Abstract
Clofazimine, a lipophilic (log P = 7.66) riminophenazine antibiotic approved by the US Food and Drug Administration (FDA) with a good safety record, was recently identified as a lead hit for cryptosporidiosis through a high-throughput phenotypic screen. Cryptosporidiosis requires fast-acting treatment as it leads to severe symptoms which, if untreated, result in morbidity for infants and small children. Consequently, a fast-releasing oral formulation of clofazimine in a water-dispersible form for pediatric administration is highly desirable. In this work, clofazimine nanoparticles were prepared with three surface stabilizers, hypromellose acetate succinate (HPMCAS), lecithin, and zein, using the flash nanoprecipitation (FNP) process. Drug encapsulation efficiencies of over 92% were achieved. Lyophilization and spray-drying were applied and optimized to produce redispersible nanoparticle powders. The release kinetics of these clofazimine nanoparticle powders in biorelevant media were measured and compared with those of crystalline clofazimine and the currently marketed formulation Lamprene. Remarkably improved dissolution rates and clofazimine supersaturation levels up to 90 times equilibrium solubility were observed with all clofazimine nanoparticles tested. Differential scanning calorimetry indicated a reduction of crystallinity of clofazimine in nanoparticles. These results strongly suggest that the new clofazimine nanoparticles prepared with affordable materials in this low-cost nanoparticle formulation process can be used as viable cryptosporidiosis therapeutics.
Keywords: clofazimine, flash nanoprecipitation, cryptosporidiosis, hypromellose acetate succinate, lecithin, zein, lyophilization, spray-drying
Introduction
Clofazimine presents with a red color and is a lipophilic riminophenazine antibiotic which has been recommended by the World Health Organization (WHO) and prescribed with other medicines for the treatment of leprosy as a multidrug therapy (MDT) for over four decades. Due to its low water solubility and bioavailability, it has been formulated in gelatin capsules as a microcrystalline suspension in an oil–wax base, marketed under the trade name Lamprene. The hydrophobicity compromises bioavailability, and peak plasma concentration was reached only after 8 h when taken with food while 12 h was needed if ingested without food.1−3
Recently, clofazimine was also identified as a potential new treatment of cryptosporidiosis through a high-throughput phenotypic screen.4 Cryptosporidiosis is a leading cause of diarrhea in children in the developing world, caused by Cryptosporidium infections in the intestine. Unlike leprosy, which is a chronic disease and long-term infection, cryptosporidiosis requires fast-acting treatment, as it leads to severe symptoms such as dehydration, vomiting, and fever, contributing to morbidity in infants and small children.5,6 Given the relatively slow absorption of Lamprene and high interpatient variability, a faster-releasing and more bioavailable formulation of clofazimine is highly desirable.7
Clofazimine is categorized as a Biopharmaceutics Classification System (BCS) class II drug because of its poor aqueous solubility and high permeability.8 Consequently, the absorption of clofazimine is dissolution-limited and the focus of formulation development is solubility enhancement.9,10 To improve the oral bioavailability and dissolution rate of poorly water-soluble drugs, two strategies are usually applied: increasing the specific surface area (i.e., surface area/mass) and producing amorphous forms of the drug, which improve both dissolution kinetics and supersaturation levels.11,12 However, for many class II drugs, reduction of particle size to the micrometer range by conventional techniques (e.g., milling) is not adequate to overcome the low bioavailability.13,14
Flash nanoprecipitation (FNP) is a copolymer-directed assembly process that can produce nanometer-sized particles with an active pharmaceutical ingredient (API) partitioned into the core in a substantially amorphous state. In the FNP process, amphiphilic stabilizers and hydrophobic APIs are molecularly dissolved in an organic phase and mixed rapidly with an antisolvent stream to drive controlled precipitation with tunable submicrometer particle size (∼50–500 nm) and narrow size distribution.15,16 In this work, instead of expensive copolymers,17 three affordable amphiphilic stabilizers, namely, hypromellose acetate succinate (HPMCAS), lecithin, and zein, were investigated to prepare clofazimine nanoparticles (NPs) with optimal drug dissolution rate enhancement. HPMCAS has been widely used as a carrier in solid dispersions formed by spray-drying18−20 and hot melt extrusion.21 Lecithin is a well-established pharmaceutical excipient of natural origin which functions as a surfactant.22−24 Zein is a low cost, generally regarded as safe (GRAS) prolamin protein from corn. It is water-insoluble owing to its high content (>50%) of nonpolar amino acids, but has much improved solubility in binary solvents containing aliphatic alcohol and water (50–95%).25,26 Thus, it functions as a barrier layer and steric stabilizer. Zein has a specific interaction with the protein casein, and the two are commonly used in combination to produce steric stabilization.
