Abstract
Adipose-derived stem cells (ADSCs) are abundant and readily obtained, and have been studied for their clinical applicability in regenerative medicine. Some surface antigens have been identified as markers of different ADSC subpopulations in mice and humans. However, it is unclear whether functionally distinct subpopulations exist in dogs. To address this issue, we evaluated aldehyde dehydrogenase (ALDH) activity—a widely used stem cell marker in mice and humans—by flow cytometry. Approximately 20% of bulk ADSCs showed high ALDH activity. Compared to cells with low activity (ALDHLo), the high-activity (ALDHHi) subpopulation exhibited a higher capacity for adipogenic and osteogenic differentiation. This is the first report of distinct ADSC subpopulations in dogs that differ in terms of adipogenic and osteogenic differentiation potential.
Keywords: adipose-derived stem cells, aldehyde dehydrogenase activity, flow cytometry, osteogenic differentiation, adipogenic differentiation
Adipose-derived stem cells (ADSCs) are a type of mesenchymal stem cells (MSCs) that exist in adipose tissues and are progenitors of adipocytes. MSCs are multipotent and can differentiate into adipocytes, osteocytes, chondrocytes, and vascular cells [17]. ADSCs and bone marrow-derived stem cells (BMSCs) have many advantages over embryonic stem (ES) cells and induced pluripotent stem (iPS) cells for clinical applications [11, 14, 15], despite having more limited differentiation potentials. For example, MSCs are readily obtained from autologous cells and have low risk of malignant transformation. Additionally, ADSCs can be obtained less invasively than BMSCs. As such, ADSCs show great promise for use in human and veterinary regenerative medicine.
ADSCs are heterogeneous and comprise distinct subpopulations, including cells exhibiting high levels of the marker cluster of differentiation (CD) 90 (CD90Hi) that exhibit high tube-forming ability, as well as low CD90-expressing (CD90Lo) cells that have high adipogenic potential in mice [13]. The CD90Hi subpopulation also exhibits a higher efficiency of iPS cells induction than CD90Lo cells [6]. Human ADSCs also contain a CD105Lo subpopulation that has high osteogenic potential [7]. Different ADSC subpopulations have been identified based on surface antigen markers; however, it is unclear how these (e.g., CD90 and CD73) are functionally related to cell differentiation. Additionally, there are no reports to date describing specific ADSC subpopulations in dogs.
Aldehyde dehydrogenases (ALDH) are a family of 19 intracellular enzymes responsible for oxidizing aldehydes [10]. High ALDH activity has been reported in hematopoietic and cancer stem cells, among other cell types [1, 5]. Only one study to date has investigated ALDH activity in human ADSCs. However, a subpopulation with high ALDH activity showed no significant difference in terms of chondrogenic differentiation potential relative to unsorted ADSCs [4]. To confirm and extend these findings, the present study examined the differentiation potential of canine ADSCs in relation to ALDH activity by flow cytometry.
Subcutaneous adipose tissue was obtained from the back region of two clinically healthy laboratory beagles (an 8-year-old, 9.3-kg female and a 6-year-old, 9.0-kg male). Anesthesia was induced in the animals with 7 mg/kg propofol (Intervet, Tokyo, Japan) and maintained with 1.3% isoflurane (DS Pharma Animal Health Co., Osaka, Japan) in oxygen. Analgesia was induced by 20 µg/kg buprenorphine (Otsuka Pharmaceutical, Tokyo, Japan) and 0.2 mg/kg meloxicam (Boehringer Ingelheim, Tokyo, Japan). Animal experiments were approved by the institutional animal experiment ethics committee and were in accordance with institutional guidelines of Yamaguchi University.
