Abstract
To investigate the molecular pathways involved in successful embryo implantation in mammals, we developed a novel method for gene transduction into the murine endometrium using in vivo electroporation. Plasmid DNA with an enhanced green fluorescence protein (EGFP) gene was injected into the uterine cavity of non-pregnant female mice, and electrical pulses were subsequently applied to the uterine horn using plate electrodes. EGFP expression was found only in the uterine luminal epithelium (LE), but not in the stroma. EGFP fluorescence in the LE was limited to the site where the positive side of the electrodes was placed during electric stimulation. These results demonstrated that our novel method enabled us to transduce a gene into a desired location of the murine uterus.
Keywords: EGFP, embryo implantation, in vivo electroporation, uterus
A reciprocal interaction between the blastocysts and receptive endometrium is required for successful embryo implantation in mammals [21]. During early pregnancy, the molecular signals that are derived from maternal tissues and/or blastocysts influence the morphological characteristics of the uterine luminal epithelium (LE) to achieve the endometrial receptivity and support a successful attachment with the blastocysts [7, 21].
An important issue in embryo implantation is how embryo positioning is determined in the uterus. The distribution of embryos in the uterus, usually called “the spacing of the embryos”, precedes the attachment of the embryos to the maternal endometrium [1]. Abnormal localization of embryos in the uterine cavity causes adverse effects on the process of implantation and mostly results in abortion, emphasizing the importance of this phenomenon for ongoing pregnancy [2]. In mice, embryos are evenly distributed along the longitudinal axis of the uterine horns and then attach to the anti-metrial side of the uterus along the vertical axis [1]. Although the molecular regulations instructing the positioning of the embryos are poorly understood, several genes expressed in the murine uterus show unique expression patterns in a spatiotemporal manner during the peri-implantation period, implying the participation of those genes in navigating the embryos to the proper site [7, 21]. Therefore, the development of a method to spatiotemporally control gene expression in the endometrium is important to elucidate the regulatory mechanisms involved in the spacing of embryos in mammals.
Here, we used in vivo electroporation for the transduction of genes into the murine endometrium. Electroporation employs high-intensity electrical pulses that are assumed to transiently increase the permeability of the plasma membrane, which then facilitates the uptake of extracellular small molecules, including nucleotides, enzymes and antibodies [10]. The electroporation method was initially invented for gene delivery into cultured cells and now can be employed to introduce genes to several organs of animals in vivo [14]. In this study, we transduced plasmid DNA with the enhanced green fluorescent protein (EGFP) gene into the endometrium of nonpregnant mice by in vivo electroporation and then monitored for the expression of the fluorescent protein.
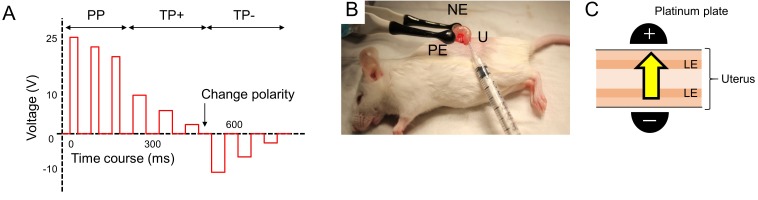
The experiment was approved by the Committee for Animal Welfare at Nagoya University (approval number: 2015060301). Electroporation was performed on the uterus of mature (over 6 weeks age) and virgin females of ICR mice (Japan SLC, Hamamatsu, Japan) using the NEPA21 electroporator (NEPA GENE, Ichikawa, Japan). Female mice were anesthetized with isoflurane. After removing the dorsal hair, the uterus was exteriorized and surgically tied up in the cervical region with sutures to prevent any leakage of the injected DNA reagents. One hundred µg of plasmid DNA with the EGFP gene (pCAGGS-EGFP, kindly provided by Mr. Yasuhiko Hayakawa, NEPA GENE) in 50 µl of phosphate buffer saline (PBS) was injected into the uterine lumen from the ovarian side using a 30G needle. Immediately after injection of DNA plasmid, the uterus was held with platinum plate electrodes (CUY650P5 or CUY654-15 × 10, NEPA GENE), and 1 or 3 sets of electric pulses were applied to the uterus (Fig. 1A). Each set of electrical pulses was composed of a poring pulse (PP) and a transfer pulse (TP), which generated a higher or lower voltage, respectively (Fig. 1B). The values of electrical currents which flowed through the tissues and those of electrical resistance were recorded, and they were within a range of 100–320 mA in electrical currents and 100−200 Ω in electrical resistance in all trials.
