streptococcus dysgalactiae (n=18/18), and Escherichia coli (n=14/18). There were no indifferent or antagonistic effects between CEZ and nisin A. In the time-kill assay, the CEZ-nisin A combination at 0.5 × or 1 × minimum inhibitory concentration exhibited synergistic reduction of bacterial growth by over 3 log10 colony forming units per ml relative to that observed with either antimicrobial substance alone. These results suggest that the CEZ-nisin A combination can be used for developing an intramammary infusion for mastitis treatment, with lower antibiotic concentrations than normal.
Keywords: cattle, cephalosporin, mastitis, nisin, synergism
Bovine mastitis is an inflammatory condition of the mammary gland, most often caused by intramammary microbial infection. It might necessitate treatment, culling, discarding of milk, and reduced milk quality and yield, which can lead to increased production costs and impose a huge economic strain on the industry [1, 38]. Over 150 different types of bacteria have been isolated from animals with bovine mastitis [6]. On the basis of the bacterial etiologic agents, bovine mastitis can be classified as the contagious or environmental type. While Staphylococcus aureus and Streptococcus agalactiae are categorized as contagious pathogens [37], coagulase-negative staphylococcus (CNS) [16], Escherichia coli [37], S. dysgalactiae [37], and Enterococcus faecalis [39] are categorized as environmental pathogens. Many intramammary infections caused by these pathogens lead to subclinical and chronic mastitis. Intramammary infection caused by CNS is especially common in many dairy farms around the world; it has caused herd problems such as elevated bulk milk somatic cell count (SCC) and decreased milk quality [35, 44]. In the dairy industry, mastitis is one of the major reasons for the use of antibiotics [29, 33]. Intramammary infusion of antibiotics is the most common therapy for mastitis in dairy farms worldwide [6, 32].
Cephalosporins, a type of β-lactam antibiotics, are considered to be the most important semisynthetic antibiotics for mastitis treatment in dairy cattle [6, 15]. Cephalosporins exert bacteriocidal effects by inhibiting bacterial cell wall synthesis [11, 15]. First-generation cephalosporins are most frequently used for intramammary treatment of mastitis [6, 15], where they generally exhibit good activity [15, 17, 19]. However, the emergence of antibiotic-resistant bacteria due to overuse of antibiotics against mastitis pathogens has been reported previously [3, 34]. Moreover, antibiotic usage results in the presence of antibiotic residues in milk, which is used for human consumption [2, 20, 29]. Therefore, in order to minimize the use of antibiotics, there is an urgent need for alternative antibiotic therapy approaches for bovine mastitis.
Nisin A, a class-I bacteriocin produced by Lactococcus lactis, is an antibacterial peptide comprising 34 amino acids. It has been used as a food preservative for over 50 years [23]. Nisin Z, variant of nisin A possessing a different amino acid residue at position 27, has recently been revealed to be a possible alternative for treatment of bovine mastitis caused by Gram-positive bacteria [10, 46]. However, nisin A and Z are generally more active against Gram-positive bacteria than Gram-negative bacteria and exert their bactericidal effect at the cytoplasmic membrane of the target organism [7, 25]. Therefore, the combination of cephalosporin and nisin A might provide an extended activity spectrum against mastitis pathogens and reduce the antibiotic dose for mastitis treatment.
This study aimed to evaluate the combined effect of a first-generation cephalosporin and nisin A against mastitis pathogens using the checkerboard and time-kill assays.
MATERIALS AND METHODS
Bacterial strains
All mastitis pathogen isolates including S. aureus (n=20), S. intermedius (n=20), S. agalactiae (n=10), S. dysgalactiae (n=18), E. faecalis (n=18), and E. coli (n=18) were obtained from clinical or subclinical cases in commercial dairy herds located in different geographical areas of Fukuoka Prefecture, Japan, between 2006 and 2012. These isolates were identified on the basis of colony morphology, Gram-staining properties, and catalase and coagulase test findings and by using commercial kits (API® STAPH, API® 20 STREP, and API® 20 E, bioMérieux Industry, Marcy-l’Étoile, France). Isolates maintained at −80°C in tryptic soy broth with 20% glycerol were retrieved and plated onto Mueller–Hinton agar (MHA) plates supplemented with 5% sheep blood and incubated at 37°C for 24 hr [15].
Antimicrobial substance
Cefazolin (CEZ), which is a first-generation cephalosporin, was purchased from Meiji Seika Pharma Co., Ltd. (Tokyo, Japan). For use in this study, CEZ stock solutions were prepared at a concentration of 10 mg/ml.
Nisin A was manufactured by Omu Milk Products Co., Ltd. (Fukuoka, Japan). The nisin A-producing L. lactis strain was grown in MRS broth (Oxoid Ltd., Basingstoke, U.K.) for 16 hr at 30°C. Nisin A was recovered from the culture supernatant and purified as previously described, with some modifications [47]. Briefly, the culture supernatant was applied to a hydrophobic resin column, and the adsorbed nisin A was eluted with 40–70% ethanol and separated by evaporation. The compound was further purified by reverse-phase high-performance liquid chromatography (HPLC). The concentration of purified nisin A was determined from the HPLC peak area with a standard curve prepared by commercial nisin preparation (Sigma-Aldrich, St. Louis, MO, U.S.A.) [13].
