Abstract
10-Hydroxy-2-decenoic acid (10H2DA) is a fatty acid found in royal jelly (RJ). In healthy mice, it activates 5’-AMP-activated protein kinase (AMPK) and increases glucose transporter 4 (GLUT4) translocation. Therefore, we examined whether 10H2DA has a potential therapeutic effect against type 2 diabetes in obese/diabetic KK-Ay mice. 10H2DA (3 mg/kg body weight) was administered to female KK-Ay mice for 4 weeks by oral gavage. Phenotypes for body weight, plasma glucose by oral glucose tolerance test and insulin levels were measured. mRNA and protein levels were determined using qRT-PCR and Western blot analyses, respectively. Long-term administration of 10H2DA significantly improved hyperglycemia and insulin resistance in KK-Ay mice, but did not prevent obesity. 10H2DA increased the expression of phosphorylated AMPK (pAMPK) protein in skeletal muscles; however, this expression did not correlate with increased GLUT4 translocation. Furthermore, 10H2DA neither enhanced the expression of adiponectin receptor mRNA nor activated the insulin signaling cascade, such as GSK-3β phosphorylation, in the liver. We found that 10H2DA-treated mice had a significant increase in the expression of peroxisome proliferator-activated receptor gamma coactivator 1 alpha (Pgc-1α) mRNA in skeletal muscles compared with non-treated group (P=0.0024). These findings suggest that 10H2DA is involved in the improvement of type 2 diabetes, at least in part via activation of Pgc-1α expression, but does not prevent obesity.
Keywords: 10-Hydroxy-2-decenoic acid, hyperglycemia, insulin resistance, KK-Ay mouse, type 2 diabetes
Obesity is a major risk factor for developing insulin resistance and type 2 diabetes [12, 31, 33]. As insulin resistance precedes the onset of hyperglycemia, it is considered to be the primary cause of type 2 diabetes onset [5, 13]. Currently, the approach to ameliorate and control type 2 diabetes in obese individuals includes a combination of pharmacotherapy and lifestyle. Recent efforts have focused on dietary ingredients useful for the complementary treatment of type 2 diabetes [30]. Berberine, a natural plant product, improves diabetic and insulin-resistant states via activation of 5′-AMP-activated protein kinase (AMPK) [14]. Dietary anthocyanin-rich bilberry extract also ameliorates hyperglycemia and insulin sensitivity via activation of AMPK [25]. Go et al. [6] recently reported that yam and allantoin exert antidiabetic effects via promotion of glucagon-like peptide-1 and activation of antioxidant defence.
Royal jelly (RJ) supplementation has various pharmacological effects, such as weight loss and decreased fasting blood glucose levels in females with diabetes [20, 21] and improved glucose tolerance in healthy volunteers [18]. Our previous study revealed that although RJ administration improves hyperglycemia via suppression of glucose-6 phosphatase (G6Pase) mRNA expression by activation of AMPK in the livers of KK-Ay mice, it did not improve insulin resistance [32]. RJ comprises 60–70% water, 10–12% carbohydrates, 12–15% proteins and 3–7% lipids [20]. However, the components of RJ that are effective for diabetes remain unclear. Recently, Takikawa et al. [26] reported that 10-hydroxy-2-decenoic acid (10H2DA), which is a lipid component specifically found in RJ [3], activates AMPK and increases the translocation of glucose transporter 4 (GLUT4) to the plasma membrane in healthy mice. Because an increase in GLUT4 translocation leads to glucose uptake in skeletal muscles, the report may suggest that 10H2DA could be useful as a therapeutic agent for type 2 diabetes.
To the best of our knowledge, no reports have been published on the effects of 10H2DA on type 2 diabetes. Because RJ administration did not improve insulin resistance [32], we are interested in determining whether 10H2DA can ameliorate insulin resistance in KK-Ay mice. Prior et al. [22] reported that consumption of whole blueberries did not prevent obesity. However, extracted blueberry anthocyanins significantly reduced body fat accumulation in mice. Therefore, 10H2DA, which is a purified product of RJ, may show biological functions different from those of RJ. In fact, we show that long-term administration of 10H2DA markedly improves hyperglycemia and insulin resistance in KK-Ay mice without reducing obesity. In addition, we investigate the preliminary mechanism by which 10H2DA administration improves insulin resistance and hyperglycemia.
MATERIALS AND METHODS
Animals
Female obese/diabetic KK-Ay mice [4, 11] were purchased at 5 weeks of age (CLEA Japan Inc., Tokyo, Japan). All mice were maintained under specific pathogen-free conditions at 23 ± 2°C and housed in plastic cages containing sterilized woodchips for bedding in a 12-hr light/dark cycle (7:00–19:00 hr). They had free access to water and standard laboratory chow (MF, Oriental Yeast Co., Tokyo, Japan). The Institutional Animal Care and Use Committee of Kyoto Sangyo University approved the protocols for animal care and experimentation.