Fig. 1.
The gene transduction using in vivo electroporation. (A) The line graph of electric pulses by in vivo electroporation. Electric pulses consisted of three poring pulses (PP), three positive transfer pulses (TP+) and three negative transfer pulses (TP-). (B) In vivo electroporation with solely platinum plate electrode. PE; positive electrode, NE; negative electrode, U; uterus. (C) The schema of electroporation. Yellow allow indicates the predictive direction of the electric current. LE; luminal epithelium.
Two days after electroporation, the mice were perfused with 4% paraformaldehyde (PFA) in 0.1M phosphate buffer. The excised uteri were fixed in 4% PFA at 4°C for 1 day and then dehydrated by submerging them in 30% sucrose in 10 mM PBS at 4°C for 1 day. These samples were then embedded in OCT compound (Sakura Finetek, Tokyo, Japan) for cryostat sectioning, and 15 µm sections were prepared. These sections were analyzed under the LEICA DM5000B fluorescent microscope (Leica Camera AG, Solms, Germany). All fluorescent images were always captured under the same conditions completely including exposure of the light using the Leica Application Suite software (Leica Camera AG).
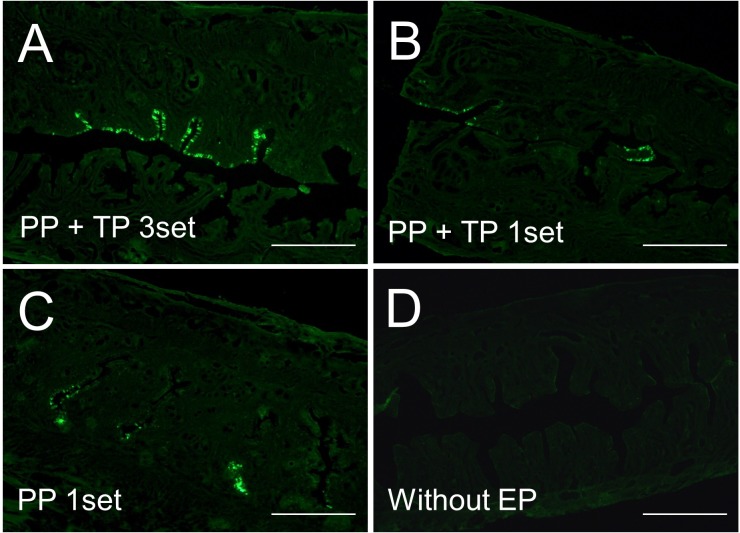
EGFP fluorescence was observed in the LE cells 2 days after the in vivo electroporation (Fig. 2). The most effective results were obtained after 3 sets of electrical pulses were performed on the uteri (n=10, Fig. 2A). The specimens that were electroporated with only 1 set of PP or 1 set of the electrical pulse (PP and TP) also exhibited EGFP fluorescence, but the frequency of it was lower than that provided with 3 sets of the pulses (n=3 each, Fig. 2B and 2C). The uterus 2 days after injection of DNA without electroporation did not show any EGFP fluorescence (n=4, Fig. 2D).
Fig. 2.
EGFP expression in the uterine luminal epithelium (LE). The highly effective transfection of EGFP gene in the LE cells was observed when 3 sets of electric pulses were performed (A), compared with that when only 1 set of pulses (B), or only 1 set of poring pulse (C) was performed. The uterus injected with DNA plasmid without electroporation did not show any EGFP fluorescence (D). PP, poring pulse; TP, transfer pulse; EP, electroporation. Scale bar: 400 µm.
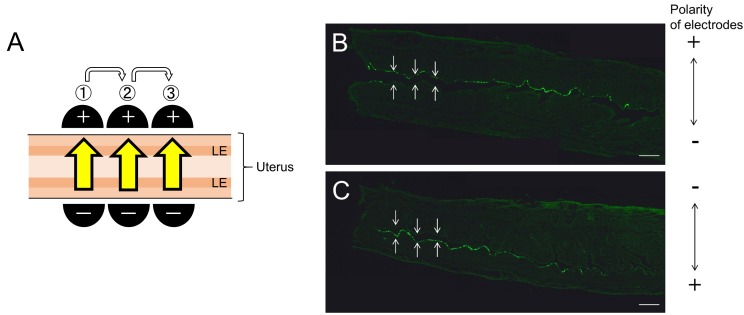
Furthermore, EGFP gene transfer was more efficient at the LE where the positive side of the electrode was faced at the time of electroporation (Fig. 3). When we applied electrical stimuli at three adjacent points along the longitudinal axis of the uterine horn (Fig. 3A), approximately a quarter of the whole length of the uterine horn exhibited EGFP fluorescence in the LE (Fig. 3B and 3C).