Determination of minimum inhibitory concentration (MIC)
The MICs of CEZ and nisin A against mastitis pathogens were determined by the microdilution method, in accordance with the Clinical and Laboratory Standards Institute guidelines [14]. Briefly, target strains were inoculated onto MHA plates supplemented with 5% sheep blood and incubated overnight at 37°C. The cells were diluted in Mueller–Hinton broth (MHB) to an approximate final concentration of 5 × 105 colony forming units (CFUs) per ml. Fifty microliters each of the antimicrobial agent dilutions and bacterial inocula were dispensed into individual wells of a 96-well microtiter plate. The plate was then incubated at 37°C for 24 hr. Minimum inhibitory concentration was defined as the lowest concentration of each antibiotic that completely inhibited bacterial growth, as apparent to the unaided eye.
Checkerboard assay
The interactions between CEZ and nisin A against mastitis pathogens were evaluated by the microbroth checkerboard method in 96-well microtiter plates containing MHB, as described in previous reports [28, 43]. Briefly, CEZ and nisin A were serially diluted along the y and x axes, respectively. The final antimicrobial substance concentrations (after two-fold dilution) ranged from 1/16 to 4 times the MIC for CEZ and from 1/256 to 4 times the MIC for nisin A. The checkerboard plates were inoculated with bacteria at an approximate concentration of 5 × 105 CFU/ml and incubated at 37°C for 24 hr, following which bacterial growth was assessed visually. To evaluate the effect of the combination treatment, the fractional inhibitory concentration (FIC) index for each combination was calculated as follows: FIC index = FIC of CEZ + FIC of nisin A, where FIC of CEZ (or nisin A) was defined as the ratio of MIC of CEZ (or nisin A) in combination and MIC of CEZ (or nisin A) alone. The FIC index values were interpreted as follows: ≤0.5, synergistic; >0.5 to ≤1.0, additive; >1.0 to ≤2.0, indifferent; and >2.0, antagonistic effects [18, 30].
Time-kill assay
The findings of in vitro interaction determined through the checkerboard assay were confirmed through the time-kill assay, which was performed by the broth dilution method, as described in previous studies [36, 41]. Briefly, the experiment included the control, CEZ, nisin A, and combination (CEZ-nisin A) groups. For bacterial inoculation, three isolates were arbitrarily selected from the most dominant FIC index group for each mastitis pathogen. Equal proportions of the three isolates from each group were combined to obtain a three-isolate mixture of each mastitis pathogen species [1]. The MICs of the three-isolate mixtures were measured by the same method as described above. Five milliliters of MHB without antimicrobial substances was used as the control, while MHB (5 ml) with CEZ or nisin A alone at concentrations of 0.5 ×, 1 × and 2 × MIC was added separately into the corresponding tubes. The two antimicrobial substances were added in combination to the corresponding tubes at the following concentrations: 0.5 × MIC CEZ + 0.5 × MIC nisin A; 1 × MIC CEZ + 1 × MIC nisin A; and 2 × MIC CEZ + 2 × MIC nisin A. The bacterial inocula were diluted to approximately 5 × 105 CFU/ml in a 5-ml final volume of MHB. At 0, 3, 6, 9 and 24 hr of incubation with agitation at 37°C, 100-µl aliquots of culture supernatant were collected from each tube, diluted tenfold, and inoculated onto MHA plates with 5% sheep blood. The plates were incubated at 37°C for 24 hr, following which the colony counts were determined. The lower limit for detection of bacterial count was 2.3 log10 CFU/ml (i.e., 200 CFU/ml). Experiments were performed in triplicate. A growth curve was plotted with the average bacterial count at each time point. Bactericidal effect was defined as a reduction of ≥3 log10 CFU/ml relative to the starting inoculum. Synergism was defined as a reduction of ≥2 log10 CFU/ml observed at 24 hr post-incubation with the CEZ-nisin A combination relative to that observed with either antimicrobial substance. Additive effect was defined as a reduction of 1 to <2 log10 CFU/ml observed at 24 hr post-incubation with the CEZ-nisin A combination relative to that observed with either antimicrobial substance. Indifferent effect was defined as an increase or decrease of <1 log10 CFU/ml, and antagonism was defined as an increase of >2 log10 CFU/ml observed at 24 hr post-incubation with the CEZ-nisin A combination relative to that observed with either antimicrobial substance [36, 41].
RESULTS
MIC and checkerboard assay
Table 1 presents a summary of MICs of CEZ and nisin A, alone and in combination, against mastitis pathogens. Cefazolin was very active against S. aureus, S. intermedius, S. agalactiae, and S. dysgalactiae, with MIC50 values ranging from 0.13 to 0.5 µg/ml. While CEZ exhibited good activity against E. coli (MIC50, 2 µg/ml), it was less active against E. faecalis (MIC50, 32 µg/ml). Nisin A was very active against S. intermedius and S. agalactiae (MIC50, 0.06 and 0.25 µg/ml, respectively); while the compound exhibited good activity against S. aureus (MIC50, 1 µg/ml), S. dysgalactiae (MIC50, 1 µg/ml), and E. faecalis (MIC50, 4 µg/ml), it was not active against E. coli (MIC50, 128 µg/ml). The MIC50 values of CEZ and nisin A in combination with each other were 2- to 8-times lower than those of either antimicrobial substance alone.