Animal groups and treatments
KK-Ay mice were divided into two groups, and orally administered 10H2DA (3 mg/kg; approximately 16 µmol/kg body weight in 25% ethanol) or vehicle (25% ethanol alone) for 4 weeks. Purified 10H2DA from RJ (>98%) was purchased from Nagara Science Co., Ltd. (Gifu, Japan).
Oral glucose tolerance test (OGTT)
OGTT was performed by the oral gavage of glucose (2 g glucose/kg body weight) in overnight-fasted mice after 4 weeks of administration of 10H2DA. Blood samples were obtained from the tail veins at 0 (fasting), 30, 60, 90 and 120 min. Blood glucose levels were determined directly using the glucose oxidase method with Glutest Neo test strips (Sanwa Chemical Co., Nagoya, Japan). Glucose area under the curve (glucose AUC) was calculated according to the trapezoid rule from the glucose measurements at each time and is expressed as min × mg/dl. The blood samples were collected from tail veins using heparinized capillary tubes at 0 (fasting), 15 and 30 min, and then centrifuged to obtain plasma as described previously [23]. Plasma insulin levels were determined using an ELISA (Shibayagi Co., Ltd., Shibukawa, Japan), as described previously [23]. Homeostatic model assessment-insulin resistance (HOMA-IR) was calculated according to the formula; fasting plasma insulin (µU/ml) ×fasting plasma glucose (mg/dl)/405.
Sample collection
On the next day after completing 10H2DA administration, mice, which were fasted overnight, were anesthetized. Blood was collected from caudal vena cava. The liver, skeletal muscle, mesenteric fat pad and retroperitoneal fat pad were removed and then stored at −80°C. Plasma membrane fractions were prepared from both gastrocnemius and soleus muscles in KK-Ay mice as described previously [19].
Quantitative real-time PCR (qRT-PCR)
Tissues were homogenized using ISOGEN II reagent (Wako Pure Chemical Industries Ltd., Osaka, Japan), and RNA was obtained from each tissue using ethanol precipitation methods. qRT-PCR reactions were performed using Fast SYBR Green Master Mix (Applied Biosystems, Tokyo, Japan), and a calibration curve method was used to analyze the data, as described previously [23]. The following primers were used: 5′-tctgaccacaaacgatgacc-3′ (forward) and 5′-cgaagcacatttgtctctgc-3′ (reverse) for mouse Pgc-1α, 5′-atgactttgggatccagtcg-3′ (forward) and 5′-tggaaccagatgggaaagag3′ (reverse) for mouse glucose-6 phosphatase (G6Pase), 5′-aaaacgccttgaacctgaaa-3′ (forward) and 5′-gtaagggaggtcggtgttga-3′ (reverse) for mouse phosphoenolpyruvate carboxykinase (Pck1), 5′-tgtgttgtctccactgtttgc-3′ (forward) and 5′-ctcattcgctgaccacacc-3′ (reverse) for mouse adiponectin receptor 1 (AdipoR1), and 5′-cccgttgaacaagaaagtcag-3′ (forward) and 5′-gcacaggtcttgcaaatgg-3′ (reverse) for mouse AdipoR2. The primers for mouse Gapdh were purchased (Takara Bio Inc., Otsu, Japan).
Western blot
Western blot analysis was performed as described by Towbin et al. [27] with slight modifications. Tissue homogenates were centrifuged, and the supernatants were subjected to electrophoresis in 10 or 15% SDS-PAGE; the proteins separated in the gel were then transferred electrophoretically to a polyvinyl difluoride (PVDF) membrane sheet (Immobilon-P, Millipore Co., Billerica, MA, U.S.A.), which was blocked with 5% nonfat dry milk/0.1% Tween 20 in PBS, as described previously [23]. After washing, the membrane was incubated with antibodies against AMPK [1:2,000; #2063, Cell Signaling Tech (CST) Japan, K.K., Tokyo, Japan], pAMPK (Thr172) (1:2,000; #4188, CST Japan, K.K.), glucose transporter-4 (GLUT4) (1:2,000; #2213, CST Japan, K.K.), β-actin (1:10,000; #017–24573, Wako Pure Chemical Industries Ltd.), Glycogen synthetase kinase-3β (GSK-3β) (1:2,000; #12456, CST Japan, K.K.), pGSK-3β (Ser9) (1:2,000; #5558, CST Japan, K.K.), Glycogen synthetase (GS) (1:2,000; #3886, CST Japan, K.K.), or pGS (Ser641) (1:2000; #3891, CST Japan, K.K.). Antigen-antibody complexes were detected using peroxidase-conjugated secondary antibodies (1:5,000; SC-3837, Santa Cruz Biotechnology, Inc., Dallas, TX, U.S.A. and 474–1806, Kirkegaard & Perry Laboratories, Inc., Washington, D.C., U.S.A.). Bands were analyzed using a Molecular Imager ChemiDoc XRS+ (Bio-Rad Laboratories, Inc., Berkeley, CA, U.S.A.).