Oral dosage forms (solid or liquid) are the preferred drug administration route owing to ease of ingestion and high patient compliance, when the pharmacokinetics of the drug permits.27,28 Compared with liquid dispersions of drug nanoparticles, which are susceptible to degradation, aggregation, sedimentation, and recrystallization, solid dosage forms are generally preferred. Dry nanoparticle powders that can be readily reconstituted prior to administration are especially attractive for pediatric formulations.29 Upon successful preparation of clofazimine nanoparticles with the three stabilizers via FNP, lyophilization and spray-drying conditions were optimized to obtain redispersible nanoparticle powders, which were subsequently tested in simulated gastric and intestinal fluids in vitro to study the influence of stabilizer types on the release profile of clofazimine.
Materials and Methods
Materials
Clofazimine (Cfz), casein sodium salt (NaCas) from bovine milk, mannitol, sucrose, trehalose, and solvents (HPLC grade) were purchased from Sigma-Aldrich (Milwaukee, WI) and used as received. Zein Pharma grade (Non-GMO) was purchased from Flo Chemical Corporation (Ashburnham, MA). AFFINISOL hypromellose acetate succinate (HPMCAS) 126, 716, and 912 and METHOCEL HPMC E3 (viscosity of 2.4–3.6 mPa at 2% solution in water at 20 °C) were a gift from Dow Chemical Company (Midland, MI). l-α-Lecithin was purchased from Fisher Scientific (Waltham, MA). FaSSIF/FeSSIF/FaSSGF and FeSSIF-V2 powders were purchased from Biorelevant.com (London, U.K.). Deionized (DI) water (18.2 MΩ·cm) was prepared by a NANOpure Diamond UV ultrapure water system (Barnstead International, Dubuque, IA).
Clofazimine Solubility
An excess of clofazimine powder was added to simulated fasted-state gastric fluid (FaSSGF), simulated fasted-state intestinal fluid (FaSSIF), and simulated feasted-state intestinal fluid (FeSSIF) buffer, respectively, followed by slow rotation using a Glas-Col rotator (Terra Haute, IN) for 24 h to allow equilibration.9 The resulting solution was centrifuged at 28000g for 2 min to remove undissolved drug. The supernatant was then transferred and analyzed by UV–vis spectrometry. The respective clofazimine concentration was calculated using a calibration curve with known standard solutions (Figure S1).
Nanoparticle Fabrication and Characterization
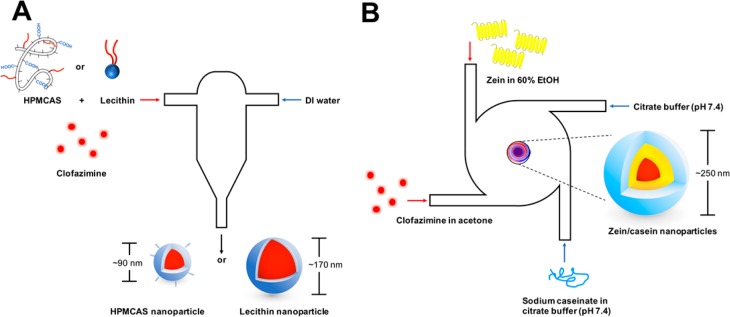
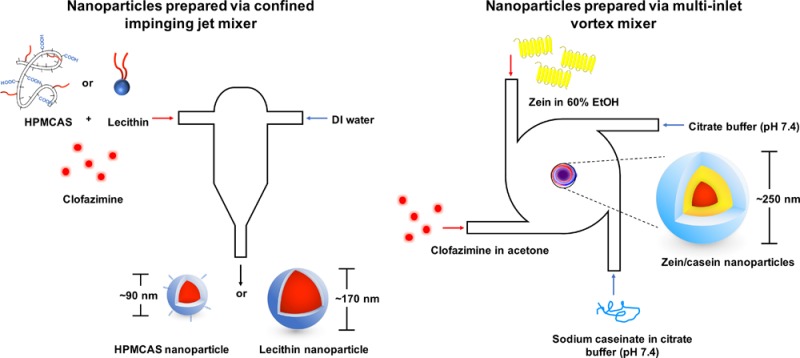
Nanoparticles were prepared via FNP as described previously.30 Specifically, HPMCAS and lecithin stabilized nanoparticles were prepared with a confined impinging jet (CIJ) mixer (Scheme 1). In brief, clofazimine and stabilizers were dissolved in 0.5 mL of either acetone (HPMCAS) or tetrahydrofuran (THF, lecithin), followed by rapid mixing against an antisolvent DI water stream (0.5 mL) via a CIJ mixer. The mixture was subsequently dispersed in 4 mL of DI water, decreasing the organic solvent to 10 vol %.