Table 1. Summary of MIC50 values of cefazolin (CEZ) and nisin A, alone and in combination, against bovine mastitis pathogens.
| Organism | n | MIC50 (range) of compound (µg/ml) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Alone |
In combination |
||||||||
| CEZ | Nisin A | CEZ | Nisin A | ||||||
| Staphylococcus aureus | 20 | 0.5 | (0.25–1) | 1 | (0.25–16) | 0.06 | (0.03–0.13) | 0.25 | (0.03–1) |
| Staphylococcus intermedius | 20 | 0.5 | (0.25–4) | 0.06 | (0.02–4) | 0.13 | (0.03–0.5) | 0.03 | (0.002–2) |
| Streptococcus agalactiae | 10 | 0.13 | (0.06–0.25) | 0.25 | (0.03–0.25) | 0.06 | (0.02–0.13) | 0.13 | (0.008–0.13) |
| Streptococcus dysgalactiae | 18 | 0.13 | (0.03–1) | 1 | (0.06–32) | 0.06 | (0.02–0.13) | 0.5 | (0.03–4) |
| Enterococcus faecalis | 18 | 32 | (1–64) | 4 | (0.5–8) | 8 | (0.25–16) | 0.5 | (0.02–4) |
| Escherichia coli | 18 | 2 | (1–8) | 128 | (64–128) | 1 | (0.06–2) | 32 | (4–64) |
MIC50 values were defined as the lowest concentration of the antimicrobial substance at which 50% of the each mastitis pathogens were inhibited.
Table 2 presents results of the checkerboard assay of CEZ and nisin A against mastitis pathogens. CEZ and nisin A exhibited synergistic or additive interactions, with FIC index values ranging from 0.19 to 1. There were no instances of indifferent or antagonistic interaction between the two compounds. The CEZ-nisin A interaction in S. aureus (100%) and E. faecalis (72.2%) cultures was predominantly synergistic, while that in S. intermedius (60%), S. agalactiae (100%), S. dysgalactiae (100%) and E. coli (77.8%) was mostly additive.
Table 2. Combined effects of cefazolin (CEZ) and nisin A determined by the checkerboard assay.
| Organism | n | FIC index | No. of strains (%) |
|||
|---|---|---|---|---|---|---|
| Synergism | Additive | Indifferent | Antagonism | |||
| (≤0.5) | ( >0.5 to ≤1.0) | ( >1.0 to ≤2.0) | ( >2.0) | |||
| Staphylococcus aureus | 20 | 0.19–0.5 | 20 (100) | 0 (0) | 0 (0) | 0 (0) |
| Staphylococcus intermedius | 20 | 0.19–1.0 | 8 (40) | 12 (60) | 0 (0) | 0 (0) |
| Streptococcus agalactiae | 10 | 0.63–1.0 | 0 (0) | 10 (100) | 0 (0) | 0 (0) |
| Streptococcus dysgalactiae | 18 | 0.56–1.0 | 0 (0) | 18 (100) | 0 (0) | 0 (0) |
| Enterococcus faecalis | 18 | 0.19–1.0 | 13 (72.2) | 5 (27.8) | 0 (0) | 0 (0) |
| Escherichia coli | 18 | 0.5 –1.0 | 4 (22.2) | 14 (77.8) | 0 (0) | 0 (0) |
The FIC index values were interpreted as follows: ≤0.5, synergistic; >0.5 to ≤1.0, additive; >1.0 to ≤2.0, indifferent; and >2.0, antagonistic effects.
Time-kill assay
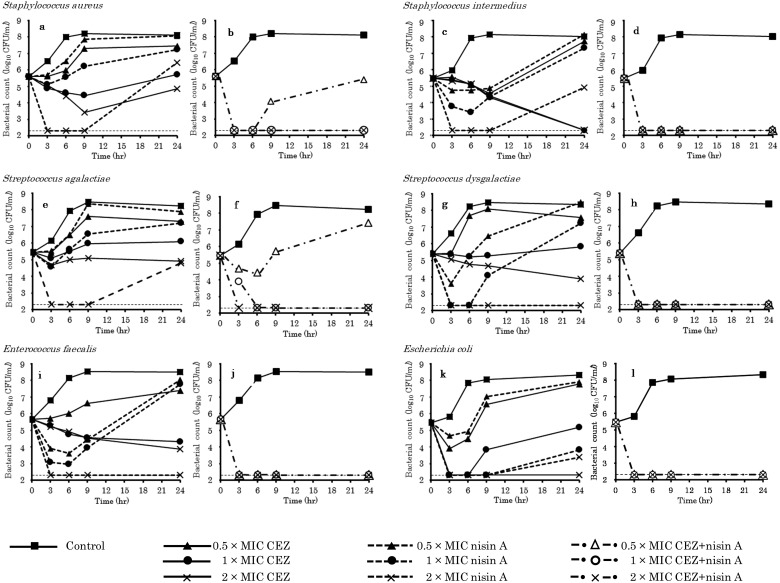
Figure 1 presents the results of the time-kill assay. The MICs of CEZ and nisin A against the three-isolate mixtures of both S. aureus and S. intermedius were 0.5 and 1 µg/ml, respectively. Against the S. aureus strains, CEZ, alone, exhibited no bactericidal effect at any time point in the incubation period. In contrast, nisin A, alone, exhibited transient bactericidal effects at 2 × MIC (Fig. 1a). However, the CEZ-nisin A combination at ≥1 × MIC exhibited bactericidal activity within 3 hr of incubation, with effects lasting for 24 hr (Fig. 1b). Against the S. intermedius strains, CEZ, alone, exhibited bactericidal activity at ≥1 × MIC (Fig. 1c). In contrast, incubation with nisin A, alone, resulted in a clear concentration-dependent decrease in bacterial count within 6 hr after exposure; this, however, did not prevent the regrowth of the surviving bacteria. However, the CEZ-nisin A combination at ≥0.5 × MIC exhibited bactericidal activity within 3 hr of incubation, with effects lasting for 24 hr (Fig. 1d).
Fig. 1.
Time-kill curves for cefazolin (CEZ) and nisin A, alone and in combination, against mastitis pathogens. a, c, e, g, i, k, CEZ and nisin A alone; b, d, f, h, j, l, CEZ-nisin A combination. Thin dotted lines indicate the limit of detection.