Statistical analysis
Data were analyzed using two-way repeated-measures ANOVA with Shaffer’s modified sequentially rejective Bonferroni as post-hoc test or 2-tailed Student’s unpaired t test. A value of P<0.05 was defined as statistically significant. Data are presented as the mean ± SEM.
RESULTS
Effect of long-term administration of 10H2DA on insulin resistance and hyperglycemia
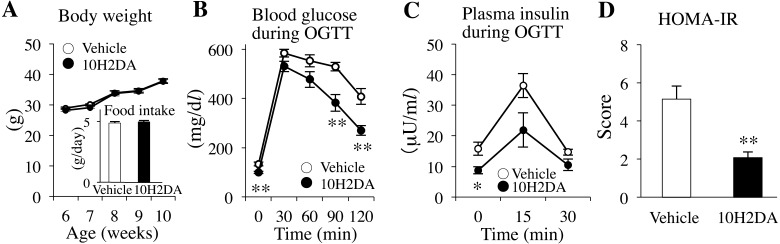
No differences in body weight and food intake were observed between the 10H2DA-treated and vehicle-treated groups during the 4 weeks of 10H2DA administration period (Fig. 1A). OGTTs were performed the next day after 4 weeks of administration of 10H2DA. The blood glucose levels were significantly decreased in 10H2DA-treated KK-Ay mice compared with those in the vehicle-treated KK-Ay mice at 0 min (P=0.0045), 90 min (P=0.0066) and 120 min (P=0.0036) after glucose loading (Fig. 1B). There was a significant difference in the glucose area under the curve (AUC) between the 10H2DA-treated (47,193 ± 2,587 mg/dl) and vehicle-treated (58,090 ± 1,802 mg/dl) groups (P=0.011). These results show that 10H2DA administration has the ability to improve glucose intolerance in obese/diabetic KK-Ay mice.
Fig. 1.
KK-Ay mice were orally administered with 10H2DA (3 mg/kg in body weight in 25% ethanol) or vehicle (25% ethanol) for 4 weeks. A: No differences in body weight and food intake were observed between 10H2DA-treated and vehicle-treated mice (n=8). B: Blood glucose levels after 4 weeks of administration of 10H2DA or vehicle were measured during an OGTT in KK-Ay mice fasted overnight (n=6–8). C: Plasma insulin levels during the OGTT were determined in the mice (n=6). D: The HOMA-IR was calculated using the fasting glucose levels from Fig. 1B and insulin levels from Fig. 1C. Data are presented as the mean ± SEM. *P<0.05, **P<0.01 vs. vehicle.
The plasma insulin levels of 10H2DA-treated mice were significantly reduced at 0 min during OGTT as compared with vehicle-treated mice (P=0.032) (Fig. 1C). The HOMA-IR score based on plasma levels of the fasting glucose and insulin obtained from Fig. 1B and 1C, respectively, was significantly decreased in 10H2DA-treated mice (2.08 ± 0.29) compared with vehicle-treated mice (5.14 ± 0.68) (P=0.0036) (Fig. 1D). These results suggest that 10H2DA markedly improves insulin resistance in obese/diabetic KK-Ay mice.
Increased expression of key enzymes regulating insulin sensitivity and glucose homoeostasis by 10H2DA in skeletal muscles
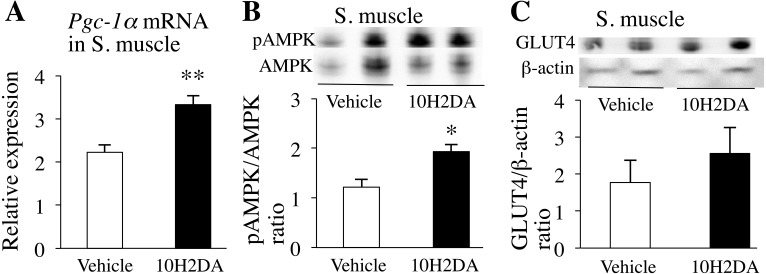
To elucidate the molecular mechanisms by which 10H2DA administration markedly improved insulin resistance and hyperglycemia, we investigated expressions of enzymes related to insulin sensitivity and glucose homeostasis in skeletal muscles. We found that 10H2DA-treated mice had a significant increase in the expression of Pgc-1α mRNA in skeletal muscles compared with vehicle-treated mice (P=0.0024) (Fig. 2A), suggesting improved muscle insulin sensitivity. We also observed that pAMPK expression levels in skeletal muscles were significantly higher in 10H2DA-treated KK-Ay mice than in vehicle-treated ones (P=0.021) (Fig. 2B). 10H2DA intake tended to increase GLUT4 translocation in skeletal muscle, although differences between 10H2DA- and vehicle-treated mice were not statistical significance (Fig. 2C).