Canine ADSCs were isolated as previously described in mice [8]. Adipose tissue was washed with Dulbecco’s phosphate-buffered saline (DPBS) (Wako, Osaka, Japan), and cut into small pieces that were incubated at 37.5°C for 1 hr with shaking in high-glucose Dulbecco’s modified Eagle’s medium (DMEM) (Wako) supplemented with 10% fetal bovine serum (FBS) (Sigma-Aldrich, St. Louis, MO, U.S.A.), penicillin (100 U/ml)/streptomycin (100 µg/ml) (PSM), amphotericin B (0.25 µg/ml) (100 × Antibiotic-Antimycotic Mixed Stock Solution; Nacalai Tesque, Kyoto, Japan), and collagenase type I (1.0 mg/ml) (Sigma-Aldrich). The digested tissue was filtered through a sterile 100-µm nylon mesh (EASYstrainer, 100 µm; Greiner Bio-one Japan, Tokyo, Japan) followed by centrifugation at 1,800 rpm for 5 min in 30 ml of DPBS with 1% FBS and 1 mM EDTA·3Na (Wako). The pellet was resuspended in DMEM and seeded on culture plates. When cultures reached 80–90% confluence, ADSCs were replated using trypsin/EDTA (0.05 w/v% Trypsin and 0.53 mmol/l EDTA 4 Na solution with Phenol Red; Wako).
Adherent cells from passage 4 were dissociated with trypsin/EDTA and 1 × 106 cells were resuspended and incubated for 5 min on ice in 1 ml of DPBS with 1% FBS and 1 mM EDTA·3Na containing 2 µl of Fc receptor-blocking reagent (FcX Blocker; Biolegend, San Diego, CA, U.S.A.). Cells were stained with 1 µl of reagent for 20 min at room temperature to exclude dead cells (Zombie NIR; Biolegend). ALDH activity was measured with an Aldefluor kit (Stem Cell Technologies, Tokyo, Japan) according to the manufacturer’s instructions. Briefly, 1 × 106 cells were resuspended in 1 ml assay buffer and 5 µl Aldefluor reagent was added. After thorough mixing, 0.5 ml of the cell suspension was transferred to a new tube with 5 µl of diethylaminobenzaldehyde reagent, followed by incubation for 30 min at 37°C and then centrifugation and resuspension in 0.5 ml assay buffer. Cells were sorted by flow cytometry (Accuri C6; BD Japan, Tokyo, Japan) and data were analyzed with FlowJo software (Tree Star, Ashland, OR, U.S.A.).
To assess the viability of ADSC subpopulations, we used Cell Counting Kit (CCK)-8 (Dojindo Laboratories, Kumamoto, Japan). Cells were seeded in a 96-well plate at a density of 1.5 × 103 cells/well. At each time point, 100 µl of fresh medium containing 10 µl CCK-8 solution were added to each well followed by incubation at 37°C for 1 hr. The absorbance at 450 nm was measured on an Epoch spectrophotometer (Biotek Japan, Tokyo, Japan). Six replicates were prepared for each group.
The adipogenic and osteogenic differentiation potential of ADSCs was analyzed on an SH800 cell sorter (Sony, Tokyo, Japan), using a cell differentiation kit (Mouse Mesenchymal Stem Cell Functional Identification Kit; R&D Systems, Minneapolis, MN, U.S.A.) according to the manufacturer’s instructions. For adipogenic differentiation, cells (3 × 103/well) were cultured at 37°C and 5% CO2 in a 96-well plate in 100 µl adipogenic differentiation medium composed of 5 ml α-Minimal Essential Medium (MEM) with 10% FBS, 1% PSM, l-glutamine, and Phenol Red (Wako) (α-MEM basal medium) supplemented with 50 µl adipogenic supplement (containing hydrocortisone, isobutylmethylxanthine, and indomethacin). The medium was replaced every 3–4 days for 15 days. For osteogenic differentiation, cells (3 × 103/well) were cultured at 37°C and 5% CO2 in a 96-well plate in 100 µl osteogenic differentiation medium composed of 5 ml α-MEM basal medium containing 250 µl mouse/rat osteogenic supplement (with ascorbate-phosphate, β-glycerolphosphate, and recombinant human bone morphogenetic protein-2). The medium was replaced every 2–3 days for 15 days.