The MICs of CEZ and nisin A against the three-isolate mixture of S. agalactiae were 0.13 and 0.25 µg/ml, respectively. Cefazolin, alone, exhibited no bactericidal effect at any time point in the incubation period. In contrast, nisin A, alone, exhibited transient bactericidal effects at 2 × MIC (Fig. 1e). The CEZ-nisin A combination exhibited concentration-dependent bactericidal activity; at ≥1 × MIC, the antimicrobial substance combination exhibited bactericidal activity within 6 hr of incubation, with effects lasting for 24 hr (Fig. 1f).
The MICs of CEZ and nisin A against the three-isolate mixture of S. dysgalactiae were 0.25 and 32 µg/ml, respectively. Cefazolin, alone, exhibited no bactericidal effect at any time point in the incubation period. In contrast, nisin A, alone, at 2 × MIC exhibited bactericidal effects within 3 hr of incubation, which lasted for 24 hr (Fig. 1g). The CEZ-nisin A combination at ≥0.5 × MIC exhibited bactericidal activity within 3 hr, with effects lasting for 24 hr (Fig. 1h).
The MICs of CEZ and nisin A against the three-isolate mixture of E. faecalis were 32 and 8 µg/ml, respectively. Cefazolin, alone, exhibited no bactericidal effect at any time point in the incubation period. In contrast, treatment with nisin A, alone, resulted in a concentration-dependent decrease in bacterial count within 6 hr after exposure; at 2 × MIC, nisin A exhibited bactericidal effects that lasted for 24 hr post-incubation (Fig. 1i). The CEZ-nisin A combination at ≥0.5 × MIC exhibited bactericidal activity within 3 hr of incubation, with effects lasting for 24 hr (Fig. 1j).
The MICs of CEZ and nisin A against the three-isolate mixture of E. coli were 2 and 128 µg/ml, respectively. Cefazolin, alone, exhibited bactericidal activity at 2 × MIC, while nisin A, alone, exhibited transient bactericidal effects at 1 × and 2 × MIC; this effect, however, did not prevent the regrowth of the surviving bacteria (Fig. 1k). The CEZ-nisin A combination at ≥0.5 × MIC exhibited bactericidal activity within 3 hr of incubation, with effects lasting for 24 hr (Fig. 1l).
Table 3 presents a summary of the changes in bacterial count in the time-kill assay at 24 hr post-incubation. Incubation with CEZ or nisin A, alone, at 0.5 × or 1 × MIC resulted in increased bacterial counts at 24 hr post-incubation. However, incubation with the CEZ-nisin A combination at 0.5 × or 1 × MIC resulted in a reduction in bacterial count by over 3 log10 CFU/ml relative to the initial count at 0 hr; at 24 hr post-incubation, this combination also resulted in a synergistic reduction of bacterial counts by 3.39 to 6.18 log10 CFU/ml relative to the count observed with either antimicrobial substance alone.
Table 3. Changes in bacterial count at 24 hr post-incubation with cefazolin (CEZ) and nisin A, alone and in combination.
| Organism | × MIC | Changes in bacterial count (log10 CFU/ml) from 0 hr |
a − c | b − c | Interpretation | ||
|---|---|---|---|---|---|---|---|
| CEZ (a) | Nisin A (b) | CEZ-nisin A (c) | |||||
| Staphylococcus aureus | 1 | 0.10 | 1.64 | –3.29 | 3.39 | 4.93 | S |
| Staphylococcus intermedius | 0.5 | 2.26 | 2.68 | –3.17 | 5.43 | 5.85 | S |
| Streptococcus agalactiae | 1 | 0.50 | 1.69 | –3.31 | 3.81 | 5.00 | S |
| Streptococcus dysgalactiae | 0.5 | 2.14 | 3.08 | –3.10 | 5.24 | 6.18 | S |
| Enterococcus faecalis | 0.5 | 1.72 | 2.67 | –3.36 | 5.08 | 6.03 | S |
| Escherichia coli | 0.5 | 2.31 | 2.45 | –3.16 | 5.47 | 5.61 | S |
Bacterial count was measured by the change in log10 CFU/ml from 0 to 24 hr post-incubation, obtained from the data in Fig. 1. (i.e., log change = log10 CFU24 ‒ log10 CFU0). The results are calculated at 0.5 × MIC, except in the case of S. aureus and S. agalactiae (1 × MIC). S, synergism.
DISCUSSION
First-generation cephalosporins such as cefazolin have been used for the treatment of bovine mastitis caused by Gram-positive and negative bacteria [6, 32]. The antibacterial peptide nisin, including nisin A, has generally been used in food preservatives because of its high antibacterial activity and nontoxicity [21, 23]. As such, some studies have suggested that nisin is effective against mastitis pathogens [10, 40, 46]. The CEZ-nisin A combination can, therefore, be expected to reduce the antibiotic dose for mastitis treatment by extending the activity spectrum by means of synergistic effects against mastitis pathogens. However, there is no information on the synergistic effect of CEZ and nisin A. The present in vitro study investigated the antibacterial activity of the CEZ-nisin A combination against mastitis pathogens for bovine mastitis treatment.
First-generation cephalosporins have generally been reported as exhibiting good bactericidal activity against mastitis bacterial pathogens [15, 17, 19]. However, Tong et al. [43] suggested that E. faecalis possesses both intrinsic and acquired resistance to a variety of antibiotics. In our study, CEZ exhibited MIC50 values of 0.13–2 µg/ml against all mastitis pathogens, except E. faecalis (32 µg/ml). These results reasonably correspond with those of previous studies [15, 17, 19, 43].