Fig. 3.
One side of the luminal epithelium (LE) shows EGFP fluorescence. (A) The schema of electroporation where the electrical stimuli were applied at three adjacent points along the longitudinal axis of the uterine horns. Yellow allow indicates the predictive direction of the electric current. (B, C) Both samples showed EGFP fluorescence at one side of the LE cells that were placed with positive paddle of electrode. White arrow shows the boundary between the LE and the stroma. Scale bar: 400 µm.
To confirm whether applying the electrical stimuli to the uterus adversely affected the fertility of females or not, virgin mice that received electroporation in the condition described above were housed with mature ICR males. One month later, all females delivered viable offspring, indicating that the electrical stimuli to the uterus in our method did not disturb the reproductive performance of the female mice (n=4, data not shown).
Electrical pulses create transient pores in the cell membrane that allow for the entry of exogenous molecules into the cytoplasm [10]. The degree of cell permeabilization can be controlled by the pulse number and/or the pulse duration [9]. A more effective transfection to the endometrium was observed when 3 sets of electrical pulses were applied to the uterus when compared to only 1 set of the pulse or when only a poring pulse was performed (Fig. 2). The intensity of the voltage also contributes to the outcomes of the transfections. Adequate values of voltage for a successful transfection differ depending on the cell type, but generally, electroporation with a high voltage pulse seems to produce better results than with low voltage [9]. Excess voltage often causes serious injury or death for the cell (e.g. irreversible electroporation) [16]. In our trials, the maximum value for the voltage was 25 V, and no damages were observed in any of the manipulated uteri after electroporation. When 75 V of PPs were applied to the uterus, the uterus was severely damaged and showed unusual inflammation and bleeding (data not shown). Consequently, the conditions that we determined here were appropriate for gene transfection to the murine uterus.
Previous studies have shown that the estrous cycle can affect the efficiency of transgene expression in the murine endometrium when liposomes are used [18]. Furthermore, an effective transfection to the murine uterus was observed in metestrus, whereas the uteri in other stages of the cycle showed only slight expression of the transgene [18]. Gene transfection to the rabbit uterus also showed that the transduction pattern in the endometrium distinctly varied according to the reproductive cycle [13]. Although we did not assess the effect of the estrus cycle in our study, we observed clear EGFP expressions in the LE of all specimens (n=10) that were randomly selected among nonpregnant mice without assessing their estrus cycles.
In addition, the promoter activity in a given vector is also a factor that can affect the efficiency of the transgene expression. The pCAGGS construct, which was used in the present study, has a CAG promoter consisting of the cytomegalovirus (CMV) enhancer and chicken β-actin promoter, and strong expression with this promoter has been reported in mammalian cells [15]. Previous reports have shown that it is preferred to use the CMV promoter for gene expression when transducing DNA vectors into the uterus using liposomes [13, 18]. We have also tried to use a construct with the CMV promoter, but the results were not reproducible (data not shown). Based on our data, we showed that electroporation with the pCAGGS vector is a powerful method for gene transfection into the endometrium.
An advantage of in vivo electroporation for gene transfection is that we can easily manipulate the region of gene expression. When the platinum plate electrodes were solely used for in vivo electroporation, we observed EGFP fluorescence on only one side of the LE, which was the side that was attached to the positive electrode (Fig. 3). The transgene expression was observed in the region, where the positive paddle of the electrodes was placed in the fetal brain [19]. It was assumed that negatively charged-DNA electrostatically flowed toward the positive electrode in the present study. Using the present method, we could designate the tissue region for transfection by simply altering the positioning of the electrodes. In pregnant mice, spatiotemporal patterns of gene expressions in the uterus can be observed. For example, Hbegf is expressed specifically at the implantation sites along the longitudinal axis of the uteri [4]. Ep3, Fgf10 and Noggin are expressed in the endometrium at the mesometrial side during the preimplantation period [17, 22]. The importance of these expression patterns for implantation is still unknown, but can be confirmed in future studies by controlling target gene expression at a specific site via in vivo electroporation.