Scheme 1. Schematic Representation of Nanoparticles Made with CIJ Mixer Using HPMCAS or Lecithin (A) and MIVM Using Zein as Surface Stabilizer (B).
Zein nanoparticles were prepared as previously described with slight adaptions using a multi-inlet vortex mixer (MIVM).31 Zein was dissolved at 6 mg/mL in 60% ethanol (EtOH) in DI water while NaCas was dissolved at 1 mg/mL in citrate buffer at pH 7.4 (10 mM sodium citrate, pH adjusted with citric acid). In addition, clofazimine was dissolved in acetone at 6 mg/mL. The clofazimine stream and zein stream (12 mL/min) were mixed against a NaCas stream and a citrate buffer stream (36 mL/min) within the MIVM, resulting in a final organic concentration of 20 vol %. As organic solvents increase the mobility of drug and compromise the stability of nanoparticle suspensions, the resultant suspension was further diluted with the same volume of citrate buffer to decrease organic solvents to 10 vol %.
Nanoparticle size and polydispersity index (PDI) were assessed by dynamic light scattering (DLS) using a Zetasizer Nano-ZS (Malvern Instruments, Southboro, MA) at 25 °C with a detection angle of 173° in triplicate. The Z-average size is the intensity-weighted diameter obtained from fitting of the correlation function, and PDI is the polydispersity index obtained from the cumulant fitting program, and reported by the DLS instrument.32
Zeta potential was analyzed in zeta potential mode using the DLS instrument’s built-in Smoluchowski model. All results were reported as mean ± standard deviation around the mean. PDI data is provided in the Supporting Information.
Nanoparticle Lyophilization
Lyophilization was carried out using a benchtop VirTis Advantage (Gardiner, NY) with and without cryoprotectants (i.e., trehalose, sucrose, mannitol, and HPMC E3). 0.5 mL nanoparticle solutions were mixed with 0.1 mL cryoprotectant solutions at different concentrations to afford various final NP:cryoprotectant weight ratios up to 1:30. The mixtures were then flash frozen by fast immersion in a dry ice/acetone cooling bath (−78 °C) for 1 min with mild agitation. The frozen samples were then immediately transferred to a lyophilizer with shelf temperature at −20 °C under vacuum (<1 × 10–3 bar). After 2 days, dried powders were removed, sealed, and stored at −20 °C. The effects of cryoprotectants at different concentrations were examined by reconstituting lyophilized nanoparticle powders in DI water at room temperature and subsequently analyzing by DLS. Sonication assistance was used when necessary to disperse powders for DLS measurements.
Zein Nanoparticle Spray Drying
The zein nanoparticle dispersion was spray-dried using a mini spray drier B-290 (BÜCHI Corporation, New Castle, DE). A number of process factors were optimized, including inlet and outlet temperatures, aspirator rate, spraying gas (N2) flow rate, and liquid feed rate. Spray-dried nanoparticle powders were collected in scintillation vials, sealed, and stored at −20 °C before use. To determine particle size, the powders were deposited on a microscope slide and observed under a bright-field microscope (Nikon Eclipse E200, Minato, Tokyo, Japan) with 40× magnification.
Loading Capacity (LC) and Encapsulation Efficiency (EE)
To determine the LC and EE of clofazimine in lyophilized or spray-dried samples, powders with known mass (∼5 mg) were dissolved in 1 mL of THF. Due to the poor solubility of zein in THF, clofazimine remained trapped in zein nanoparticles and led to incomplete solubilization. Thus, 0.1 mL of 80% EtOH in water was first added to dissolve the zein coatings before further dilution with 0.9 mL of THF. The insoluble inorganic salts were removed by centrifugation at 5000g for 2 min (Eppendorf Centrifuge 5430R, Eppendorf, Hamburg, Germany). The supernatant was further diluted with THF as necessary, and the concentration of clofazimine was determined on a UV–vis spectrophotometer at 450 nm (Evolution 300 UV–vis, Thermo Electron, Waltham, MA) and quantified based on a calibration curve of clofazimine from known standard solutions (Figure S1). The LC and EE of samples were calculated with the following equations:
Differential Scanning Calorimetry (DSC)
DSC measurements were performed on a TA Instruments Q200 (New Castle, DE). Samples (5–10 mg) were weighed in aluminum pans and equilibrated at 20 °C under dry N2 atmosphere (50 mL/min). Subsequently, the samples were heated from 20 to 250 °C at a heating rate of 5 °C/min. The scan was analyzed by TA Instruments Universal Analysis 2000 software.