Although early studies have reported the MICs of nisin against major Gram-positive mastitis pathogens, the reported values were high and exhibited a wide range (10–250 µg/ml) [8]. Nisin has since then been incorporated into commercially available teat-dipping formulations for mastitis prevention. It has been reported to exhibit mean log reductions of 3.90 and 4.22 against S. aureus and E. coli, respectively, after exposure for 1 min [40]. Furthermore, Cao et al. [10] and Wu et al. [46] reported the therapeutic efficacy of intramammary nisin infusion in lactating dairy cows with clinical or subclinical mastitis caused by several mastitis pathogens, including staphylococci and streptococci. Although these reports suggest the efficacy of nisin against mastitis pathogens, objective evidence of its antibacterial activity against various mastitis pathogens seems to be insufficient. In our study, the MIC50 values of nisin A against staphylococci and streptococci ranged from 0.06 to 1 µg/ml. Even against cephalosporin-resistant enterococci, nisin A exhibited relatively low MIC50 values (4 µg/ml). These results demonstrate the antimicrobial efficacy of nisin A against Gram-positive mastitis pathogens and support the results of previous studies [10, 40, 46]. On the other hand, nisin A exhibited very low activity against E. coli. An accumulating body of evidence shows that nisin exhibits high antibacterial activity against Gram-positive bacteria but not against Gram-negative species. However, previous studies have suggested that nisin A [22] and Z [27] do exhibit MICs against E. coli. Although further research is required to confirm these findings, the present results support the relevance of these previous findings. E. coli can cause mammary gland inflammation in dairy cows around the time of parturition and during early lactation, with striking local, and sometimes severe, systemic clinical symptoms [9]. Understandably, it is desirable that therapeutic substances used for mastitis treatment possess bactericidal properties against both Gram-positive and negative bacteria. Accordingly, we hypothesized that a combination of CEZ and nisin A would be effective against both Gram-positive and negative mastitis pathogens. Furthermore, we expected that the synergistic effect of this combination would reduce the antibiotic dose for mastitis treatment in dairy cows.
In the present study, the synergistic effect was evaluated on the basis of MIC values obtained by the checkerboard and time-kill assays. The MIC50 of the CEZ-nisin A combination against mastitis pathogens was 2- to 8-fold lower than that observed with either compound alone. In the checkerboard assay, all interactions between CEZ and nisin A were synergistic or additive, with FIC index values ranging from 0.19 to 1. In the checkerboard assay, the CEZ-nisin A interactions against S. aureus and E. faecalis strains were mainly synergistic, while those against the other strains were mainly additive. In the time-kill assay, incubation with the CEZ-nisin A combination for 24 hr resulted in a 103-fold greater synergistic reduction in bacterial count relative to that observed with either compound at 0.5 × or 1 × MIC. Furthermore, at 0.5 × or 1 × MIC, the CEZ-nisin A combination exhibited bactericidal effects against all pathogen strains within 6 hr of incubation. The checkerboard and time-kill assay methods are among the most widely used techniques for in vitro assessment of synergistic effects [45]. However, some studies have reported discordance between the findings of these two methods [12, 45]. In the present study, S. aureus and E. faecalis strains, which were selected from the synergistic-effect group in the checkerboard assay, were also susceptible to the synergistic effect of CEZ-nisin A in the time-kill assay. However, the S. intermedius, S. agalactiae, S. dysgalactiae, and E. coli strains that were selected from the additive-effect group in the checkerboard assay exhibited susceptibility to the synergistic effect in the time-kill assay. Despite this discordance in results, both assays revealed synergistic or additive effects of CEZ-nisin A, thus indicating the efficacy of this combination.
Cephalosporins are β-lactam antimicrobials that exert bactericidal properties by disruption of bacterial cell-wall synthesis [11, 15]. The mode of action of nisin A involves interaction with the membrane-bound cell-wall precursor lipid II concomitant with pore formation in the cytoplasmic membrane of the target organism, resulting in loss of membrane potential and leakage of intracellular metabolites [7, 25].
In the present study, the CEZ-nisin A combination exhibited synergistic or additive effects against both Gram-positive and negative mastitis pathogens. It may, therefore, be inferred that the bactericidal effect of CEZ-nisin A against Gram-positive bacteria results from the interference of CEZ with bacterial cell-wall synthesis and cytoplasmic membrane pore formation by nisin A. On the other hand, the poor sensitivity of Gram-negative bacteria to nisin A might be attributed to the large size of the peptide, which would restrict its passage through the outer membrane of Gram-negative bacteria [24]. In this respect, some reports [5, 24] suggest the use of the metal-chelating agent ethylenediaminetetraacetic acid (EDTA) for the enhancement of nisin A sensitivity; EDTA removes stabilizing cations from the outer membrane and destroys the membrane function as a penetration barrier. Although the mechanism of action of nisin A against Gram-negative bacteria is not completely understood, it is supposed that CEZ initially mediates the inhibition of Gram-negative bacterial cell-wall synthesis, following which nisin A causes pore formation in the cytoplasmic membrane and leakage of intracellular metabolites.
In the present study, the CEZ-nisin A combination exhibited synergistic or additive effects, which suggests that the two antimicrobial substances can together achieve mastitis pathogen control even at low concentrations. Furthermore, this combination might provide extended activity spectrum against mastitis pathogens such as enterococci or E. coli, which are not sufficiently inhibited by either compound individually. An early study [26] had reported that milk fat inhibits the antibacterial effect of nisin. However, Bhatti et al. [4] did not observe any decrease in the antibacterial effect of nisin in non-homogenized milk products such as raw milk. Although Szweda et al. [42] reported a decreased susceptibility to nisin in antibiotic-resistant S. aureus isolated from bovine mastitis, Okuda et al. [31] suggested that nisin A that forms stable pore on biofilm cells is highly potent for the treatment of biofilm-associated infections. These findings, together with the present results, suggest that the CEZ-nisin A combination can serve as an alternative therapy for bovine mastitis in the form of intramammary infusion, with lower antibiotic concentrations than normal.