It is noteworthy that genes could be transduced specifically into the LE cells, since the LE is the site where the uterus makes first contact with the blastocysts during embryo implantation [5]. The LE cells dramatically undergo morphological alterations consistent with changes in expressions of several molecules to receive embryos during the peri-implantation period [5]. Previous studies have shown that the apical-basal polarity of the LE cells is lost in the receptive phase, which would allow for the proper organization of cell surface adhesion molecules to interact with the embryo during implantation [20]. Using our in vivo electroporation method, we would be able to confirm the function of adhesion molecules that are assumed to be important for interactions with the embryo.
We propose that this method could be applied for studies involving endometrial cancer in women, which primarily originates from the uterine epithelial cells [11]. The pathology and the etiology of endometrial cancer have been studied using transgenic rodents that have uterus-specific gene mutations, such as a loss of function mutation in Pten [3, 8]. Although these mice actually display a progressive cancer phenotype in the endometrium, the oncogenic gene mutation is introduced in the whole of uterine horn and the mutation already exists at the time of birth in these models [3, 8]. In human endometrial cancer, however, the gene mutation that sporadically occurs in the epithelial cells causes tumor progression, and these are normally observed in adult patients [6, 8]. Therefore, the conventional cancer models do not mimic the conditions of the endometrial cancer in humans. In combination with new genomic editing tools, such as CRISPR/-Cas9 system [12], it is possible to establish new cancer models in rodents by inducing the LE specific gene mutation for oncogenesis only at a given site using in vivo electroporation. Taken together, the present method is an easy and powerful tool to investigate not only the mechanism of embryo implantation, but also pathological aspects of the uteri, such as endometrial cancer.
Acknowledgments
The authors thank Mr. Yasuhiko Hayakawa for kindly providing pCAGGS-EGFP vector and giving accurate advices about in vivo electroporation. This study was supported by Grants-in-Aid for Scientific Research (No. 23380172) from Japan Society for the Promotion of Science (JSPS).
REFERENCES
- 1.Chen Q., Zhang Y., Elad D., Jaffa A. J., Cao Y., Ye X., Duan E.2013. Navigating the site for embryo implantation: biomechanical and molecular regulation of intrauterine embryo distribution. Mol. Aspects Med. 34: 1024–1042. doi: 10.1016/j.mam.2012.07.017 [DOI] [PubMed] [Google Scholar]
- 2.Chen Q., Zhang Y., Peng H., Lei L., Kuang H., Zhang L., Ning L., Cao Y., Duan E.2011. Transient beta2-adrenoceptor activation confers pregnancy loss by disrupting embryo spacing at implantation. J. Biol. Chem. 286: 4349–4356. doi: 10.1074/jbc.M110.197202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daikoku T., Hirota Y., Tranguch S., Joshi A. R., DeMayo F. J., Lydon J. P., Ellenson L. H., Dey S. K.2008. Conditional loss of uterine Pten unfailingly and rapidly induces endometrial cancer in mice. Cancer Res. 68: 5619–5627. doi: 10.1158/0008-5472.CAN-08-1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Das S. K., Wang X. N., Paria B. C., Damm D., Abraham J. A., Klagsbrun M., Andrews G. K., Dey S. K.1994. Heparin-binding EGF-like growth factor gene is induced in the mouse uterus temporally by the blastocyst solely at the site of its apposition: a possible ligand for interaction with blastocyst EGF-receptor in implantation. Development 120: 1071–1083. [DOI] [PubMed] [Google Scholar]
- 5.Davidson L. M., Coward K.2016. Molecular mechanisms of membrane interaction at implantation. Birth Defects Res. C Embryo Today 108: 19–32. doi: 10.1002/bdrc.21122 [DOI] [PubMed] [Google Scholar]
