Abstract
Hydroxyethyl starches (HES) are commonly used synthetic colloidal solution in veterinary medicine. Despite of possible adverse effect to kidney injury in human, there is no report about nephrotoxic effects of HES in dogs. HES was administered to a Golden retriever (4-year-old, intact male) with ascites in order to increase plasma osmolality. Initially, the dog was mild azotemic, however, kidney function was rapidly deteriorated after several days of HES administration. Finally, histopathological examination revealed remarkable osmotic nephrosis. In the case reported herein, acute kidney injury was remarkably developed after HES administration. Clinical and histopathologic findings of acute kidney injury support nephrotoxic effects of HES to a dog.
Keywords: acute kidney injury, dog, hydroxyethyl starch, osmotic nephrosis
Hydroxyethyl starches are modified polysaccharides with long chain shaped molecular structures derived from amylopectin [2]. Hydroxyethyl starch molecules are hydrolyzed mainly by serum alpha-amylase [21]. The degradation rate of hydroxyethyl starch molecules by serum amylase is determined by their molecular weight, C2/C6 ratio, concentration and degree of molar substitution [17]. Degraded molecules in the blood are excreted through the kidneys, phagocytosed by reticuloendothelial cells or absorbed by various cells as a form of pinocytosis [22]. Cellular lysosomes lack amylase, therefore hydroxyethyl starch molecules can remain in cells without degradation, and this tissue storage may be potentially harmful to the host [23].
Hydroxyethyl starches have been recognized as relatively safe synthetic colloidal solutions. However, based on many on-going reports of adverse effects and conflicting results from randomized-controlled trials, the use of hydroxyethyl starches in human medicine is still controversial [5]. As a result, the use of hydroxyethyl starch solutions is not recommended for specific circumstances, such as critically ill patients, sepsis, burn-injury, patients with impaired renal function or coagulopathy. The Food and Drug Administration has classified hydroxyethyl starches as a boxed warning drug since 2013 [11], and the Surviving Sepsis Campaign banned its use in 2012 based on reports that there were no significant differences in therapeutic effect, but more adverse effects, compared to the use of crystalloid in septic patients [1, 9].
While human medicine has limited the use of hydroxyethyl starches, there are ongoing debates over the use of HES in veterinary medicine, because of a lack of species-specific data. In 201 cases where 6% tetrastarch was administered, the creatinine concentration did not increase significantly [24], while 180 cases receiving 10% pentastarch were associated with an increased risk of kidney injury and mortality rate [15]. These two research papers have conflicting results, and a consensus about the use of HES in veterinary medicine has not been established.
The known adverse effects of HES in human medicine include acute kidney injury, coagulopathy, immune reaction, reticuloendothelial dysfunction and hepatopathy [14]. Moreover, the incidence of renal adverse effects by HES is increasing as reported by several large randomized controlled trials. In the Volume Substitution and Insulin Therapy in Severe Sepsis and the Crystalloids Morbidity Associated with Severe Sepsis trials, severe septic patients treated with HES had increased rates of acute kidney injury and renal replacement therapy [4, 13]. The Crystalloid versus Hydroxyethyl Starch trial found that use of HES in critically ill patients significantly increased the need for renal replacement therapy [12].
In veterinary medicine, the adverse effects of HES have rarely been investigated and only coagulopathy has been reported [6, 8, 10]. Therefore, there is insufficient evidence for the renal adverse effects of HES in veterinary medicine [1].
The following case report describes acute kidney injury after the administration of hydroxyethyl starch.
A 4-year-old intact male Golden retriever, weighing 32 kg, was admitted to a local animal hospital, because of abdominal distention, melena, lethargy and anorexia. The dog was raised indoors, and there was no possible toxicant exposure determined from its history.
