Abstract
During the autumn migration of many waterfowls, body mass is lowest upon arrival at the wintering area and gradually increases during the winter. Consequently, body mass is highest before the spring migration. We studied the pattern of body mass changes in the Black-Headed Gull (Larus ridibundus) from December 2010 to December 2016 in the Shinhama area of Chiba, Japan. Based on 327 captured animals, body mass increased during the wintering period, but tended to decrease before migration. In 2014–2016, a muscle mass reduction in females was observed, explaining the change in body mass. However, the observed weight loss may be caused by many factors, which may be related to the migratory ecology of the regional population.
Keywords: Black-Headed Gull, body mass change, Larus ridibundus, migration, wintering period
The Black-Headed Gull (Larus ridibundus) is a small gull with a body length of approximately 38 cm; it belongs to the order Charadriiformes and the family Laridae [15]. Black-Headed Gulls breed in the United Kingdom, Iceland and northern Eurasia; however, they move from North Africa to the eastern coast of North America in the winter [8]. In Japan, they are observed as passage migrants in Hokkaido [16] and as winter migrants in the south of Houshu [30]. They live in lakes, rivers, estuaries [22] and coasts, and feed on insects, small fishes, lugworms and dead fish [18]. They are designated as a species of least concern (LC) in the International Union for Conservation of Nature (IUCN) Red List [17], although there are various concerns that are considered in its status, including outbreaks of avian influenza [20], overfishing of eggs and contamination with chemical pollutants [14] and oil pollution [13]. Regarding this last point especially, Black-Headed Gulls are registered as “sea birds with a high possibility of being affected by oil pollution” by the Ministry of the Environment [23], and in fact, oil exposure has often been observed in this species.
Black-Headed Gulls migrate annually between their fledging areas (i.e., breeding areas) and their first wintering areas [28]. The order Charadriiformes, which includes the red-necked phalarope (Phalaropus lobatus) [21] and western sandpiper (Calidris mauri) [29], exhibits increased body mass, mainly attributed to increased fat, before migration. Based on a survey of Black-Headed Gulls in Madrid, Spain, the body mass is lowest after the autumn migration and increases before the spring migration [5]. Regarding migratory ecology, migration in Laridae may differ from that in other Charadriiformes families, including Scolopacidae and Charadriidae, which migrate without resting [1]. The migration ecology of Black-Headed Gulls has not been examined in Japan. In addition, waterfowl are reported to be more highly susceptible to environmental pollution than field birds [31]; accordingly, it is necessary to understand the physiological conditions of these species for rescue and monitoring efforts when they are affected by human accidents, such as oil pollution. In this study, we analyzed the body mass of Black-Headed Gulls during the winter. When assessing body mass, keel score and fat score which change similarly to body mass were also evaluated. And, we evaluated the molting status which is said to affect body mass [3], fat score and keel score [4].
MATERIALS AND METHODS
The study was conducted from December 2010 to December 2016 in the Shinhama area of Ichikawa City, Chiba Prefecture, Japan (N35°, E139°). Birds were captured twice daily (11:30 to 12:00 and 15:00 to 15:30) by using bread to attract them. To minimize adverse effects, birds were captured by hand. The handling of captured birds complied with the guidelines for the use of wild birds in research [12]. After they were captured, birds were placed in a purse bag for holding, body mass (g) was quickly obtained using a digital balance (HL-3000WP, A&D, Tokyo, Japan), and ages were determined. From December 2014 to December 2016, pubic bone width measurements were obtained, and blood sampling was performed for sex identification. Blood was collected from the wing vein using a heparinized 27 G needle and a 1-ml syringe (TOP needle and TOP syringe, TOP, Tokyo, Japan) to obtain an amount that was less than 1% of the body mass [10]. Blood was stored at 4°C until the examination. After a metal ring and colored ring were placed on the tarsus, birds were released. From December 2014 to December 2016, molt, fat located from the clavicle to the trailing edge of the sternum (transverse abdominal fat) [26], and muscle (keel score) were examined [2]. Molt was judged according to head feathers; if black feathers were observed, the bird was evaluated as “molted (1),” otherwise it was considered “pre-molt (0)”. Fat was scored on a scale of 1 to 5. A score of 1 indicated no fat on the particular area, 2 indicated little fat, 3 indicated fat was present on half of the clavicle, 4 indicated fat was present on over half of the clavicle, and 5 indicated that, in addition to the criterion for 4, fat also extended to the sternum. The criteria for determining keel score (from 1 to 5) were as follows: 1 indicated that the pectoral muscle was minimal and the sternal keel could be easily felt when pinched, 2 indicated that the pectoral muscle was slightly more developed and only the edge of the sternum could be easily felt, 3 indicated that some pectoral muscle was detected and sharpness was lacking, 4 indicated that the pectoral muscle was attached at the same height as the tip of the keel protrusion, and 5 was the state in which the pectoral muscle exceeded the keel protrusion. A fat score was estimated for the back between the scapula and thoracic vertebra. In this area, 1 indicated no fat, 2 indicated that fat occupied less than half the area between the scapula and thoracic vertebrae, 3 indicated that fat occupied more than half of the area, 4 indicated more than half of the scapula and thoracic vertebrae and not above the scapula, and 5 was above the scapula.