Release Kinetics in Vitro
Simulated gastric fluid (FaSSGF) and intestinal fluids (FaSSIF and FeSSIF) were prepared according to the manufacturer’s instructions. Each formulation was evaluated in triplicate with a release medium swap assay. Additionally, dissolution tests were also performed with clofazimine powder and Lamprene as controls.
Release under Gastric Conditions
Nanoparticle powder samples were resuspended in prewarmed FaSSGF (37 °C) to achieve a drug concentration of ∼75 μg/mL by pipetting up and down vigorously multiple times. The samples were incubated at 37 °C (NesLab RTE-111 bath circulator, Thermo Fisher Scientific, Waltham, MA) for 30 min without agitation to mimic physiological gastric conditions and transition time in the stomach. Aliquots were taken at 1, 5, 10, 15, 20, and 30 min. To analyze the free drug concentration, each aliquot was centrifuged at 28000g for 5 min to pellet nanoparticles. The supernatant was diluted further with FaSSGF to fall within the calibration range, and clofazimine concentration was determined by UV–vis spectrometer at 491 nm.
Release under Intestinal Conditions
After passing through the 30 min FaSSGF protocol, the solutions were further diluted with 1.1× FaSSIF (pH 6.5) or FeSSIF (pH 5.8), resulting in a final clofazimine concentration lower than its solubility limit in both buffers. Aliquots were taken at 15, 30, 45, 60, 120, 240, and 360 min after the pH shift and were centrifuged at 28000g for 10 min. The drug concentration in the supernatant was analyzed via UV–vis spectrometer at 491 nm and calculated based on a calibration curve.
Results and Discussion
Clofazimine Nanoparticles
HPMCAS is a cellulosic polymer synthesized by esterification of HPMC with acetic anhydride and succinic anhydride. It has been widely utilized to prepare stable amorphous solid dispersions of poorly soluble drugs,19 particularly through spray-drying processes.20 However, few efforts have been made to fully exploit the potential of HPMCAS as surface stabilizers to make nanoparticles.33 Therefore, three HPMCAS polymers with different substitution ratios of succinyl and acetyl groups were studied in order to compare their ability to stabilize clofazimine nanoparticles during the FNP process. HPMCAS 126 has the highest acetyl substitution level and is therefore the most hydrophobic of the three HPMCAS polymers tested. HPMCAS 716 is the most hydrophilic.
When the organic solvent and antisolvent stream are impinged together during FNP, sufficiently high clofazimine supersaturation must be achieved to drive nucleation and nanoparticle formation. Thus, common water-miscible organic solvents (e.g., acetone, THF, dimethyl sulfoxide) were screened as candidates for the organic stream in the FNP process. Acetone was selected due to the low solubility of clofazimine in the final mixed solvent. Using acetone as the organic solvent, all HPMCAS polymers were able to form clofazimine nanoparticles, with sizes ranging from 70 to 100 nm, and narrow particle size distributions (PDI 0.19–0.26). The small particle size indicated fast precipitation of hydrophobic clofazimine from the acetone stream upon homogeneous mixing with the antisolvent aqueous stream in the confined mixing chamber of CIJ mixer. The net negative zeta potential (−25 to −29 mV), due to the ionized HPMCAS surface coating, provided electrostatic stabilization of the nanoparticles. However, the size stability among the three nanoparticle formulations was significantly different at room temperature, as shown in Figure 1. For HPMCAS 716 nanoparticles, particle size increased rapidly within 3 h, indicating aggregation. In contrast, HPMCAS 126 nanoparticles remained at a constant size for at least 6 h, allowing sufficient time for processing into dry powders. This observed disparity in stability is likely attributed to the stronger hydrophobic interactions of HPMCAS 126 with the clofazimine core, owing to its higher degree of acetyl group substitution. Due to the superior size stability of clofazimine nanoparticles stabilized by it, HPMCAS 126 was selected for further study.
Figure 1.