In conclusion, the results of the checkerboard and time-kill assays in the present study indicated that CEZ and nisin A exert synergistic or additive bactericidal effects against bovine mastitis pathogens. These results suggest that the CEZ-nisin A combination is effective in reducing the antibiotic dose in intramammary infusions formulated for mastitis treatment in dairy cattle. Further studies are required for in vivo assessment of microbial response to this antimicrobial substance combination.
Acknowledgments
We thank the members of the Livestock Hygiene Service Center of Fukuoka prefecture for their assistance of collecting mastitis pathogens. This work was partially supported by “Research project for utilizing advanced technologies in agriculture, forestry and fisheries” of Ministry of Agriculture, Forestry and Fisheries of Japan.
REFERENCES
- 1.Ananda Baskaran S., Kazmer G. W., Hinckley L., Andrew S. M., Venkitanarayanan K.2009. Antibacterial effect of plant-derived antimicrobials on major bacterial mastitis pathogens in vitro. J. Dairy Sci. 92: 1423–1429. doi: 10.3168/jds.2008-1384 [DOI] [PubMed] [Google Scholar]
- 2.Andrew S. M., Moyes K. M., Borm A. A., Fox L. K., Leslie K. E., Hogan J. S., Oliver S. P., Schukken Y. H., Owens W. E., Norman C.2009. Factors associated with the risk of antibiotic residues and intramammary pathogen presence in milk from heifers administered prepartum intramammary antibiotic therapy. Vet. Microbiol. 134: 150–156. doi: 10.1016/j.vetmic.2008.09.022 [DOI] [PubMed] [Google Scholar]
- 3.Berghash S. R., Davidson J. N., Armstrong J. C., Dunny G. M.1983. Effects of antibiotic treatment of nonlactating dairy cows on antibiotic resistance patterns of bovine mastitis pathogens. Antimicrob. Agents Chemother. 24: 771–776. doi: 10.1128/AAC.24.5.771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhatti M., Veeramachaneni A., Shelef L. A.2004. Factors affecting the antilisterial effects of nisin in milk. Int. J. Food Microbiol. 97: 215–219. doi: 10.1016/j.ijfoodmicro.2004.06.010 [DOI] [PubMed] [Google Scholar]
- 5.Boziaris I. S., Adams M. R.1999. Effect of chelators and nisin produced in situ on inhibition and inactivation of gram negatives. Int. J. Food Microbiol. 53: 105–113. doi: 10.1016/S0168-1605(99)00139-7 [DOI] [PubMed] [Google Scholar]
- 6.Bradley A. J., Green M. J.2009. Factors affecting cure when treating bovine clinical mastitis with cephalosporin-based intramammary preparations. J. Dairy Sci. 92: 1941–1953. doi: 10.3168/jds.2008-1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breukink E., van Heusden H. E., Vollmerhaus P. J., Swiezewska E., Brunner L., Walker S., Heck A. J., de Kruijff B.2003. Lipid II is an intrinsic component of the pore induced by nisin in bacterial membranes. J. Biol. Chem. 278: 19898–19903. doi: 10.1074/jbc.M301463200 [DOI] [PubMed] [Google Scholar]
- 8.Broadbent J. R., Chou Y. C., Gillies K., Kondo J. K.1989. Nisin inhibits several gram-positive, mastitis-causing pathogens. J. Dairy Sci. 72: 3342–3345. doi: 10.3168/jds.S0022-0302(89)79496-0 [DOI] [PubMed] [Google Scholar]
- 9.Burvenich C., Van Merris V., Mehrzad J., Diez-Fraile A., Duchateau L.2003. Severity of E. coli mastitis is mainly determined by cow factors. Vet. Res. 34: 521–564. doi: 10.1051/vetres:2003023 [DOI] [PubMed] [Google Scholar]
- 10.Cao L. T., Wu J. Q., Xie F., Hu S. H., Mo Y.2007. Efficacy of nisin in treatment of clinical mastitis in lactating dairy cows. J. Dairy Sci. 90: 3980–3985. doi: 10.3168/jds.2007-0153 [DOI] [PubMed] [Google Scholar]
- 11.Caprile K. A.1988. The cephalosporin antimicrobial agents: a comprehensive review. J. Vet. Pharmacol. Ther. 11: 1–32. doi: 10.1111/j.1365-2885.1988.tb00117.x [DOI] [PubMed] [Google Scholar]
- 12.Chan E. L., Zabransky R. J.1987. Determination of synergy by two methods with eight antimicrobial combinations against tobramycin-susceptible and tobramycin-resistant strains of Pseudomonas. Diagn. Microbiol. Infect. Dis. 6: 157–164. doi: 10.1016/0732-8893(87)90101-5 [DOI] [PubMed] [Google Scholar]
- 13.Chinachoti N., Matsusaki H., Sonomoto K., Ishizaki A.1998. Nisin Z Production by Lactococcus lactis IO-1 Using Xylose as a Carbon Source. Biosci. Biotechnol. Biochem. 62: 1022–1024. doi: 10.1271/bbb.62.1022 [DOI] [PubMed] [Google Scholar]
- 14.Clinical and Laboratory Standards Institute. 2012. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard-Ninth Edition (M07-A8). Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 15.Cortinhas C. S., Oliveira L., Hulland C. A., Santos M. V., Ruegg P. L.2013. Minimum inhibitory concentrations of cephalosporin compounds and their active metabolites for selected mastitis pathogens. Am. J. Vet. Res. 74: 683–690. doi: 10.2460/ajvr.74.5.683 [DOI] [PubMed] [Google Scholar]