To detect adipogenic differentiation by immunocytochemistry, cells were fixed for 20 min in 4% paraformaldehyde phosphate buffer solution (Wako), washed three times with DPBS, and blocked with DPBS supplemented with 0.3% Triton X-100 non-ionic surfactant (Sigma-Aldrich) and 10% FBS for 45 min. Cells were then incubated for 1 hr in DPBS containing 10 µg/ml of goat anti-mouse fatty acid-binding protein (FABP) 4 polyclonal antibody to label adipocytes. A negative control was run using DPBS with no primary antibody. Cells were washed with DPBS and incubated for 1 hr in DPBS containing phycoerythrin (PE)-conjugated secondary antibody (rabbit F (ab’) 2 anti-goat IgG H&L (PE), pre-adsorbed; Abcam Japan, Tokyo, Japan). After washing with DPBS, cells were mounted with a solution containing 5 µg/ml Hoechst 33342 (Dojindo Laboratories) to label nuclei. To detect osteogenic differentiation by immunocytochemistry, cells were fixed for 20 min in 4% paraformaldehyde, washed three times with DPBS, and blocked for 45 min in DPBS supplemented with 0.3% Triton X-100 and 10% FBS. Cells were then incubated for 1 hr in DPBS containing 10 µg/ml of goat anti-mouse osteopontin polyclonal antibody to label osteocytes. A negative control was run using DPBS with no primary antibody. After washing with DPBS, cells were incubated for 1 hr in DPBS containing PE-conjugated rabbit anti-goat secondary antibody, washed with DPBS, and mounted as described above.
Images were captured using an IN Cell Analyzer 2200 (GE Healthcare, Piscataway, NJ, U.S.A.). The program was set to capture nine images per well at 16.7 ms/30% gain and 2,000 ms/50% gain for the Hoechst and PE channels, respectively. Image analysis was performed using Workstation 3.4 for INcell 1000 (GE Healthcare).
Statistical analysis was performed using Prism 4.0 software (GraphPad Inc., San Diego, CA, U.S.A.). Results are expressed as mean ± standard error. Comparisons of two groups were carried out with the unpaired Student’s t-test. Multiple comparisons were carried out with one-way ANOVA. A P-value <0.05 was considered statistically significant.
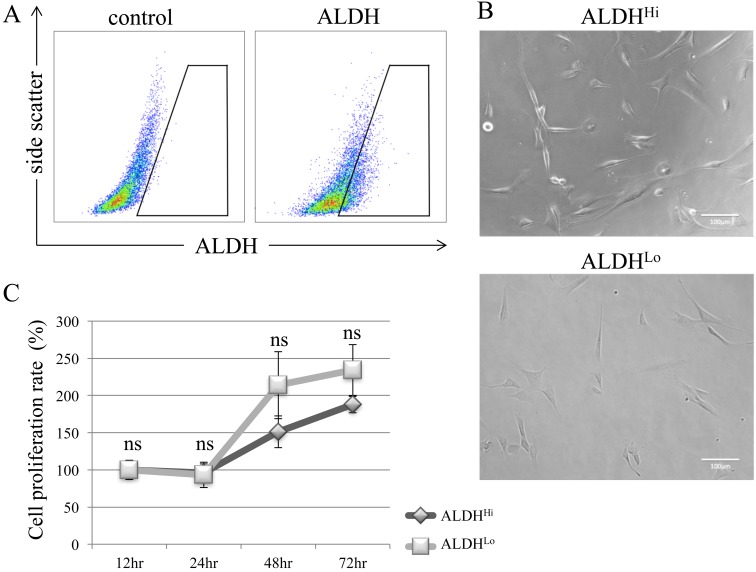
To identify different subpopulations of canine ADSCs, we evaluated ALDH activity by flow cytometry. Approximately 20% of ADSCs were ALDH-positive (Fig. 1A). After 24 hr of incubation, sorted ALDHHi and ALDHLo cells became attached to the culture plates (Fig. 1B); both subpopulations showed similar morphologies. There was no difference in proliferation rate between ALDHHi and ALDHLo subpopulations (Fig. 1C).