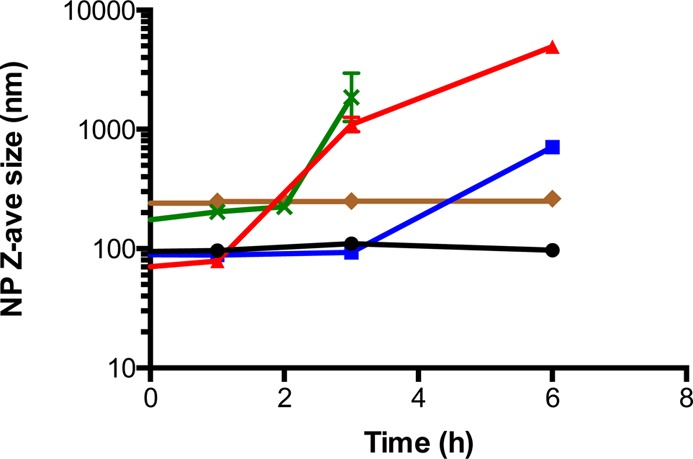
Size stability of clofazimine nanoparticles with various surface coatings in 10 vol % organics (HPMCAS 126, black ●; HPMCAS 716, red ▲; HPMCAS 912, blue ■; lecithin, green ×; zein, brown ◆).
Lecithin by itself or in combination with other polymers (e.g., chitosan) has been exploited to prepare nanoparticles for various drug delivery applications.34,35 Lecithin is a generic term for a mixture of phospholipids derived from plant or animal sources; this study was carried out using l-α-Lecithin derived from soybean. Owing to the poor solubility of lecithin in acetone, the preferred solvent for clofazimine precipitation, THF was instead used to dissolve both drug and amphiphilic stabilizer lecithin. After FNP, lecithin formed nanoparticles 175 nm in diameter (PDI 0.16). After 3 h, particles increased in size and became too large for accurate DLS sizing. The reduced size stability may be attributed to the fact that lecithin, unlike polymeric HPMCAS, is a small molecular weight molecule with short hydrophobic tail and zwitterionic headgroup, which creates only a thin protective layer on the particle surface. The high clofazimine solubility in 10 vol % THF along with ease of migration through thin lecithin stabilizer layer likely resulted in the fast recrystallization of clofazimine in the aqueous phase. The shorter stability time, relative to HPMCAS-based clofazimine particles, necessitated rapid removal of solvents from the lecithin nanoparticles. Accordingly, lyophilization of lecithin clofazimine nanoparticles for subsequent release assays was carried out immediately after FNP.
Zein has a characteristic solubility in 50–90% aqueous ethanol36 whereas clofazimine is only slightly soluble in this solvent mixture. Therefore, another mixing geometry, the MIVM with four feed streams, was used to accommodate different solvents requirements of the API and zein. The zein was introduced in a mixed EtOH:water stream, and the clofazimine was introduced in an acetone stream.
A second protein, NaCas, was introduced in citrate buffer stream as a secondary stabilizer. NaCas absorbs on zein surfaces, thereby reducing surface hydrophobicity and providing electrostatic stabilization.25 Most notably, it has been demonstrated that significantly improved redispersibility of lyophilized37 and spray-dried38 zein nanoparticles was achieved after treatment with NaCas. The resulting nanoparticle dispersion from the MIVM, containing zein/NaCas particles with encapsulated clofazimine, consisted of 20 vol % organics (acetone and EtOH) that led to appreciable Ostwald ripening and clofazimine recrystallization.39 However, if the suspension was diluted 2-fold with citrate buffer immediately after preparation (10% organics, Figure 1), zein nanoparticles had greatly enhanced stability and only a minor size increase from 240 to 262 nm after 6 h.
Drying of Clofazimine Nanoparticles and Redispersion
Lyophilization, or freeze-drying, is a standard procedure to produce dry pharmaceutical powders. However, mechanical stresses during freezing often irreversibly induce nanoparticle aggregation.40 As a result, cryoprotectants are required as excipients to inhibit interactions between nanoparticles during the freezing process and thus preserve redispersibility.