- 16.De Visscher A., Supré K., Haesebrouck F., Zadoks R. N., Piessens V., Van Coillie E., Piepers S., De Vliegher S.2014. Further evidence for the existence of environmental and host-associated species of coagulase-negative staphylococci in dairy cattle. Vet. Microbiol. 172: 466–474. doi: 10.1016/j.vetmic.2014.06.011 [DOI] [PubMed] [Google Scholar]
- 17.Demon D., Ludwig C., Breyne K., Guédé D., Dörner J. C., Froyman R., Meyer E.2012. The intramammary efficacy of first generation cephalosporins against Staphylococcus aureus mastitis in mice. Vet. Microbiol. 160: 141–150. doi: 10.1016/j.vetmic.2012.05.017 [DOI] [PubMed] [Google Scholar]
- 18.Draper L. A., Cotter P. D., Hill C., Ross R. P.2013. The two peptide lantibiotic lacticin 3147 acts synergistically with polymyxin to inhibit Gram negative bacteria. BMC Microbiol. 13: 212. doi: 10.1186/1471-2180-13-212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erskine R. J., Walker R. D., Bolin C. A., Bartlett P. C., White D. G.2002. Trends in antibacterial susceptibility of mastitis pathogens during a seven-year period. J. Dairy Sci. 85: 1111–1118. doi: 10.3168/jds.S0022-0302(02)74172-6 [DOI] [PubMed] [Google Scholar]
- 20.Fejzic N., Begagic M., Šerić-Haračić S., Smajlovic M.2014. Beta lactam antibiotics residues in cow’s milk: comparison of efficacy of three screening tests used in Bosnia and Herzegovina. Bosn. J. Basic Med. Sci. 14: 155–159. doi: 10.17305/bjbms.2014.3.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Felicio B. A., Pinto M. S., Oliveira F. S., Lempk M. W., Pires A. C., Lelis C. A.2015. Effects of nisin on Staphylococcus aureus count and physicochemical properties of Minas Frescal cheese. J. Dairy Sci. 98: 4364–4369. doi: 10.3168/jds.2015-9520 [DOI] [PubMed] [Google Scholar]
- 22.Field D., Begley M., O’Connor P. M., Daly K. M., Hugenholtz F., Cotter P. D., Hill C., Ross R. P.2012. Bioengineered nisin A derivatives with enhanced activity against both Gram positive and Gram negative pathogens. PLoS One 7: e46884. doi: 10.1371/journal.pone.0046884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hansen J. N.1994. Nisin as a model food preservative. Crit. Rev. Food Sci. Nutr. 34: 69–93. doi: 10.1080/10408399409527650 [DOI] [PubMed] [Google Scholar]
- 24.Helander I. M., Mattila-Sandholm T.2000. Permeability barrier of the gram-negative bacterial outer membrane with special reference to nisin. Int. J. Food Microbiol. 60: 153–161. doi: 10.1016/S0168-1605(00)00307-X [DOI] [PubMed] [Google Scholar]
- 25.Islam M. R., Nagao J., Zendo T., Sonomoto K.2012. Antimicrobial mechanism of lantibiotics. Biochem. Soc. Trans. 40: 1528–1533. doi: 10.1042/BST20120190 [DOI] [PubMed] [Google Scholar]
- 26.Jung D. S., Bodyfelt F. W., Daeschel M. A.1992. Influence of fat and emulsifiers on the efficacy of nisin in inhibiting Listeria monocytogenes in fluid milk. J. Dairy Sci. 75: 387–393. doi: 10.3168/jds.S0022-0302(92)77773-X [DOI] [PubMed] [Google Scholar]
- 27.Kuwano K., Tanaka N., Shimizu T., Nagatoshi K., Nou S., Sonomoto K.2005. Dual antibacterial mechanisms of nisin Z against Gram-positive and Gram-negative bacteria. Int. J. Antimicrob. Agents 26: 396–402. doi: 10.1016/j.ijantimicag.2005.08.010 [DOI] [PubMed] [Google Scholar]
- 28.Lebel G., Piché F., Frenette M., Gottschalk M., Grenier D.2013. Antimicrobial activity of nisin against the swine pathogen Streptococcus suis and its synergistic interaction with antibiotics. Peptides 50: 19–23. doi: 10.1016/j.peptides.2013.09.014 [DOI] [PubMed] [Google Scholar]
- 29.Mitchell J. M., Griffiths M. W., McEwen S. A., McNab W. B., Yee A. J.1998. Antimicrobial drug residues in milk and meat: causes, concerns, prevalence, regulations, tests, and test performance. J. Food Prot. 61: 742–756. doi: 10.4315/0362-028X-61.6.742 [DOI] [PubMed] [Google Scholar]
- 30.Naghmouchi K., Belguesmia Y., Baah J., Teather R., Drider D.2011. Antibacterial activity of class I and IIa bacteriocins combined with polymyxin E against resistant variants of Listeria monocytogenes and Escherichia coli. Res. Microbiol. 162: 99–107. doi: 10.1016/j.resmic.2010.09.014 [DOI] [PubMed] [Google Scholar]
- 31.Okuda K., Zendo T., Sugimoto S., Iwase T., Tajima A., Yamada S., Sonomoto K., Mizunoe Y.2013. Effects of bacteriocins on methicillin-resistant Staphylococcus aureus biofilm. Antimicrob. Agents Chemother. 57: 5572–5579. doi: 10.1128/AAC.00888-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oliveira L., Ruegg P. L.2014. Treatments of clinical mastitis occurring in cows on 51 large dairy herds in Wisconsin. J. Dairy Sci. 97: 5426–5436. doi: 10.3168/jds.2013-7756 [DOI] [PubMed] [Google Scholar]