Sex was identified by polymerase chain reaction (PCR) using 2550F (5′-GTTACTGATTCGTCTACGAGA-3′) for the forward primer and 2718R (5′-ATTGAAATGATCCAGTGCTTG-3′) for the reverse primer [11]. For DNA extraction, the blood was mixed well with distilled water or phosphate-buffered saline (1.37 M NaCl, 27 mM KCL, 81 mM Na2HPO4•12H2O (Na2HPO4•7H2O) and 14.4 mM KH2PO4), and DNA was extracted using the QIAamp® DNA Blood Mini Kit (QIAGEN, Hilden, Germany). The extracted DNA (<100 ng/25 µl) was mixed as template DNA with 1 × PCR Buffer for KOD Fx Neo, 0.4 mM dNTPs, 0.25 µM each primer, 1 U/25 µl KOD Fx Neo (TOYOBO, Osaka, Japan) and distilled water to reach 25 µl. The PCR program was as follows: pre-denaturation at 94°C for 2 min, denaturation at 94°C for 30 sec, annealing at 30 sec from 60 to 50°C and extension at 72°C for 30 sec for a total of 35 cycles, with a final extension at 72°C for 5 min. Electrophoresis was performed on a 3% agarose gel for 30 to 35 min at 100 V/cm (1 × TAE, 6 × Loading Buffer [6 × Loading Buffer, TaKaRa, Otsu, Japan]). The gel was stained with 0.5 µg/ml ethidium bromide for 10 min and irradiated with ultraviolet light to determine the sex [6].
Black-Headed Gulls in the area increased from the end of November to the end of December, and they tended to fly north from the end of March to the middle of April. Based on this trend, the wintering period was divided into the following categories: “Arriving”, “Staying” and “Flying away”. “Arriving” indicated the period in which Black-Headed Gulls began to arrive. The population started decreasing at the “Flying away” period, defined as the period until Black-Headed Gulls were not observed (for this reason, the dates for each period differed among survey years), and the period between “Arriving” and “Flying away” was defined as “Staying” and was the period in which population size remained constant. With respect to age, the Black-Headed Gulls were divided into “Adult” and “Yearling” according to foot color, beak color and feather color.
After confirming that the data were normally distributed by the Kolmogorov–Smirnov test, means and 95% confidence interval were obtained. Differences among values were evaluated using the Steel-Dwass test implemented in R 3.3.0, and the molting status was evaluated using the Mann-Whitney U-test (α=0.05).