Three sugars, sucrose, trehalose, and mannitol, as well as HPMC E3 (a water-soluble HPMC polymer) were screened at different NPs:cryoprotectant mass ratios. For clofazimine-encapsulating HPMCAS 126 or lecithin nanoparticles, with all cryoprotectants tested, the particle sizes were considerably larger (on the order of μm) upon redispersion, which prevented accurate size determination by DLS. Despite the size increase, lyophilization cakes (lyo cakes) with short reconstitution times were obtained from HPMCAS 126 nanoparticles protected by HPMC E3 at NP:cryoprotectant mass ratios as low as 1:0.5. In contrast, dense lyo cakes were observed for all of the simple sugar cryoprotectants. Higher cryoprotectant concentrations only marginally reduced particle size and improved size distribution for all cryoprotectants. To maximize the drug loading in final formulation while maintaining good redispersibility, HPMCAS 126 nanoparticles with HPMC E3 cryoprotectant at a ratio of 1:0.5 were used for the following dissolution tests. For the lecithin nanoparticles, the nanoparticles lyophilized with 3× mannitol produced a more desirable, less dense lyo cake; however, there was still an increase in particle size after reconstitution.
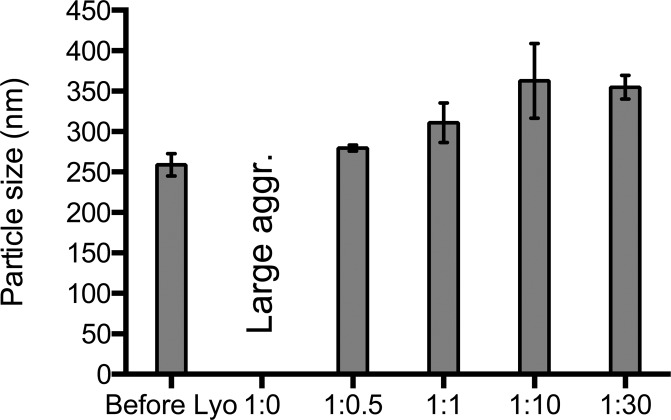
Unlike HPMCAS 126 and lecithin, lyophilized zein nanoparticles were easily redispersed to nanoscale size with mannitol, sucrose, and trehalose sugars. Mannitol was used as the cryoprotectant in the final formulation for the following dissolution study due to its low hygroscopicity and ability to form readily redispersible lyo cakes.41 At NP:mannitol mass ratios from 1:0.1 to 1:0.5, red precipitates were observed within 45 min after redispersion, suggesting compromised nanoparticle stability. Increased stability was found for ratios above 1:0.5; however, upon redispersion, there was an increase in nanoparticle size, as shown in Figure 2. Of the NP:mannitol mass ratios tested, the 1:1 ratio was deemed optimal.
Figure 2.
Particle size and PDI of clofazimine zein nanoparticles after lyophilization with different concentrations of mannitol as cryoprotectant.
Spray-drying is an alternative method to prepare nanoparticle powders from liquid suspensions. Compared with lyophilization, spray-drying is a high-throughput process that is fast, scalable, and cost-effective. The influence of processing methods on resulting solid properties was studied using zein nanoparticles made via an MIVM in 10 vol % organic solvents. The optimized conditions were as follows: inlet temperature of 160 °C, aspiration rate of 90%, spray gas flow rate at 250 NL/h, and a sample feed rate of 9 mL/min. As measured by microscopy, spray-drying produced fine particles with median diameter of 2.3 μm based on number distribution (Figure 3A). The capillary forces during spray-drying produced more dense particles and powders compared to powders from lyophilized samples.42 The spray-dried zein nanoparticles were redispersed to nanoscale size easily upon addition of DI water, with no need for mannitol (Figure 3B). Unlike zein, the spray-dried HPMCAS or lecithin solid particles could only be redispersed to micrometer-sized colloids, even when large amounts of HPMC E3 or mannitol were added as excipients. Therefore, in the following discussions, we only focused on the spray-dried zein samples without additional drying excipients.
Figure 3.
Microscope image of spray-dried zein nanoparticles (A) and particle size of fresh nanoparticle before spray-drying (SD) process and reconstitution in DI water.
LC and EE
The amount of clofazimine in the nanoparticles (i.e., LC and EE) was determined by UV–vis spectroscopy after solidification and is summarized in Table 1. Overall, high encapsulation efficiency was achieved by FNP processes for all stabilizers. HPMCAS and lecithin nanoparticles prepared by the CIJ mixer achieved 98.7% encapsulation efficiency. The slightly lower encapsulation efficiency for the lyophilized zein formulation (92.8%) was likely caused by API loss during operation as the MIVM, which has a larger holdup volume than the CIJ mixer. Spray-dried zein nanoparticles had an encapsulation efficiency (92.1%) almost identical to that of lyophilized samples, demonstrating low drug loss during the spray-drying process. HPMCAS 126 afforded the highest drug loading (32.9%) as a result of the low cryoprotectant concentration required for good redispersibility.