Fig. 1.
Detection of ALDH-positive subpopulations of canine ADSCs and evaluation of proliferation rates. (A) Flow cytometric analysis of canine ADSCs. Baseline fluorescence was established by adding the ALDH inhibitor diethylaminobenzaldehyde (control). (B) Sorted ALDHHi and ALDHLo showed similar morphologies after incubation for 24 hr. (C) There was no significant difference in cell proliferation rates between ALDHHi and ALDHLo subpopulations. Values are expressed as mean ± standard error (n=5). *P<0.05 vs. control cells; ns, not significant.
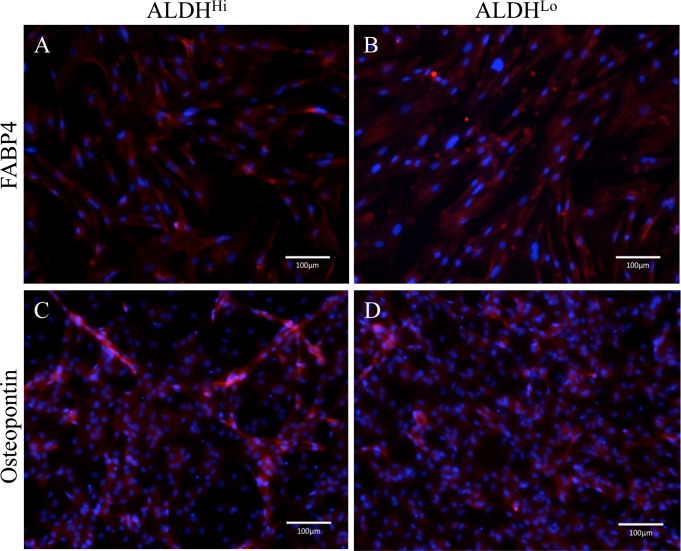
To assess adipogenic and osteogenic differentiation potential of ALDHHi and ALDHLo subpopulations, cells were cultured in adipogenic and osteogenic differentiation medium, respectively. After 15 days of culture in adipogenic differentiation medium, ADSCs appeared as large, round cells with lipid-rich cytoplasmic vacuoles; both ALDHHi and ALDHLo subpopulations were FABP4-positive, indicating adipogenic differentiation (Fig. 2A and 2B). After 15 days of culture in osteogenic differentiation medium, ADSCs were spindle-shaped with cytoplasmic granules, and both ALDHHi and ALDHLo subpopulations expressed osteopontin, indicating osteogenic differentiation (Fig. 2C and 2D).
Fig. 2.
Differentiation potentials of ALDHHi ADSCs. FABP4 (A, B) and osteopontin (C, D) expression in ADSCs (red) following adipogenic (A, B) and osteogenic (C, D) differentiation of ALDHHi and ALDHLo subpopulations, as determined by immunocytochemistry. Nuclei were stained with Hoechst 33342 (blue).
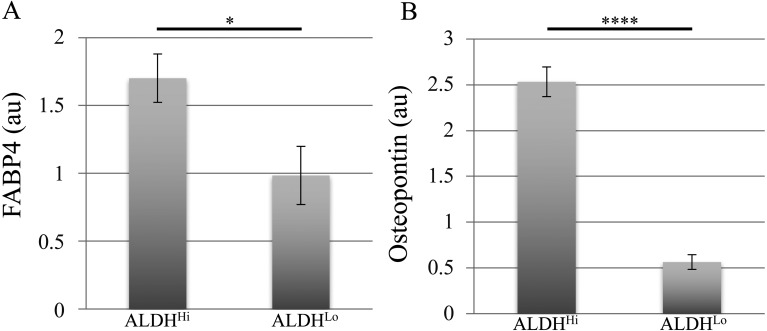
To quantify the differentiation potential, we determined immunofluorescence-positive area ratios for ALDHHi and ALDHLo subpopulations using an imaging cytometer. The ALDHHi subpopulation exhibited significantly higher adipogenic and osteogenic differentiation marker-positive area ratios and thus, a higher differentiation potential than ALDHLo cells (Fig. 3).