On day 1, azotemia and hypoalbuminemia were found by blood chemistry panel at the local animal hospital. To increase the plasma osmolality, 6% hydroxyethyl starch 130/0.4 solution (Voluven®, Fresenius-Kabi, Bad Homburg, Germany) (10 ml/kg, IV, bolus) was administered by a local veterinarian. On day 8, the creatinine level was increased about three-fold, and proteinuria was profound. This rapidly deteriorating azotemia and proteinuria persisted during hospitalization at the local animal hospital (Table 1).
Table 1. Serial result of serum albumin, BUN, serum creatinine concentration, urine specific gravity, urine protein (dipstick) and UPC.
| Analyte | Units | Reference interval | Day 1 | Day 2 | Day 3 | Day 8 | Day 10 | Day 15 |
|---|---|---|---|---|---|---|---|---|
| Albumin | g/dl | 2.3–4.0 | 1.5 | 1.5 | - | - | 1.4 | 1.9 |
| BUN | mg/dl | 7–27 | 43 | 38 | 34 | 58 | 69 | 183 |
| Creatinine | mg/dl | 0.5–1.8 | 3.1 | 3.1 | 3.4 | 9.5 | 10.7 | 17.4 |
| USG | - | - | - | 1.015 | - | 1.020 | 1.025 | 1.009 |
| Urine protein | mg/dl | - | - | n.d. | - | 30 | 15 | 100 |
| UPC | - | <0.5 | - | - | - | - | - | 1.85 |
BUN=blood urea nitrogen; USG=urine specific gravity; UPC=urine protein creatinine ratio; n.d.=not detected.
On day 15, the dog was referred to the Veterinary Medical Teaching Hospital for severe azotemia, ascites and gastrointestinal bleeding. The dog was normothermic (38.0°C), with a normal heart (120 bpm) and a normal respiratory rate (30/min), and hypertensive (Indirect systolic blood pressure 175 mmHg measured by Doppler method), possibly because of aggressive fluid administration from the local animal hospital before referral. Ecchymosis and a distended abdomen were obvious upon physical examination.
Microcytic non-regenerative anemia, neutrophilia and thrombocytopenia were found by complete blood count.
Hypoalbuminemia, hypoproteinemia, hyperbilirubinemia, increased alkaline phosphatase and gamma glutamyl transferase activity were found by blood chemistry panel. Increased fasting bile acid and mild increased activated partial thromboplastin were found by an additional liver function test. Deteriorated azotemia and hyperphosphatemia were observed.
Blood gas and electrolyte analysis indicated metabolic acidosis, hyponatremia, hyperkalemia and hypocalcemia (Table 2). Hyposthenuria and proteinuria were also observed (Table 1). Throughout the intensive care unit stay, the dog showed anuria without any response to hydration.
Table 2. Summary of clinicopathological findings in a dog after referral.
| Analyte | Units | Reference interval | Day 15 (after referral) |
|---|---|---|---|
| HCT | % | 37.3–61.7 | 16 |
| MCV | µm3 | 61.6–73.5 | 49.8 |
| MCHC | g/dl | 32.0–37.9 | 38.8 |
| Reticulocyte count | ×103/µl | - | 43 |
| Platelet count | ×103/µl | 148–484 | 71 |
| Neutrophils | ×103/µl | 2.95–11.64 | 13.71 |
| Total protein | g/dl | 5.2–8.2 | 4.9 |
| Globulin | g/dl | 2.5–4.5 | 3.0 |
| Cholesterol | mg/dl | 110–320 | 138 |
| Bilirubin | mg/dl | 0.0–0.9 | 1.7 |
| ALP | IU/l | 23–212 | 374 |
| GGT | IU/l | 0–7 | 33 |
| Bile acids | µg/ml | 0–4.9 | >12.25 |
| aPTT | sec | 72–102 | 120 |
| Phosphorus | mg/dl | 2.5–6.8 | 12.7 |
| pH | - | 7.335–7.446 | 7.328 |
| HCO3− | mEq/l | 18–24 | 11.5 |
| Sodium | mEq/l | 140–150 | 129.2 |
| Potassium | mEq/l | 3.5–5.8 | 6.54 |
| Ionized calcium | mmol/l | 1.12–1.42 | 1.12 |
HCT=hematocrit, MCV=mean corpuscular volume, MCHC=mean corpuscular hemoglobin concentration, ALP=alkaline phosphatase, GGT=gamma-glutamyltransferase, aPTT=activated partial thromboplastin time.