Fig. 2.
KK-Ay mice fasted overnight were sacrificed the next day after completing 10H2DA or vehicle administration. A: Relative pAMPK protein levels in skeletal muscles were determined by Western blotting (n=6). B: Relative mRNA expression levels of Pgc-1α in skeletal muscles (n=7–8) were quantified by qRT-PCR and normalized to the levels of Gapdh mRNA. C: Relative translocated GLUT4 protein levels in skeletal muscles were determined by Western blotting (n=6). Data are presented as the mean ± SEM. *P<0.05, **P<0.01 vs. vehicle.
Increased expression of key enzymes regulating insulin sensitivity and glucose homoeostasis by 10H2DA in the liver
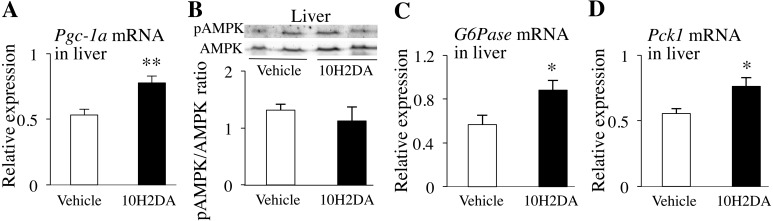
Pgc-1α mRNA expression level in the liver was also significantly higher in 10H2DA-treated mice than in vehicle-treated ones (P=0.0064) (Fig. 3A), whereas pAMPK protein expression level was almost the same between the two groups (Fig. 3B). Pgc-1α expression is considered to be induced by fasting. This effect promotes the expression of gluconeogenic genes in the liver, such as G6Pase and Pck1, which are the key enzymes of gluconeogenesis. In fact, the expression levels of G6Pase (P=0.049) and Pck1 mRNAs (P=0.028) were significantly higher in 10H2DA-treated mice than in vehicle-treated ones (Fig. 3C snd 3D).
Fig. 3.
KK-Ay mice fasted overnight were sacrificed the next day after completing 10H2DA or vehicle administration. Relative mRNA expression levels of Pgc-1α in liver (n=7–8) (A), G6Pase in liver (n=7–8) (B) and Pck1 in liver (n=7–8) (C) were quantified by qRT-PCR and normalized to the levels of Gapdh mRNA. D: Relative pAMPK protein levels in liver (n=6) were determined by Western blotting. Data are presented as the mean ± SEM. *P<0.05, **P<0.01 vs. vehicle.
Adiponectin (AdipoQ), AdipoR1 and AdipoR2 expression in the liver and fat
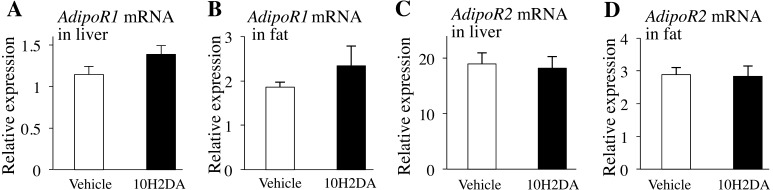
RJ administration enhanced mRNA expression of AdipoQ in fat and AdopoR1 in the liver [32]. In the present study, however, no differences in serum adiponectin (Table 1) and mRNA expression levels of AdipoR1 (Fig. 4A and 4B) and AdipoR2 (Fig. 4C and 4D) were observed between 10H2DA- and vehicle-treated mice.
Table 1. Comparison of abdominal fat weight and serum components in KK-Ay mice after 4 weeks of treatment with vehicle or 10H2DA.
| Vehicle (n=7) | 10H2DA (n=8) | |
|---|---|---|
| Fat weight (g) | ||
| Retroperitoneal fat | 4.91 ± 0.24 | 5.02 ± 0.29 |
| Mesenteric fat | 1.04 ± 0.07 | 1.17 ± 0.05 |
| Abdominal fat | 5.95 ± 0.26 | 6.19 ± 0.34 |
| Adiposity index (%) | ||
| Retroperitoneal fat | 13.21 ± 0.42 | 13.24 ± 0.46 |
| Mesenteric fat | 2.80 ± 0.16 | 3.10 ± 0.09 |
| Abdominal fat | 16.02 ± 0.42 | 16.34 ± 0.50 |
| NEFA (mEq/l) | 0.98 ± 0.09 | 0.99 ± 0.09 |
| TG (mg/dl) | 116.86 ± 11.12 | 140.84 ± 31.97 |
| TCHO (mg/dl) | 121.75 ± 5.37 | 132.93 ± 4.60 |
| Adiponectin (mg/ml) | 3.33 ± 0.32 | 3.60 ± 0.36 |
NEFA: non-esterified fatty acids; TG: triglycerides; TCHO: total cholesterol. Fat weight was measured on the day the mice were sacrifiecd. NEFA, TG, TCHO and adiponectin levels were measured in the fasting state on the day the mice were sacrificed. Data are presented as the mean ± SEM.