Fig. 3.
Quantitative analysis of differentiation marker-positive areas in differentiated ADSCs. (A, B) FABP4-positive (A) and osteopontin-positive (B) area ratios relative to respective areas of nuclear staining for ALDHHi and ALDHLo subpopulations of ADSCs after adipogenic (A) and osteogenic (B) induction. Values are expressed as mean ± standard error (n=5). *P<0.05, ****P<0.0001; au, arbitrary unit.
ADSCs can be readily obtained from adipose tissue and are therefore a convenient source of stem cells for clinical studies. In human medicine, a number of clinical trials have been carried out using ADSCs for the treatment of cardiovascular disease, spinal cord injury, cirrhosis, renal insufficiency, skin fistula with Crohn’s disease, skin fistula following surgery, and for breast reconstruction after mastectomy [9].
We report here canine ADSC subpopulations that are multipotent and have distinct differentiation potentials, as evidenced by differences in ALDH activity levels. Previous studies have reported a correlation between ALDH activity and differentiation potential; one of these showed that activation of ALDH2 enhanced adipogenesis and signaling pathways downstream of peroxisome proliferator-activated receptor (PPAR)-γ in murine ADSCs [16], suggesting that ALDH activity is correlated with adipocyte differentiation. Another report describing an ALDHHi subpopulation showed high rates of osteogenic differentiation in human BMSCs [3]. The correlation between ALDH activity and osteogenic differentiation potentials of ADSCs in humans, mice and dogs have not been previously investigated. However, the previous report and our results suggest that the ALDHHi subpopulation has a higher osteogenic differentiation potential, which may be true for other MSCs in different species.
Further elucidation of underlying molecular mechanism of higher osteogenic potential in ALDHHi subpopulation might provide useful information for future application strategy of regenerative medicine. Besides osteogenic potentials, ADSCs are reported to have ability to undergo chondrogenic, myogenic, and endothelial differentiation [2, 12, 17]; therefore, to exploit canine ADSCs for clinical applications, future studies should examine the expression of other markers and conditions that induce differentiation into other cell types.
In conclusion, canine ADSCs have an ALDHHi subpopulation that shows greater adipogenic and osteogenic differentiation potential than those with low ALDH activity.
Acknowledgments
This work was partly supported by a Japan Society for the Promotion of Science KAKENHI (grant no. 26893172).
REFERENCES
- 1.Balber A. E.2011. Concise review: aldehyde dehydrogenase bright stem and progenitor cell populations from normal tissues: characteristics, activities, and emerging uses in regenerative medicine. Stem Cells 29: 570–575. doi: 10.1002/stem.613 [DOI] [PubMed] [Google Scholar]
- 2.Brzoska M., Geiger H., Gauer S., Baer P.2005. Epithelial differentiation of human adipose tissue-derived adult stem cells. Biochem. Biophys. Res. Commun. 330: 142–150. doi: 10.1016/j.bbrc.2005.02.141 [DOI] [PubMed] [Google Scholar]
- 3.Capoccia B. J., Robson D. L., Levac K. D., Maxwell D. J., Hohm S. A., Neelamkavil M. J., Bell G. I., Xenocostas A., Link D. C., Piwnica-Worms D., Nolta J. A., Hess D. A.2009. Revascularization of ischemic limbs after transplantation of human bone marrow cells with high aldehyde dehydrogenase activity. Blood 113: 5340–5351. doi: 10.1182/blood-2008-04-154567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Estes B. T., Wu A. W., Storms R. W., Guilak F.2006. Extended passaging, but not aldehyde dehydrogenase activity, increases the chondrogenic potential of human adipose-derived adult stem cells. J. Cell. Physiol. 209: 987–995. doi: 10.1002/jcp.20808 [DOI] [PubMed] [Google Scholar]