RESULTS
Body mass and age in Black-Headed Gulls were examined over a period of 7 years. In total, 327 birds were captured, including 296 Adult and 31 Yearling as well as in a part 78 confirmed males and 66 confirmed females. In this way, Yearling did not perform statistical evaluation with Adult as it only showed the result, because the capture number was small and along with this, from 2014 to 2016 we subject only Adult to statistical evaluation. In December 2013 to March 2014, Black-Headed Gulls were not captured in “Staying”, so this period did not added in the statistical evaluation. In addition, 143 birds were classified as “Arriving”, 82 as “Staying” and 102 as “Flying away” (Table 1). Body mass of Adult was 278.5 (± SD; ± 11.7 g) in “Arriving”, 284.4 (± 10.7 g) in “Staying” and 269.3 (± 15.0 g) in “Flying away”, and that of Yearling was 300.0 (± 35.8 g) in “Arriving”, 292.9 (± 22.5 g) in “Staying” and 273.8 (± 12.3 g) in “Flying away”. However, Yearling did not change in weight during the wintering period (P>0.05), and Adult differed significantly between the “Arriving” and “Flying away” periods and between “Staying” and “Flying away” (P<0.05). At the same periods of each 7 survey years, there was a similar decreasing trend from “Staying” to “Flying away” for 7 survey years. Moreover, Steel-Dwass test was conducted, but no significant difference was confirmed (both ages: all of P>0.05 between years). Accordingly, we evaluated the data obtained for all 7 years collectively in adults.
Table 1. Body mass of 327 Black-Headed Gulls over a 7-year period.
| Arriving |
Staying |
Flying away |
|||||
|---|---|---|---|---|---|---|---|
| n | mass (g) | n | mass (g) | n | mass (g) | ||
| Age class | Adult | 134 | 278.5a (± 11.7) | 71 | 284.4b (± 10.7) | 91 | 269.3ab (± 15.0) |
| Yearling | 9 | 300.0 (± 35.8) | 11 | 292.9 (± 22.5) | 11 | 273.8 (± 12.3) | |
Summary of the capture number and average body mass (g) at each period. The same letter after a measurement indicates a significant difference (P<0.05). Age and body mass (± SD) are presented. There was no significant difference between Adult and Yearling in the wintering period, indicating that there was no difference in body mass depending on age.
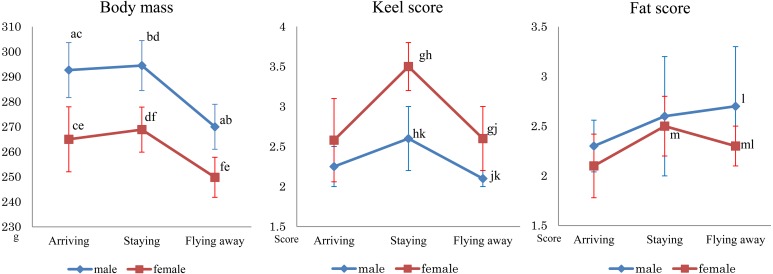
Next, the results for relationships among the sex, categories and body mass or each score from December 2014 to December 2016 were evaluated. One hundred and forty-four birds were captured, including 28 “Arriving”, 65 “Staying” and 51 “Flying away”. Sex was determined only when blood samples could be obtained. Yearling was excluded from this analysis, because of small data (only 8 numbers). In total, therefore, 63 adult females (Arriving, 14; Staying, 26; and Flying away, 23) and 73 adult males (Arriving, 14; Staying, 37; and Flying away, 22) were analyzed. The male body mass was 292.7 (± 12.1 g) in “Arriving”, 294.5 (± 14.1 g) in “Staying” and 275.2 (± 24.0 g) in “Flying away”, and female body mass was 265.0 (± 14.0 g) in “Arriving”, 268.8 (± 10.9 g) in “Staying” and 250.5 (± 9.8 g) in “Flying away” (Table 2). There was a significant difference by sex in “Arriving” and “Staying”, and it decreased significantly from “Staying” to “Flying away” for each sex (P<0.05). The average (± standard deviation) adult keel scores were 2.4 (± 0.4) for “Arriving”, 2.8 (± 0.6) for “Staying” and 2.3 (± 0.4) for “Flying away”. Regarding sex, male keel score was 2.3 (± 0.3) for “Arriving”, 2.6 (± 0.4) for “Staying” and 2.1 (± 0.1) for “Flying away”, and female keel score was 2.6 (± 0.5) for “Arriving”, 3.5 (± 0.4) for “Staying” and 2.6 (± 0.4) for “Flying away” (Fig. 1). Keel score decreased significantly from the “Staying” to “Flying away” period in sexes and showed significant difference in both “Staying” and “Flying away” between sexes (P<0.05). The fat scores were 2.1 (± 0.1) for “Arriving”, 2.6 (± 0.4) for “Staying” and 2.3 (± 0.4) for “Flying away”, and male fat score was 2.3 (± 0.3) for “Arriving”, 2.6 (± 0.7) for “Staying” and 2.7 (± 0.7) for “Flying away”, and female was 2.1 (± 0.3) for “Arriving”, 2.5 (± 0.3) for “Staying” and 2.3 (± 0.3) for “Flying away” (Fig. 1). There was no significant difference in male fat score among 3 periods, while significant differences were observed in female fat score between “Staying” and “Flying away” and between sexes in “Flying away” (P<0.05). In the “Arriving” period, the clavicle fat was reddish, and when the fat score was 3 or more, the colors of the muscle and fat at the clavicle were similar, and it was time-consuming to make a clear determination.