Too much ascites had accumulated to obtain an accurate view by ultrasound. However, the echogenicity of the cortex of both kidneys was increased. The liver could not be accurately assessed by ultrasound because of a disturbance by the severe ascites. The ascites was removed under ultrasonographic guide, because of respiratory disturbance and its characteristic was transudate.
Chronic liver failure was suspected, because of decreased functional enzymes, ascites accumulation and coagulopathy. Globulin and cholesterol levels were within reference interval, and no marked proteinuria on day 2 could exclude protein-losing enteropathy and protein-losing nephropathy from differential diagnosis of hypoalbuminemia, respectively. Acute anuric renal failure and the decreased liver function were the probable cause of the present illness. Because of the moribund state of the dog, he was euthanized, and necropsy was performed.
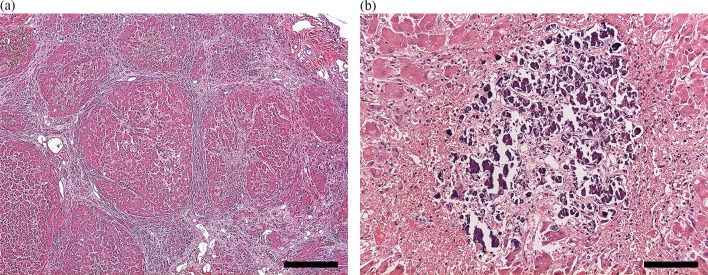
After necropsy, gross examination showed multifocal nodules bulging on the surface of the liver, mild-to-moderate petechial-to-ecchymotic hemorrhages in the heart, gastrointestinal bleeding and degeneration of the kidney cortex. Histopathological examination revealed multiple hepatic nodules surrounded by thick fibrous septa, moderate multiple calcium depositions in the necrotic areas of the hepatic nodules (Fig. 1), severe multiple linear renal tubular necrosis and moderate glomerular injury resulting in the mild transudation of albumin. Of note, osmotic nephrosis lesions were prominent in the renal tubules (Fig. 2).
Fig. 1.
Histopathological section of the liver, light microscopy. Hematoxylin and eosin stained section with (a) multiple hepatic nodules surrounded by thick fibrous septa; magnification 40×, bar=500 µm and (b) moderate multiple calcium depositions in the necrotic areas; magnification 200×, bar=100 µm.
Fig. 2.
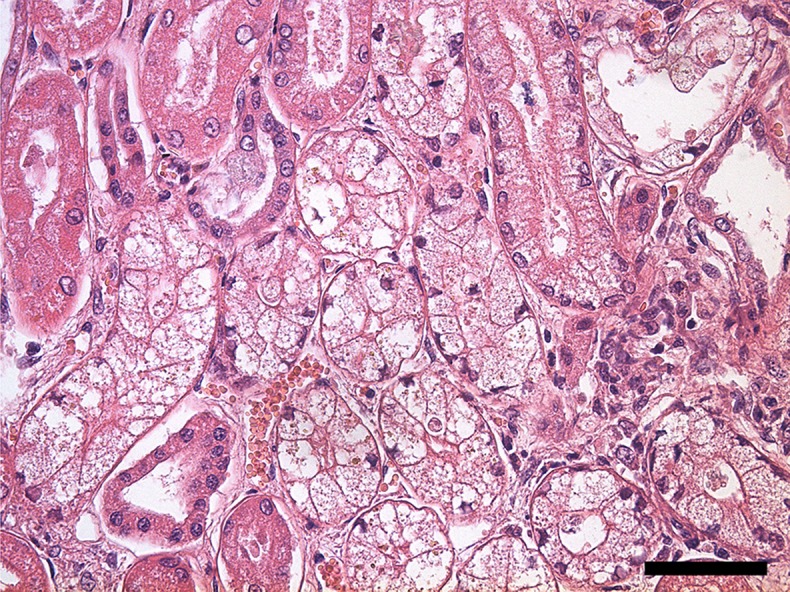
Histopathological section of the kidney, light microscopy. Hematoxylin and eosin stained section with osmotic nephrosis lesion in the tubules; magnification 400×, bar=50 µm.