- 6.Dedes K. J., Wetterskog D., Ashworth A., Kaye S. B., Reis-Filho J. S.2011. Emerging therapeutic targets in endometrial cancer. Nat. Rev. Clin. Oncol. 8: 261–271. doi: 10.1038/nrclinonc.2010.216 [DOI] [PubMed] [Google Scholar]
- 7.Dey S. K., Lim H., Das S. K., Reese J., Paria B. C., Daikoku T., Wang H.2004. Molecular cues to implantation. Endocr. Rev. 25: 341–373. doi: 10.1210/er.2003-0020 [DOI] [PubMed] [Google Scholar]
- 8.Friel A. M., Growdon W. B., McCann C. K., Olawaiye A. B., Munro E. G., Schorge J. O., Castrillon D. H., Broaddus R. R., Rueda B. R.2010. Mouse models of uterine corpus tumors: clinical significance and utility. Front. Biosci. (Elite Ed.) 2: 882–905. [DOI] [PubMed] [Google Scholar]
- 9.Gehl J.2003. Electroporation: theory and methods, perspectives for drug delivery, gene therapy and research. Acta Physiol. Scand. 177: 437–447. doi: 10.1046/j.1365-201X.2003.01093.x [DOI] [PubMed] [Google Scholar]
- 10.Heller L. C., Heller R.2006. In vivo electroporation for gene therapy. Hum. Gene Ther. 17: 890–897. doi: 10.1089/hum.2006.17.890 [DOI] [PubMed] [Google Scholar]
- 11.Kandoth C., Schultz N., Cherniack A. D., Akbani R., Liu Y., Shen H., Robertson A. G., Pashtan I., Shen R., Benz C. C., Yau C., Laird P. W., Ding L., Zhang W., Mills G. B., Kucherlapati R., Mardis E. R., Levine D. A., Cancer Genome Atlas Research Network. 2013. Integrated genomic characterization of endometrial carcinoma. Nature 497: 67–73. doi: 10.1038/nature12113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lander E. S.2016. The Heroes of CRISPR. Cell 164: 18–28. doi: 10.1016/j.cell.2015.12.041 [DOI] [PubMed] [Google Scholar]
- 13.Laurema A., Lumme S., Heinonen S. E., Heinonen S., Ylä-Herttuala S.2007. Transduction patterns and efficiencies in rabbit uterine tissues after intraluminal uterine adenovirus administration vary with the reproductive cycle. Acta Obstet. Gynecol. Scand. 86: 1035–1040. doi: 10.1080/00016340701415640 [DOI] [PubMed] [Google Scholar]
- 14.Neumann E., Schaefer-Ridder M., Wang Y., Hofschneider P. H.1982. Gene transfer into mouse lyoma cells by electroporation in high electric fields. EMBO J. 1: 841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niwa H., Yamamura K., Miyazaki J.1991. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108: 193–199. doi: 10.1016/0378-1119(91)90434-D [DOI] [PubMed] [Google Scholar]
- 16.Olweny E. O., Cadeddu J. A.2012. Novel methods for renal tissue ablation. Curr. Opin. Urol. 22: 379–384. doi: 10.1097/MOU.0b013e328355ecf5 [DOI] [PubMed] [Google Scholar]
- 17.Paria B. C., Ma W., Tan J., Raja S., Das S. K., Dey S. K., Hogan B. L.2001. Cellular and molecular responses of the uterus to embryo implantation can be elicited by locally applied growth factors. Proc. Natl. Acad. Sci. U.S.A. 98: 1047–1052. doi: 10.1073/pnas.98.3.1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Relloso M., Esponda P.2000. In-vivo transfection of the female reproductive tract epithelium. Mol. Hum. Reprod. 6: 1099–1105. doi: 10.1093/molehr/6.12.1099 [DOI] [PubMed] [Google Scholar]
- 19.Taniguchi Y., Young-Pearse T., Sawa A., Kamiya A.2012. In utero electroporation as a tool for genetic manipulation in vivo to study psychiatric disorders. Neuroscientist 18: 169–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thie M., Fuchs P., Denker H. W.1996. Epithelial cell polarity and embryo implantation in mammals. Int. J. Dev. Biol. 40: 389–393. [PubMed] [Google Scholar]
- 21.Wang H., Dey S. K.2006. Roadmap to embryo implantation: clues from mouse models. Nat. Rev. Genet. 7: 185–199. doi: 10.1038/nrg1808 [DOI] [PubMed] [Google Scholar]
- 22.Yang Z. M., Das S. K., Wang J., Sugimoto Y., Ichikawa A., Dey S. K.1997. Potential sites of prostaglandin actions in the periimplantation mouse uterus: differential expression and regulation of prostaglandin receptor genes. Biol. Reprod. 56: 368–379. doi: 10.1095/biolreprod56.2.368 [DOI] [PubMed] [Google Scholar]