- 33.Oliver S. P., Murinda S. E., Jayarao B. M.2011. Impact of antibiotic use in adult dairy cows on antimicrobial resistance of veterinary and human pathogens: a comprehensive review. Foodborne Pathog. Dis. 8: 337–355. doi: 10.1089/fpd.2010.0730 [DOI] [PubMed] [Google Scholar]
- 34.Piddock L. J.1996. Does the use of antimicrobial agents in veterinary medicine and animal husbandry select antibiotic-resistant bacteria that infect man and compromise antimicrobial chemotherapy? J. Antimicrob. Chemother. 38: 1–3. doi: 10.1093/jac/38.1.1 [DOI] [PubMed] [Google Scholar]
- 35.Pyörälä S., Taponen S.2009. Coagulase-negative staphylococci-emerging mastitis pathogens. Vet. Microbiol. 134: 3–8. doi: 10.1016/j.vetmic.2008.09.015 [DOI] [PubMed] [Google Scholar]
- 36.Ribes S., Pachón-Ibáñez M. E., Domínguez M. A., Fernández R., Tubau F., Ariza J., Gudiol F., Cabellos C.2010. In vitro and in vivo activities of linezolid alone and combined with vancomycin and imipenem against Staphylococcus aureus with reduced susceptibility to glycopeptides. Eur. J. Clin. Microbiol. Infect. Dis. 29: 1361–1367. doi: 10.1007/s10096-010-1007-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riffon R., Sayasith K., Khalil H., Dubreuil P., Drolet M., Lagacé J.2001. Development of a rapid and sensitive test for identification of major pathogens in bovine mastitis by PCR. J. Clin. Microbiol. 39: 2584–2589. doi: 10.1128/JCM.39.7.2584-2589.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rollin E., Dhuyvetter K. C., Overton M. W.2015. The cost of clinical mastitis in the first 30 days of lactation: An economic modeling tool. Prev. Vet. Med. 122: 257–264. doi: 10.1016/j.prevetmed.2015.11.006 [DOI] [PubMed] [Google Scholar]
- 39.Rysanek D., Zouharova M., Babak V.2009. Monitoring major mastitis pathogens at the population level based on examination of bulk tank milk samples. J. Dairy Res. 76: 117–123. doi: 10.1017/S0022029908003816 [DOI] [PubMed] [Google Scholar]
- 40.Sears P. M., Smith B. S., Stewart W. K., Gonzalez R. N., Rubino S. D., Gusik S. A., Kulisek E. S., Projan S. J., Blackburn P.1992. Evaluation of a nisin-based germicidal formulation on teat skin of live cows. J. Dairy Sci. 75: 3185–3190. doi: 10.3168/jds.S0022-0302(92)78083-7 [DOI] [PubMed] [Google Scholar]
- 41.Shi J., Mao N. F., Wang L., Zhang H. B., Chen Q., Liu H., Tang X., Jin T., Zhu C. T., Li F. B., Sun L. H., Xu X. M., Xu Y. Q.2014. Efficacy of combined vancomycin and fosfomycin against methicillin-resistant Staphylococcus aureus in biofilms in vivo. PLOS ONE 9: e113133. doi: 10.1371/journal.pone.0113133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Szweda P., Schielmann M., Frankowska A., Kot B., Zalewska M.2014. Antibiotic resistance in Staphylococcus aureus strains isolated from cows with mastitis in eastern Poland and analysis of susceptibility of resistant strains to alternative nonantibiotic agents: lysostaphin, nisin and polymyxin B. J. Vet. Med. Sci. 76: 355–362. doi: 10.1292/jvms.13-0177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tong Z., Zhang Y., Ling J., Ma J., Huang L., Zhang L.2014. An in vitro study on the effects of nisin on the antibacterial activities of 18 antibiotics against Enterococcus faecalis. PLOS ONE 9: e89209. doi: 10.1371/journal.pone.0089209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vanderhaeghen W., Piepers S., Leroy F., Van Coillie E., Haesebrouck F., De Vliegher S.2014. Invited review: effect, persistence, and virulence of coagulase-negative Staphylococcus species associated with ruminant udder health. J. Dairy Sci. 97: 5275–5293. doi: 10.3168/jds.2013-7775 [DOI] [PubMed] [Google Scholar]
- 45.White R. L., Burgess D. S., Manduru M., Bosso J. A.1996. Comparison of three different in vitro methods of detecting synergy: time-kill, checkerboard, and E test. Antimicrob. Agents Chemother. 40: 1914–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu J., Hu S., Cao L.2007. Therapeutic effect of nisin Z on subclinical mastitis in lactating cows. Antimicrob. Agents Chemother. 51: 3131–3135. doi: 10.1128/AAC.00629-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zendo T., Fukao M., Ueda K., Higuchi T., Nakayama J., Sonomoto K.2003. Identification of the lantibiotic nisin Q, a new natural nisin variant produced by Lactococcus lactis 61-14 isolated from a river in Japan. Biosci. Biotechnol. Biochem. 67: 1616–1619. doi: 10.1271/bbb.67.1616 [DOI] [PubMed] [Google Scholar]