In this case study, the rapid exacerbation of renal function after the administration of HES from a local animal hospital was remarkable. Creatinine levels were rapidly increased. Additional lab work and anuria indicated the dog had acute kidney injury, and histopathological examination indicated that hydroxyethyl starch had induced the AKI. Liver cirrhosis was also confirmed based on necropsy and histopathological findings.
In veterinary medicine, the use of HES is controversial, because there is no evidence that the same adverse effects in human patients might appear in animals [1]. One study reported that the prevalence of adverse outcome in animal after HES administration is considerably lower than the prevalence in human [24]. The reasons for this are that the serum amylase activity in dogs is 13 times higher than in humans [18] and that animals tend to stay in hospital for a shorter duration compared with humans, so that the total administered volume is lower.
There are a few hypotheses about the mechanism of HES-induced acute kidney injury, such as obstruction of the tubular lumen, hyperviscosity-induced GFR decrease, clearance by the renal interstitial reticuloendothelial system and osmotic nephrosis have been proposed [16, 20]. A well-known hypothesis is that osmotic nephrosis is induced by absorbed molecules through renal tubular epithelial cells. Those molecules accumulate in the tubular epithelial cells, and cause swelling and vacuolization. Dextran, mannitol, immunoglobulins and iodinated contrast agents have also been shown to cause similar lesions [3, 7]. These osmotic nephrosis lesions are reversible, but can be long lasting [19].
In this case study, histopathological examination clearly provided pathological evidence of HES-induced kidney injury. Based on the histopathological findings in the kidney, the renal function of the dog was markedly influenced by osmotic nephrosis lesions. Petechia and ecchymosis were found in the skin by physical examination, although platelet counts were not markedly decreased. This suggests that platelet dysfunction had occurred. The adverse effects of HES, liver failure or uremic toxin produced by AKI are possible causes of platelet dysfunction, but this is yet to be determined.
Additionally, liver cirrhosis might influence the incidence of acute kidney injury. According to Wiedermann et al., the cellular storage of HES might cause decreased liver function, and further research on tissue accumulation is needed [23]. We believe the dog described in this report had accelerated renal function impairment caused by an increased excretion load to the kidney caused by the insufficient removal of HES molecules by liver phagocytes.
This case report is a warning against the use of synthetic colloids in animals with decreased kidney function. Studies of HES-induced adverse effects in veterinary medicine, especially large-scale multicenter studies, should be continued.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2014R1A1A1004588).