Table 2. Body mass of 136 Adult Black-Headed Gulls by sex.
| Arriving |
Staying |
Flying away |
|||||
|---|---|---|---|---|---|---|---|
| n | mass (g) | n | mass (g) | n | mass (g) | ||
| Sex class | Male | 14 | 292.7ac (± 12.1) | 37 | 294.5bd (± 14.1) | 22 | 275.2ab (± 24.0) |
| Female | 14 | 265.0ce (± 14.0) | 26 | 268.8df (± 10.9) | 23 | 250.5fe (± 9.8) | |
Summary of the capture number and average body mass (g) at each period. The same letter after a measurement indicates a significant difference (P<0.05). From December 2014 to December 2016, 144 birds were identified sexing. Of 78 males and 66 females, 73 males and 63 females confirm a significant difference in body mass in “Arriving” and “Staying” period in each sex (P<0.05). Significant decrease was confirmed for each sex in “Arriving” and “Flying away”, “Staying” and “Flying away” (P<0.05).
Fig. 1.
Details of sex, body mass, fat score and keel score for 136 adult birds samples from December 2014 to December 2016. In adult, 73 males and 63 females were observed, and 14 males and 14 females in the “Arriving” period, 37 males and 26 females in the “Staying” period, and 22 males and 23 females in the “Flying away” period were captured during the 2 years. The left panel summarizes body mass (g), the middle panel summarizes fat scores, and the right panel summarizes keel scores. The vertical lines indicate the standard deviation. Points with the same letter were significantly different (P<0.05). Both sexes showed significantly lower body masses in the “Flying away” period than in other periods. In both sexes, the muscle score changed significantly between “Arriving” and “Staying” and between “Staying” and “Flying away.” On the other hand, in females, the fat score was significantly decreased from “Staying” to “Flying away”.
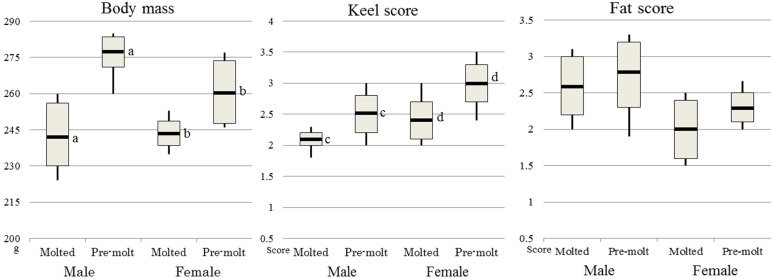
In the only “Flying away” period, 8 females were classified as molted, and 15 were pre-molt; 12 males were molted, and 10 was pre-molt. With respect to molting status (Fig. 2), the average body mass (and standard deviation) of the molted males were 240.5 g (± 7.7 g), and pre-molt males were 280.0 g (± 4.7 g). Molted females were 243.0 g (± 5.6 g), and that of pre-molt females was 260.2 g (± 13.6 g). In the comparison of keel score according to molting status, the molted males had a score of 2.1 (± 0.1), molted females had a score of 2.5 (± 0.3), pre-molt males scored 2.4 (± 0.3), and pre-molt females scored 3.0 (± 0.3), and there was a significant difference between molted and pre-molt (both sexes, P<0.05). In the comparison of fat score according to molting status, the scores were 2.6 (± 0.4) for molted males, 2.8 (± 0.5) for pre-molt males, 2.0 (± 0.4) for molted females and 2.3 (± 0.2) for pre-molt females, and there was no significant difference (both sexes, P>0.05).
Fig. 2.