Fig. 4.
KK-Ay mice fasted overnight were sacrificed the next day after completing 10H2DA or vehicle administration. Relative mRNA expression levels of AdipoR1 in liver (A) (n=7–8), AdipoR1 in retroperitoneal fat (B) (n=7–8), AdipoR2 in liver (C) (n=7–8) and AdipoR2 in retroperitoneal fat (D) (n=7–8). Data are presented as the mean ± SEM.
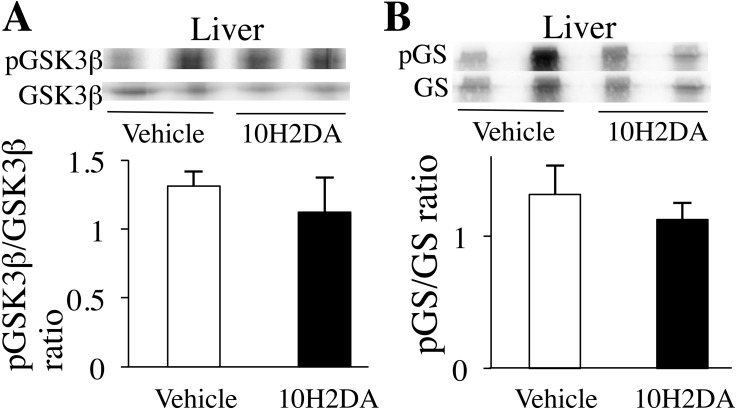
Insulin signaling protein expression in the liver
The liver is an important organ for glucose regulation. Activation of the insulin signaling cascade enhances GS through GSK3β activation, which means that glucose release from the liver is suppressed. However, phosphorylation levels of pGSK3β (Ser9) (Fig. 5A) and pGS (Ser641) (Fig. 5B) did not differ between 10H2DA- and vehicle-treated groups.
Fig. 5.
KK-Ay mice fasted overnight were sacrificed the next day after completing 10H2DA or vehicle administration. Relative protein levels of pGSK3β (A) and pGS (B) were determined by Western blotting (n=6). Data are presented as the mean ± SEM.
DISCUSSION
In the present study, we provide the primary evidence that long-term administration of 10H2DA markedly improves hyperglycemia and insulin resistance in obese/diabetic KK-Ay mice, but does not prevent obesity. Our previous study demonstrated that whole RJ administration induced weight loss and improved hyperglycemia, but did not improve insulin resistance [32]. This shows that whole RJ and its purified matter, 10H2DA, have different biological actions. Because insulin resistance precedes hyperglycemia, improving insulin resistance through 10H2DA can be a promising therapeutic regimen for type 2 diabetes.
A previous study reported that a single large-dose administration of 10H2DA significantly increases pAMPK protein and GLUT4 translocation in the skeletal muscles of healthy mice [26]. In the present study, the long-term administration of 10H2DA significantly increased pAMPK protein in skeletal muscles. GLUT4 translocation in skeletal muscle tends to increase in 10H2DA group, although it did not reach statistical significance. This may contribute to the improvement of insulin sensitivity and hyperglycemia. This discrepancy in the content of GLUT4 may be due to differences in the experimental procedure, such as in the number of 10H2DA administrations, dose and presence or absence of obesity/diabetes, because we administered 10H2DA multiple times in an amount approximately one-hundredth of the dose used by the previous report in the obese/diabetic mice [26]. In addition, it is controversial whether AMPK signaling corresponds to glucose uptake in skeletal muscles. In some cases, AMPK signaling correlates with muscle glucose uptake [8, 9], but in others, changes in AMPK signaling do not correspond well to glucose uptake (GLUT4 translocation) in skeletal muscles [17, 29]. Furthermore, metformin ameliorates diabetes without any change in skeletal muscle GLUT4 content [7, 24].
AMPK upregulation suppresses mRNA expression of gluconeogenic genes, such as G6Pase and Pck1, in the liver [28]. In the present study, no difference in pAMPK protein expression levels in the liver was observed between the 10H2DA- and vehicle-treated groups. This might mean that the expression levels of G6Pase and Pck1 mRNAs should be almost the same in both groups. However, the expression levels of G6Pase and Pck1 mRNAs were significantly higher in the 10H2DA-treated group than in the vehicle-treated one. This may suggest that 10H2DA is involved in the upregulation of G6Pase and Pck1 mRNAs via activation of enzymes other than AMPK. Pgc-1α is considered to be involved in the activation of gluconeogenic genes in the liver under fasting conditions [15]. A previous report also showed that adiponectin and AdipoR1 regulate PGC-1α [10]. However, in the present study, 10H2DA did not affect serum adiponectin levels and expression levels of AdipoR1 mRNA in the liver and fat of KK-Ay mice. These results suggest that increase of AMPK phosphorylation via AdipoR1 in 10H2DA group did not occur. This might be a reason why 10H2DA administration did not suppress expression of G6Pase and Pck1 mRNAs. In addition, 10H2DA did not affect the insulin signaling cascade in the liver, suggesting that hepatic glucose production was not suppressed.