Table 1. Characterization of Clofazimine Loaded Nanoparticles Prepared with Different Stabilizers.
|
Z-average (nm) |
PDI |
zeta
potential (mV) |
||||||
|---|---|---|---|---|---|---|---|---|
| stabilizer | before processing | redispersion | before processing | redispersion | before processing | redispersion | LC (%) | EE (%) |
| HPMCAS 126 | 94 ± 1 | micrometer sizea | 0.24 ± 0.02 | na | –28.7 ± 3.2 | na | 32.9 | 98.7 |
| HPMCAS 716 | 71 ± 1 | na | 0.26 ± 0.02 | na | –29.1 ± 3.2 | na | na | na |
| HPMCAS 912 | 89 ± 1 | na | 0.18 ± 0.02 | na | –25.1 ± 2.7 | na | na | na |
| lecithin | 175 ± 4 | micrometer sizeb | 0.16 ± 0.06 | na | –52.3 ± 2.5 | na | 16.4 | 98.7 |
| zein | 240 ± 3 | 311 ± 24c/317 ± 7d | 0.11 ± 0.04 | 0.38 ± 0.08c/0.31 ± 0.03d | –46.4 ± 0.4 | –51.0 ± 2.5c/–47.4 ± 0.8d | 11.0c/10.5d | 92.8c/92.1d |
Lyophilized HPMCAS 126 nanoparticles with 1:0.5 HPMC E3.
Lyophilized lecithin nanoparticles with 1:3 mannitol.
Lyophilized zein nanoparticles with 1:1 mannitol.
Spray-dried zein nanoparticles.
DSC
Calorimetric techniques are useful for characterizing the physical state of a drug in a polymeric matrix. The DSC curve of clofazimine crystals exhibited a sharp melting endotherm at 223.3 °C, followed by an exothermal peak due to degradation, which was in good agreement with previous reports.8 Lower crystallinity of the drug in nanoparticle form was indicated by a shifting of the melting peak toward lower temperature, as well as peak broadening.43,44 As depicted in Figure 4, small, broad melting peaks (shown in dashed box) were observed at 222.5, 200.3, and 212.0 °C, respectively, for lyophilized HPMCAS 126, lecithin, and zein nanoparticles. These decreased melting temperatures and reduced endotherms demonstrate the substantially amorphous state of the clofazimine produced in the FNP process.45
Figure 4.
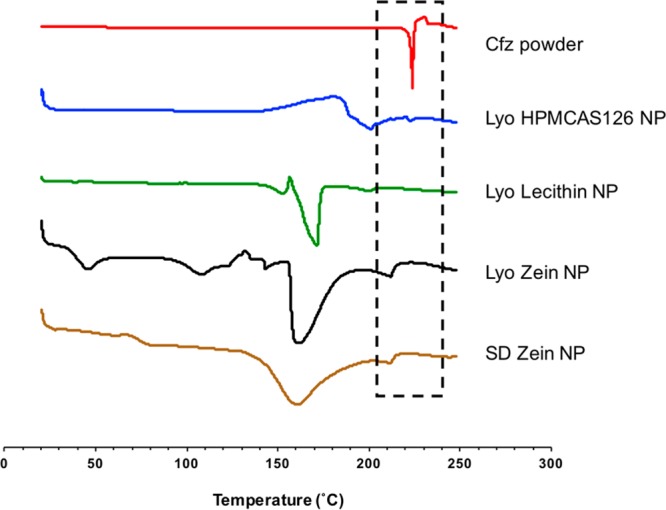
DSC thermograms of clofazimine powder and nanoparticle with different stabilizers and corresponding cryoprotectants. Lyo, lyophilization; SD, spray-drying. The region in the dashed box is the highlighted clofazimine peak. (See Figure S5 for DSC of control samples.)