Results obtained for body mass, keel score and fat score with respect to molting during the “Flying away” period from December 2014 to December 2016. Analysis of body mass with respect to sex and molting status for 12 molted males, 10 pre-molt male, 8 molted females and 15 pre-molt females. The bar in the boxplots shows the average value, vertical lines were max and minimum value, and top and bottom end of box were average value ± SD. The same letter after measurements indicates a significant difference (P<0.05). Body mass was significantly decreased in each sex by molt, and keel score was too (P<0.05), whereas fat score was not decreased by molt (P>0.05).
DISCUSSION
When comparing body mass in “Arriving” and “Staying” in the Shinhama area, significant differences were observed between males and females (P<0.05), which is the same trend as was seen in the population in Madrid [5]. However, body mass changes during the wintering season were different from those observed in Madrid [5] and in other Charadriiformes, suggesting that significant changes in the body mass of the Black-Headed Gulls were related to population-specific factors. It was said that Black-Headed Gull was a single subspecies [7], but considering the report [9] that the eastern Russia population was bigger than the European population and the characteristics about migration strategy of this study, the possibility was considered that regional subspecies existed in Black-Headed Gull. Various factors, such as migratory ecology, may be associated with the change in body mass during the wintering period in the Black-Headed Gull. Furthermore, factors, such as the migration strategy and regional conditions of populations, may explain the body mass change [1]. For example, the Herring Gull (Larus argentatus), which belongs to the same family as the Black-Headed Gull, is often observed in Hokkaido and northern Japan before reaching the Shinhama area while traveling from Russia to Japan (Sato, unpublished). It is possible that Black-Headed Gulls established a migration strategy in which the migration is not completed at once. The Lesser Black-Backed Gull (Larus fuscus) [19] makes a stopover during its migration according to GPS-based satellite telemetry data. Considering these observations for the Herring Gull and Lesser Black-Backed Gull, selecting a route with continuous land, which provides a source of food and place to rest, may be a factor explaining the ability to migrate with a decreased body mass. It is possible that gulls make stopovers when they migrate across the continent. Furthermore, the decrease in body mass may be related not only to diseases that are concerns of the IUCN, but also to differences in molting status, as observed in Barnacle Geese (Branta leucopsis) [25] and White-Throated Sparrow (Zonotrichia albicollis) [24], as well as diet quality [1]. In Barnacle Geese, a large portion of energy is lost during molting. A loss in body mass occurs via increased metabolism during molting. Degradation in body mass during migration has been reported in these birds, and it was thought that they were migrating while molting. Although body mass reduction via molting has been reported in birds, the detailed mechanism is not clear. In this survey, there was significant difference in body mass change depending on molting status in both sexes. The loss in body mass was attributed to a loss in muscle score, rather than fat (Fig. 2). In birds that eat fish and functionally deform their necks, little fat is observed in the clavicular region. For this reason, in this study, it was useful to consider the back together with the clavicular region for a comprehensive evaluation of fat score. The reason why this study resulted in a different trend for a prior report [5, 21, 29] that fat increased before migration was merely not suitable for assessment of fat score (for example, as a physiological characteristic of Black-Headed Gull, they accumulate fat by visceral fat rather than fat on the body surface, etc.) or that Black-Headed Gull is physiologically different migration strategy from other species. In this study, no difference was confirmed at the same periods of each 7 survey years about both ages and between the three periods in Yearling, but the result of Yearling seems to be caused by the small sample size, so additional investigation was necessary. Significant difference was confirmed for wintering period by the body mass of adult birds, and the body mass was lower before the spring migration than after the autumn migration.
Regarding the molting status, many birds complete their molt before or after spring migration, but the Black-Headed Gull examined in this study started before spring migration. For females which had significantly decreased fat score, they were thought to be taking different migration strategy from males, irrespective of molt. It is needed for detailed verification about specific evaluation of changes in metabolism. It was not clear why these birds were able to migrate while molting. It is necessary to determine the proximate and ultimate causes of the migratory strategy of Black-Headed Gulls in future studies (e.g., presence of subspecies or difference of the population). Additionally, in order to fully elucidate the migratory strategy of Black-Headed Gulls, it is necessary to use a blood test (blood biochemical and hematological examinations) [27] to evaluate the in-vivo changes related to migration and molt.