- 5.Jones R. J., Barber J. P., Vala M. S., Collector M. I., Kaufmann S. H., Ludeman S. M., Colvin O. M., Hilton J.1995. Assessment of aldehyde dehydrogenase in viable cells. Blood 85: 2742–2746. [PubMed] [Google Scholar]
- 6.Kawamoto K., Konno M., Nagano H., Nishikawa S., Tomimaru Y., Akita H., Hama N., Wada H., Kobayashi S., Eguchi H., Tanemura M., Ito T., Doki Y., Mori M., Ishii H.2013. CD90- (Thy-1-) high selection enhances reprogramming capacity of murine adipose-derived mesenchymal stem cells. Dis. Markers 35: 573–579. doi: 10.1155/2013/392578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leyva-Leyva M., Barrera L., López-Camarillo C., Arriaga-Pizano L., Orozco-Hoyuela G., Carrillo-Casas E. M., Calderón-Pérez J., López-Díaz A., Hernandez-Aguilar F., González-Ramírez R., Kawa S., Chimal-Monroy J., Fuentes-Mera L.2013. Characterization of mesenchymal stem cell subpopulations from human amniotic membrane with dissimilar osteoblastic potential. Stem Cells Dev. 22: 1275–1287. doi: 10.1089/scd.2012.0359 [DOI] [PubMed] [Google Scholar]
- 8.Lin J., Lindsey M. L., Zhu B., Agrawal C. M., Bailey S. R.2007. Effects of surface-modified scaffolds on the growth and differentiation of mouse adipose-derived stromal cells. J. Tissue Eng. Regen. Med. 1: 211–217. doi: 10.1002/term.27 [DOI] [PubMed] [Google Scholar]
- 9.Locke M., Feisst V., Dunbar P. R.2011. Concise review: human adipose-derived stem cells: separating promise from clinical need. Stem Cells 29: 404–411. doi: 10.1002/stem.593 [DOI] [PubMed] [Google Scholar]
- 10.Marchitti S. A., Brocker C., Stagos D., Vasiliou V.2008. Non-P450 aldehyde oxidizing enzymes: the aldehyde dehydrogenase superfamily. Expert Opin. Drug Metab. Toxicol. 4: 697–720. doi: 10.1517/17425255.4.6.697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin G. R.1981. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl. Acad. Sci. U.S.A. 78: 7634–7638. doi: 10.1073/pnas.78.12.7634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mizuno H., Zuk P. A., Zhu M., Lorenz H. P., Benhaim P., Hedrick M. H.2002. Myogenic differentiation by human processed lipoaspirate cells. Plast. Reconstr. Surg. 109: 199–209, discussion 210–211. doi: 10.1097/00006534-200201000-00030 [DOI] [PubMed] [Google Scholar]
- 13.Takahashi H., Haraguchi N., Nishikawa S., Miyazaki S., Suzuki Y., Mizushima T., Nishimura J., Takemasa I., Yamamoto H., Mimori K., Ishii H., Doki Y., Mori M.2012. Biological and clinical availability of adipose-derived stem cells for pelvic dead space repair. Stem Cells Transl. Med. 1: 803–810. doi: 10.5966/sctm.2012-0085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takahashi K., Yamanaka S.2006. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126: 663–676. doi: 10.1016/j.cell.2006.07.024 [DOI] [PubMed] [Google Scholar]
- 15.Tsuneyoshi N., Dunn N. R.2013. Guards at the gate to embryonic stem cell differentiation. Cell 153: 281–283. doi: 10.1016/j.cell.2013.03.037 [DOI] [PubMed] [Google Scholar]
- 16.Yu Y. H., Liao P. R., Guo C. J., Chen C. H., Mochly-Rosen D., Chuang L. M.2016. PKC-ALDH2 pathway plays a novel role in adipocyte differentiation. PLoS One 11: e0161993. doi: 10.1371/journal.pone.0161993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zuk P. A., Zhu M., Mizuno H., Huang J., Futrell J. W., Katz A. J., Benhaim P., Lorenz H. P., Hedrick M. H.2001. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 7: 211–228. doi: 10.1089/107632701300062859 [DOI] [PubMed] [Google Scholar]