Pgc-1α is considered a key factor regulating insulin sensitivity and glucose homeostasis in mammalian muscles. Modest Pgc-1α overexpression in the muscle improves insulin sensitivity [1, 2]. Another study also suggested that the antidiabetic drug metformin increased PGC-1α protein expression via activation of AMPK, without affecting GLUT4 content in rat skeletal muscles [24]. Consistent with these reports, the present study demonstrated that 10H2DA administration significantly increased the expression of Pgc-1α mRNA and pAMPK protein in skeletal muscles of KK-Ay mice. Although increased expression of pAMPK did not affect GLUT4 expression, significantly, this does not exclude the possibility that increased pAMPK is involved in Pgc-1α expression in skeletal muscles.
The present findings showed that Pgc-1α mRNA expression also increased in the liver. This resulted in the increased expression of the gluconeogenic genes, G6Pase and Pck1, suggesting an increase in hepatic glucose production. It is paradoxical that insulin resistance and hyperglycemia are improved in vivo, despite increased gluconeogenic mRNAs in 10H2DA-treated mice. Consistent with our findings, Liang et al. [16] reported that moderate whole-body overexpression of Pgc-1α in transgenic (TG) mice has an opposite effect on hepatic and muscle insulin sensitivity. Despite the increased expression of G6Pase and Pck1 mRNAs in the liver, glucose tolerance improved in TG mice [16]. The mechanism causing the dichotomous effect of Pgc-1α overexpression in the liver and skeletal muscles remains unknown. Activation of Pgc-1α mRNA by 10H2DA administration is postulated to lead to partial or systemic improvement of glucose uptake in peripheral tissues, including muscles, by improving insulin resistance. Expression level of Pgc-1α mRNA in fat did not differ significantly between 10H2DA- and vehicle-treated mice (data not shown), suggesting that abdominal fat may not contribute to insulin sensitivity in 10H2DA. However, a further study is required to clarify this mechanism. Taken together, these findings suggest that the increased expression of Pgc-1α mRNA in muscle and liver is important in improving insulin resistance and hyperglycemia in an in vivo diabetic state.
In conclusion, 10H2DA, a purified natural component of RJ, is potently involved in the improvement of hyperglycemia and insulin resistance, at least in part via the activation of Pgc-1α mRNA expression, but does not prevent obesity.
REFERENCES
- 1.Benton C. R., Nickerson J. G., Lally J., Han X. X., Holloway G. P., Glatz J. F., Luiken J. J., Graham T. E., Heikkila J. J., Bonen A.2008. Modest PGC-1alpha overexpression in muscle in vivo is sufficient to increase insulin sensitivity and palmitate oxidation in subsarcolemmal, not intermyofibrillar, mitochondria. J. Biol. Chem. 283: 4228–4240. doi: 10.1074/jbc.M704332200 [DOI] [PubMed] [Google Scholar]
- 2.Benton C. R., Holloway G. P., Han X. X., Yoshida Y., Snook L. A., Lally J., Glatz J. F., Luiken J. J., Chabowski A., Bonen A.2010. Increased levels of peroxisome proliferator-activated receptor gamma, coactivator 1 alpha (PGC-1alpha) improve lipid utilisation, insulin signalling and glucose transport in skeletal muscle of lean and insulin-resistant obese Zucker rats. Diabetologia 53: 2008–2019. doi: 10.1007/s00125-010-1773-1 [DOI] [PubMed] [Google Scholar]
- 3.Blum M. S., Novak A. F., Taber S., 3rd.1959. 10-Hydroxy-delta 2-decenoic acid, an antibiotic found in royal jelly. Science 130: 452–453. doi: 10.1126/science.130.3373.452 [DOI] [PubMed] [Google Scholar]
- 4.Bonini J. A., Colca J. R., Dailey C., White M., Hofmann C.1995. Compensatory alterations for insulin signal transduction and glucose transport in insulin-resistant diabetes. Am. J. Physiol. 269: E759–E765. [DOI] [PubMed] [Google Scholar]
- 5.Cline G. W., Petersen K. F., Krssak M., Shen J., Hundal R. S., Trajanoski Z., Inzucchi S., Dresner A., Rothman D. L., Shulman G. I.1999. Impaired glucose transport as a cause of decreased insulin-stimulated muscle glycogen synthesis in type 2 diabetes. N. Engl. J. Med. 341: 240–246. doi: 10.1056/NEJM199907223410404 [DOI] [PubMed] [Google Scholar]