REFERENCES
- 1.Adamik K. N., Yozova I. D., Regenscheit N.2015. Controversies in the use of hydroxyethyl starch solutions in small animal emergency and critical care. J. Vet. Emerg. Crit. Care (San Antonio) 25: 20–47. doi: 10.1111/vec.12283 [DOI] [PubMed] [Google Scholar]
- 2.Boldt J.2009. Modern rapidly degradable hydroxyethyl starches: current concepts. Anesth. Analg. 108: 1574–1582. doi: 10.1213/ane.0b013e31819e9e6c [DOI] [PubMed] [Google Scholar]
- 3.Boldt J., Priebe H. J.2003. Intravascular volume replacement therapy with synthetic colloids: is there an influence on renal function? Anesth. Analg. 96: 376–382. [DOI] [PubMed] [Google Scholar]
- 4.Brunkhorst F. M., Engel C., Bloos F., Meier-Hellmann A., Ragaller M., Weiler N., Moerer O., Gruendling M., Oppert M., Grond S., Olthoff D., Jaschinski U., John S., Rossaint R., Welte T., Schaefer M., Kern P., Kuhnt E., Kiehntopf M., Hartog C., Natanson C., Loeffler M., Reinhart K., German Competence Network Sepsis (SepNet). 2008. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N. Engl. J. Med. 358: 125–139. doi: 10.1056/NEJMoa070716 [DOI] [PubMed] [Google Scholar]
- 5.Cazzolli D., Prittie J.2015. The crystalloid-colloid debate: Consequences of resuscitation fluid selection in veterinary critical care. J. Vet. Emerg. Crit. Care (San Antonio) 25: 6–19. doi: 10.1111/vec.12281 [DOI] [PubMed] [Google Scholar]
- 6.Chohan A. S., Greene S. A., Grubb T. L., Keegan R. D., Wills T. B., Martinez S. A.2011. Effects of 6% hetastarch (600/0.75) or lactated Ringer’s solution on hemostatic variables and clinical bleeding in healthy dogs anesthetized for orthopedic surgery. Vet. Anaesth. Analg. 38: 94–105. doi: 10.1111/j.1467-2995.2010.00589.x [DOI] [PubMed] [Google Scholar]
- 7.Cittanova M. L., Leblanc I., Legendre C., Mouquet C., Riou B., Coriat P.1996. Effect of hydroxyethylstarch in brain-dead kidney donors on renal function in kidney-transplant recipients. Lancet 348: 1620–1622. doi: 10.1016/S0140-6736(96)07588-5 [DOI] [PubMed] [Google Scholar]
- 8.Classen J., Adamik K. N., Weber K., Rubenbauer S., Hartmann K.2012. In vitro effect of hydroxyethyl starch 130/0.42 on canine platelet function. Am. J. Vet. Res. 73: 1908–1912. doi: 10.2460/ajvr.73.12.1908 [DOI] [PubMed] [Google Scholar]
- 9.Dellinger R. P., Levy M. M., Rhodes A., Annane D., Gerlach H., Opal S. M., Sevransky J. E., Sprung C. L., Douglas I. S., Jaeschke R., Osborn T. M., Nunnally M. E., Townsend S. R., Reinhart K., Kleinpell R. M., Angus D. C., Deutschman C. S., Machado F. R., Rubenfeld G. D., Webb S., Beale R. J., Vincent J. L., Moreno R., Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup. 2013. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 39: 165–228. doi: 10.1007/s00134-012-2769-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Falco S., Bruno B., Maurella C., Bellino C., D’Angelo A., Gianella P., Tarducci A., Zanatta R., Borrelli A.2012. In vitro evaluation of canine hemostasis following dilution with hydroxyethyl starch (130/0.4) via thromboelastometry. J. Vet. Emerg. Crit. Care (San Antonio) 22: 640–645. doi: 10.1111/j.1476-4431.2012.00816.x [DOI] [PubMed] [Google Scholar]
- 11.FDA. Department of Health and Human Services U.S. Food and Drug Administration. 2013. Press release: FDA Safety Communication: Boxed warning on increased mortality and severe renal injury and risk of bleeding. https://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm358349.htm [accessed April 7, 2017].