Acknowledgments
We deeply appreciate the members of the Friends of the Gyotoku Bird Observatory NPO for their helpful suggestions. We express our gratitude to Mr. Y. Sawa (Investigator of Bird Life International Tokyo) and Mr. H. Sugawa (Japanese Bird Banding Association) for providing information on the captured Glack-Headed Gull. We are also grateful to the members of the Laboratory of Wildlife Medicine, Nippon Veterinary and Life Science University for their assistance with analyses.
REFERENCES
- 1.Alerstam T., Lindstrom A.1990. Optimal bird migration: The relative importance of time, energy, and safety. pp. 331–351. In: Bird Migration. Springer-verlag Berlin, Heidelberg. [Google Scholar]
- 2.Bairlein F.1995. Manual of Field Methods. European-african songbird migration network. revised ed., European Science Foundation, Wihelmshaven [Google Scholar]
- 3.Bluhm C. K., Schwabl H., Schwabl I., Perera A., Follett B. K., Goldsmith A. R., Gwinner E.1991. Variation in hypothalamic gonadotrophin-releasing hormone content, plasma and pituitary LH, and in-vitro testosterone release in a long-distance migratory bird, the garden warbler (Sylvia borin), under constant photoperiods. J. Endocrinol. 128: 339–345. doi: 10.1677/joe.0.1280339 [DOI] [PubMed] [Google Scholar]
- 4.Brown R. E., Saunders D. K.1998. Regulated changes in body mass and muscle mass in molting Blue-winged teal for an early return to flight. Can. J. Zool. 76: 26–32. doi: 10.1139/z97-164 [DOI] [Google Scholar]
- 5.Cantos F. J., Alonso-Gomez A. L., Delgado M. J.1994. Seasonal changes in fat and protein reserves of the Black-headed gull, Larus ridibundus in relation to migration. Comp. Biochem. Physiol. Part A. Physiol. 108: 117–122. doi: 10.1016/0300-9629(94)90062-0 [DOI] [Google Scholar]
- 6.Cheng Y. H., Kuo T. F., Lee D. N., Weng C. F.2006. Sex Identification of the Black-faced Spoonbill (Platalea minor). Zool. Stud. 45: 104–113. [Google Scholar]
- 7.Cramp S., Simmons K. E. L.1983. Handbook of the Birds of Europe, the Middle East and North Africa. Vol.3. Oxford Univ. Press, Oxford. [Google Scholar]
- 8.Del Hoyo J., Elliott A., Sargatal J.1996. Laridae gulls. pp. 572–623. In: Handbook of the Birds of the World, vol. 3: Lynx ed., Barcelona. [Google Scholar]
- 9.Dwight J.1925. Gulls (Laridae) of the world, their plumages, moults, variation, relationships and distribution. Bull. Am. Mus. Nat. Hist. 52: 63–408. [Google Scholar]
- 10.Fair J. M., Paul E., Jones J., Clark A. B., Davie C., Kaiser G.2010. Guidelines to the Use of Wild Birds in Research. 3rd ed., The Ornithological Council, Washington, D.C. [Google Scholar]
- 11.Fridolfsson A. K., Ellegren H.1999. A simple and universal method for molecular sexing of non-ratite birds. J. Avian Biol. 30: 116–121. doi: 10.2307/3677252 [DOI] [Google Scholar]
- 12.Fudge A. M.2000. Laboratory Medicine: Avian and Exotic Pets. W.B. Saunders, Philadelphia. [Google Scholar]
- 13.Gorski W., Jakuczun B., Nitecki C., Petryna A.1977. Investigation of oil pollution on the Polish Baltic coast in 1974–1975. Przeglad Zoologiczny. 21: 20–23. [Google Scholar]
- 14.Guglielmo C. G., Williams T. D., Zwingelstein G., Brichon G., Weber J. M.2002. Plasma and muscle phospholipids are involved in the metabolic response to long-distance migration in a shorebird. J. Comp. Physiol. B 172: 409–417. doi: 10.1007/s00360-002-0266-z [DOI] [PubMed] [Google Scholar]
- 15.Harrison P.1985. Black-headed gull. p. 448. In: Seabirds: An Identification Guide, Revised ed., Christopher Helm, London. [Google Scholar]