- 6.Go H. K., Rahman M. M., Kim G. B., Na C. S., Song C. H., Kim J. S., Kim S. J., Kang H. S.2015. Antidiabetic Effects of Yam (Dioscorea batatas) and Its Active Constituent, Allantoin, in a Rat Model of Streptozotocin-Induced Diabetes. Nutrients 7: 8532–8544. doi: 10.3390/nu7105411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Handberg A., Kayser L., Høyer P. E., Voldstedlund M., Hansen H. P., Vinten J.1993. Metformin ameliorates diabetes but does not normalize the decreased GLUT 4 content in skeletal muscle of obese (fa/fa) Zucker rats. Diabetologia 36: 481–486. doi: 10.1007/BF02743261 [DOI] [PubMed] [Google Scholar]
- 8.Hayashi T., Hirshman M. F., Kurth E. J., Winder W. W., Goodyear L. J.1998. Evidence for 5′ AMP-activated protein kinase mediation of the effect of muscle contraction on glucose transport. Diabetes 47: 1369–1373. [DOI] [PubMed] [Google Scholar]
- 9.Hayashi T., Hirshman M. F., Fujii N., Habinowski S. A., Witters L. A., Goodyear L. J.2000. Metabolic stress and altered glucose transport: activation of AMP-activated protein kinase as a unifying coupling mechanism. Diabetes 49: 527–531. doi: 10.2337/diabetes.49.4.527 [DOI] [PubMed] [Google Scholar]
- 10.Iwabu M., Yamauchi T., Okada-Iwabu M., Sato K., Nakagawa T., Funata M., Yamaguchi M., Namiki S., Nakayama R., Tabata M., Ogata H., Kubota N., Takamoto I., Hayashi Y. K., Yamauchi N., Waki H., Fukayama M., Nishino I., Tokuyama K., Ueki K., Oike Y., Ishii S., Hirose K., Shimizu T., Touhara K., Kadowaki T.2010. Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by Ca2+ and AMPK/SIRT1. Nature 464: 1313–1319. doi: 10.1038/nature08991 [DOI] [PubMed] [Google Scholar]
- 11.Iwatsuka H., Shino A., Suzuoki Z.1970. General survey of diabetic features of yellow KK mice. Endocrinol. Jpn. 17: 23–35. doi: 10.1507/endocrj1954.17.23 [DOI] [PubMed] [Google Scholar]
- 12.Kopelman P. G.2000. Obesity as a medical problem. Nature 404: 635–643. [DOI] [PubMed] [Google Scholar]
- 13.Lauro D., Kido Y., Castle A. L., Zarnowski M. J., Hayashi H., Ebina Y., Accili D.1998. Impaired glucose tolerance in mice with a targeted impairment of insulin action in muscle and adipose tissue. Nat. Genet. 20: 294–298. doi: 10.1038/3112 [DOI] [PubMed] [Google Scholar]
- 14.Lee Y. S., Kim W. S., Kim K. H., Yoon M. J., Cho H. J., Shen Y., Ye J. M., Lee C. H., Oh W. K., Kim C. T., Hohnen-Behrens C., Gosby A., Kraegen E. W., James D. E., Kim J. B.2006. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes 55: 2256–2264. doi: 10.2337/db06-0006 [DOI] [PubMed] [Google Scholar]
- 15.Liang H., Ward W. F.2006. PGC-1alpha: a key regulator of energy metabolism. Adv. Physiol. Educ. 30: 145–151. doi: 10.1152/advan.00052.2006 [DOI] [PubMed] [Google Scholar]
- 16.Liang H., Balas B., Tantiwong P., Dube J., Goodpaster B. H., O’Doherty R. M., DeFronzo R. A., Richardson A., Musi N., Ward W. F.2009. Whole body overexpression of PGC-1alpha has opposite effects on hepatic and muscle insulin sensitivity. Am. J. Physiol. Endocrinol. Metab. 296: E945–E954. doi: 10.1152/ajpendo.90292.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maarbjerg S. J., Jørgensen S. B., Rose A. J., Jeppesen J., Jensen T. E., Treebak J. T., Birk J. B., Schjerling P., Wojtaszewski J. F., Richter E. A.2009. Genetic impairment of AMPKalpha2 signaling does not reduce muscle glucose uptake during treadmill exercise in mice. Am. J. Physiol. Endocrinol. Metab. 297: E924–E934. doi: 10.1152/ajpendo.90653.2008 [DOI] [PubMed] [Google Scholar]
- 18.Morita H., Ikeda T., Kajita K., Fujioka K., Mori I., Okada H., Uno Y., Ishizuka T.2012. Effect of royal jelly ingestion for six months on healthy volunteers. Nutr. J. 11: 77. doi: 10.1186/1475-2891-11-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishiumi S., Ashida H.2007. Rapid preparation of a plasma membrane fraction from adipocytes and muscle cells: application to detection of translocated glucose transporter 4 on the plasma membrane. Biosci. Biotechnol. Biochem. 71: 2343–2346. doi: 10.1271/bbb.70342 [DOI] [PubMed] [Google Scholar]