- 12.Gattas D. J., Dan A., Myburgh J., Billot L., Lo S., Finfer S. and CHEST Management Committee. 2013. Fluid resuscitation with 6% hydroxyethyl starch (130/0.4 and 130/0.42) in acutely ill patients: systematic review of effects on mortality and treatment with renal replacement therapy. Intensive Care Med. 39: 558–568. doi: 10.1007/s00134-013-2840-0 [DOI] [PubMed] [Google Scholar]
- 13.Guidet B., Martinet O., Boulain T., Philippart F., Poussel J. F., Maizel J., Forceville X., Feissel M., Hasselmann M., Heininger A., Van Aken H.2012. Assessment of hemodynamic efficacy and safety of 6% hydroxyethylstarch 130/0.4 vs. 0.9% NaCl fluid replacement in patients with severe sepsis: the CRYSTMAS study. Crit. Care 16: R94. doi: 10.1186/cc11358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hartog C. S., Bauer M., Reinhart K.2011. The efficacy and safety of colloid resuscitation in the critically ill. Anesth. Analg. 112: 156–164. doi: 10.1213/ANE.0b013e3181eaff91 [DOI] [PubMed] [Google Scholar]
- 15.Hayes G., Benedicenti L., Mathews K.2016. Retrospective cohort study on the incidence of acute kidney injury and death following hydroxyethyl starch (HES 10% 250/0.5/5:1) administration in dogs (2007−2010). J. Vet. Emerg. Crit. Care (San Antonio) 26: 35–40. doi: 10.1111/vec.12412 [DOI] [PubMed] [Google Scholar]
- 16.Hüter L., Simon T. P., Weinmann L., Schuerholz T., Reinhart K., Wolf G., Amann K. U., Marx G.2009. Hydroxyethylstarch impairs renal function and induces interstitial proliferation, macrophage infiltration and tubular damage in an isolated renal perfusion model. Crit. Care 13: R23. doi: 10.1186/cc7726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jungheinrich C., Neff T. A.2005. Pharmacokinetics of hydroxyethyl starch. Clin. Pharmacokinet. 44: 681–699. doi: 10.2165/00003088-200544070-00002 [DOI] [PubMed] [Google Scholar]
- 18.Mocharla H., Mocharla R., Hodes M. E.1990. Alpha-amylase gene transcription in tissues of normal dog. Nucleic Acids Res. 18: 1031–1036. doi: 10.1093/nar/18.4.1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pillebout E., Nochy D., Hill G., Conti F., Antoine C., Calmus Y., Glotz D.2005. Renal histopathological lesions after orthotopic liver transplantation (OLT). Am. J. Transplant. 5: 1120–1129. doi: 10.1111/j.1600-6143.2005.00852.x [DOI] [PubMed] [Google Scholar]
- 20.Schortgen F., Brochard L.2009. Colloid-induced kidney injury: experimental evidence may help to understand mechanisms. Crit. Care 13: 130. doi: 10.1186/cc7745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Treib J., Haass A., Pindur G., Grauer M. T., Wenzel E., Schimrigk K.1996. All medium starches are not the same: influence of the degree of hydroxyethyl substitution of hydroxyethyl starch on plasma volume, hemorrheologic conditions, and coagulation. Transfusion 36: 450–455. doi: 10.1046/j.1537-2995.1996.36596282590.x [DOI] [PubMed] [Google Scholar]
- 22.Westphal M., James M. F., Kozek-Langenecker S., Stocker R., Guidet B., Van Aken H.2009. Hydroxyethyl starches: different products--different effects. Anesthesiology 111: 187–202. doi: 10.1097/ALN.0b013e3181a7ec82 [DOI] [PubMed] [Google Scholar]
- 23.Wiedermann C. J., Joannidis M.2014. Accumulation of hydroxyethyl starch in human and animal tissues: a systematic review. Intensive Care Med. 40: 160–170. doi: 10.1007/s00134-013-3156-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yozova I. D., Howard J., Adamik K. N.2016. Retrospective evaluation of the effects of administration of tetrastarch (hydroxyethyl starch 130/0.4) on plasma creatinine concentration in dogs (2010−2013): 201 dogs. J. Vet. Emerg. Crit. Care (San Antonio) 26: 568–577. doi: 10.1111/vec.12483 [DOI] [PubMed] [Google Scholar]