- 16.Hujimaki Y.2010. Black-Headed Gull. p. 74. In: Check-List of Birds in Hokkaido, 3rd ed., Far Eastern Avian Study Group, Bibai (in Japanese). [Google Scholar]
- 17.IUCN. 2012. Larus ridibundus, Black-headed gull. online. In: The IUCN Red List of Threatened Species. Birdlife international, Cambridge. [Google Scholar]
- 18.Kanouchi T., Abe K., Ueda H.2013. Black-headed gull. pp. 306–307. In: Japanese Birds, New ed., Yama-kei Publishers, Tokyo (in Japanese). [Google Scholar]
- 19.Klaassen R. H. G., Ens B. J., Shamoun-Baranes J., Exo K., Bairlein F.2011. Migration strategy of flight generalist, the Lesser Black-backed gull Larus fuscus. Behav. Ecol. 23: 58–68. doi: 10.1093/beheco/arr150 [DOI] [Google Scholar]
- 20.Melville D. S., Shortridge K. F.2006. Migratory waterbirds and avian influenza in the East Asian-Australasian Flyway with particular reference to the 2003–2004 H5N1 outbreak. pp. 432–438. In: Waterbirds Around the World. The Stationery Office, Edinburgh. [Google Scholar]
- 21.Mercier F. M.1985. Fat reserves and migration of Red-necked Phalaropes (Phalaropus lobatus) in the Quoddy region, New Brunswick, Canada. Can. J. Zool. 63: 2810–2816. doi: 10.1139/z85-420 [DOI] [Google Scholar]
- 22.Ministry of the Environment. 2002. Black-headed gull. pp. 72–73. In: Atlas of Japanese Migratory Birds from 1961 to 1995. Ministry of the Environment, Tokyo (in Japanese). [Google Scholar]
- 23.Ministry of the Environment. 2006. Classification and main types of seabirds highly likely to suffer from oil pollution damage. p. 35. In: Ministry of the Environment 2006. Oil Pollution Relief Manual “Wild Birds guidelines for rescue of Oil Polluted Birds” Specified nonprofit organization wildlife rescue veterinary association, Tokyo (in Japanese). [Google Scholar]
- 24.Odum E. P., Perkinson J. D., Jr.1951. Relation of lipid metabolism to migration in birds; seasonal variation in body lipids of the migratory white-throated sparrow. Physiol. Zool. 24: 216–230. doi: 10.1086/physzool.24.3.30152115 [DOI] [PubMed] [Google Scholar]
- 25.Portugal S. J., Green J. A., Butler P. J.2007. Annual changes in body mass and resting metabolism in captive barnacle geese (Branta leucopsis): the importance of wing moult. J. Exp. Biol. 210: 1391–1397. doi: 10.1242/jeb.004598 [DOI] [PubMed] [Google Scholar]
- 26.Redfern C. P. F., Slough A. E. J., Dean B., Brice J. L., Jones P. H.2000. Fat and body condition in migrating Redwings Turdus iliacus. J. Avian Biol. 31: 197–205. doi: 10.1034/j.1600-048X.2000.310211.x [DOI] [Google Scholar]
- 27.Spano J. S., Pedersoli W. M., Kemppainen R. J., Krista L. M., Young D. W.1987. Baseline hematologic, endocrine, and clinical chemistry values in ducks and roosters. Avian Dis. 31: 800–803. doi: 10.2307/1591034 [DOI] [PubMed] [Google Scholar]
- 28.Sugawa W.1984. Increase in the population of Black-headed gull in Far East Asia. Marine Sciences Monthly 16: 194–198 (in Japanese). [Google Scholar]
- 29.Summers R., Piersma T., Strann K., Wiersma P.1998. How do purple sandpipers Calidris maritima survive the winter north of the arctic circle? Ardea 86: 51–58. [Google Scholar]
- 30.Takano S.2007. Black-headed gull. p. 375. In: A Field Guide to the Birds of Japan, Revised new ed., Wild Bird Society of Japan, Tokyo (in Japanese). [Google Scholar]
- 31.Ueda F., Mochizuki M.2000. Wildlife as a monitor for the environmental pollution. J. Med. Sci. 53: 723–727 (in Japanese). [Google Scholar]