- 20.Pourmoradian S., Mahdavi R., Mobasseri M., Faramarzi E., Mobasseri M.2012. Effects of royal jelly supplementation on body weight and dietary intake in type 2 diabetic females. Health Promot. Perspect. 2: 231–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pourmoradian S., Mahdavi R., Mobasseri M., Faramarzi E., Mobasseri M.2014. Effects of royal jelly supplementation on glycemic control and oxidative stress factors in type 2 diabetic female: a randomized clinical trial. Chin. J. Integr. Med. 20: 347–352. doi: 10.1007/s11655-014-1804-8 [DOI] [PubMed] [Google Scholar]
- 22.Prior R. L., Wu X., Gu L., Hager T. J., Hager A., Howard L. R.2008. Whole berries versus berry anthocyanins: interactions with dietary fat levels in the C57BL/6J mouse model of obesity. J. Agric. Food Chem. 56: 647–653. doi: 10.1021/jf071993o [DOI] [PubMed] [Google Scholar]
- 23.Sasaki D., Kotoh J., Watadani R., Matsumoto K.2015. New animal models reveal that coenzyme Q2 (Coq2) and placenta-specific 8 (Plac8) are candidate genes for the onset of type 2 diabetes associated with obesity in rats. Mamm. Genome 26: 619–629. doi: 10.1007/s00335-015-9597-4 [DOI] [PubMed] [Google Scholar]
- 24.Suwa M., Egashira T., Nakano H., Sasaki H., Kumagai S.2006. Metformin increases the PGC-1alpha protein and oxidative enzyme activities possibly via AMPK phosphorylation in skeletal muscle in vivo. J. Appl. Physiol. 101: 1685–1692. doi: 10.1152/japplphysiol.00255.2006 [DOI] [PubMed] [Google Scholar]
- 25.Takikawa M., Inoue S., Horio F., Tsuda T.2010. Dietary anthocyanin-rich bilberry extract ameliorates hyperglycemia and insulin sensitivity via activation of AMP-activated protein kinase in diabetic mice. J. Nutr. 140: 527–533. doi: 10.3945/jn.109.118216 [DOI] [PubMed] [Google Scholar]
- 26.Takikawa M., Kumagai A., Hirata H., Soga M., Yamashita Y., Ueda M., Ashida H., Tsuda T.2013. 10-Hydroxy-2-decenoic acid, a unique medium-chain fatty acid, activates 5′-AMP-activated protein kinase in L6 myotubes and mice. Mol. Nutr. Food Res. 57: 1794–1802. [DOI] [PubMed] [Google Scholar]
- 27.Towbin H., Staehelin T., Gordon J.1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. U.S.A. 76: 4350–4354. doi: 10.1073/pnas.76.9.4350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Viollet B., Guigas B., Leclerc J., Hébrard S., Lantier L., Mounier R., Andreelli F., Foretz M.2009. AMP-activated protein kinase in the regulation of hepatic energy metabolism: from physiology to therapeutic perspectives. Acta Physiol. (Oxf.) 196: 81–98. doi: 10.1111/j.1748-1716.2009.01970.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wadley G. D., Lee-Young R. S., Canny B. J., Wasuntarawat C., Chen Z. P., Hargreaves M., Kemp B. E., McConell G. K.2006. Effect of exercise intensity and hypoxia on skeletal muscle AMPK signaling and substrate metabolism in humans. Am. J. Physiol. Endocrinol. Metab. 290: E694–E702. doi: 10.1152/ajpendo.00464.2005 [DOI] [PubMed] [Google Scholar]
- 30.Wang H. X., Ng T. B.1999. Natural products with hypoglycemic, hypotensive, hypocholesterolemic, antiatherosclerotic and antithrombotic activities. Life Sci. 65: 2663–2677. doi: 10.1016/S0024-3205(99)00253-2 [DOI] [PubMed] [Google Scholar]
- 31.Wild S., Roglic G., Green A., Sicree R., King H.2004. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 27: 1047–1053. doi: 10.2337/diacare.27.5.1047 [DOI] [PubMed] [Google Scholar]
- 32.Yoshida M., Hayashi K., Watadani R., Okano Y., Tanimura K., Kotoh J., Sasaki D., Matsumoto K., Maeda A.2017. Royal jelly improves hyperglycemia in obese/diabetic KK-Ay mice. J. Vet. Med. Sci. 79: 299–307. doi: 10.1292/jvms.16-0458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zimmet P., Alberti K. G., Shaw J.2001. Global and societal implications of the diabetes epidemic. Nature 414: 782–787. doi: 10.1038/414782a [DOI] [PubMed] [Google Scholar]