Interestingly, the additional heating step during spray-drying resulted in different DSC traces of zein/NaCas proteins between 20 and 150 °C. In comparison with lyophilized zein nanoparticles, the absence of endotherms for spray-dried zein nanoparticles around 45 and 108 °C indicated denaturing of the zein and/or casein during spray-drying.46
Release Kinetics in Vitro
Clofazimine is practically insoluble in water (log P = 7.66), but has high permeability. Consequently, dissolution rate is the key determinant in the bioavailability of clofazimine.9 The 24 h solubility of clofazimine in FaSSGF, FaSSIF, and FeSSIF was determined to be 0.36, 6.20, and 29.60 μg/mL, respectively. The significantly enhanced bioavailability in FaSSIF and FeSSIF is contributed by the presence of bile salt micelles in intestinal fluids, which is a well-studied phenomenon.47
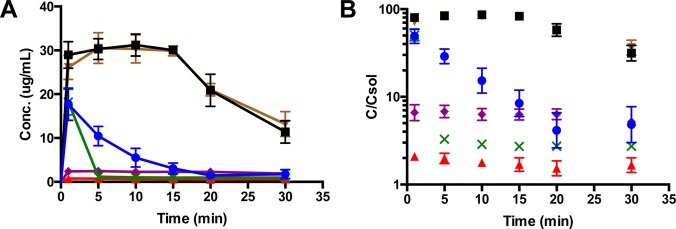
Biorelevant dissolution tests, which simulate physiological conditions in the gastrointestinal (GI) tract, were carried out to compare release kinetics of clofazimine nanoparticles prepared with different surface stabilizers. Dissolution in FaSSGF was studied by dispersing lyophilized or spray-dried clofazimine samples at concentration equal to ∼208× the solubility of free clofazimine powder, with the results shown in Figures 5A and 5B. As expected, the clofazimine powder had extremely low bioavailability. The clofazimine waxy suspension withdrawn from Lamprene capsules had a solubility that plateaued at ∼2.2 μg/mL (∼6× solubility) throughout the 30 min incubation at 37 °C. HPMCAS 126 and lecithin samples reached maximum drug concentration almost instantly (t = 1 min) after dispersion, with concentrations corresponding to a nearly 50-fold solubility enhancement. The decay in supersaturation for lecithin was considerably faster than for the HPMCAS 126 stabilizer. This result is consistent with the known inhibitory effect of HPMCAS on recrystallization of API from a supersaturated solution.19
Figure 5.
Released clofazimine concentration and supersaturation level of clofazimine nanoparticles with different stabilizers (Lyo HPMCAS 126 NP, blue ●; Lyo lecithin NP, green × ; Lyo zein NP, black ■; SD zein NP, brown ▼), compared to free clofazimine (Cfz, red ▲) powder and commercial product Lamprene (purple ◆) in FaSSGF. The supersaturation level was calculated as released clofazimine concentration divided by clofazimine solubility in FaSSGF.
The zein nanoparticles were by far the best stabilizer for clofazimine in the gastric fluid dissolution test. They exhibited a much higher supersaturation (80× for Lyo and 72× for SD) than HPMC or lecithin. The area under the curve (AUC) of clofazimine concentration over time from zein NPs was 4 times higher than from HPMC and 6.5 times higher than from lecithin (668 μg·min/mL, 170 μg·min/mL, and 100 μg·min/mL for zein, HPMCAS, and lecithin, respectively).
A control experiment, suspending nanoparticle powders directly with DI water, was also performed at 37 °C for 30 min to investigate the influence of pH on clofazimine dissolution rate (Figure S6). A much lower drug concentration (∼2–5 μg/mL) was detected for all formulations, and no precipitation of clofazimine was observed based on visual inspection. Therefore, if clofazimine powders were dispersed in water for pediatric administration, there would be negligible drug release until the powders entered the stomach. Due to the enhanced solubility of the weak base clofazimine under the low pH condition in the stomach, the drug would begin release and dissolution.
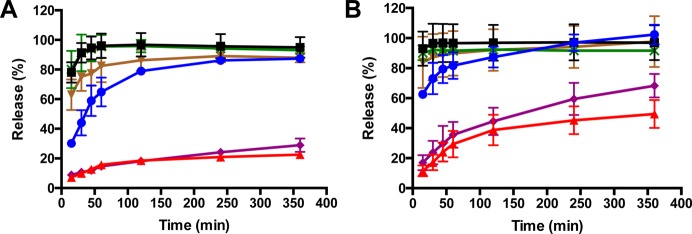
After 30 min initial exposure at pH 1.6 to simulate stomach conditions, a pH shift experiment was carried out by diluting the NP/gastric fluid solution into FaSSIF or FeSSIF, as shown in Figures 6A and 6B for fasted conditions and feasted conditions, respectively. In this experiment, the entire contents of the sample from the gastric incubation were transferred into the FaSSIF or FeSSIF media. This means that the released clofazimine at the end of the gastric fluid incubation, recrystallized clofazimine, and clofazimine still encapsulated in NPs were all included in the 6 h intestinal fluid runs. As a result of 5× higher bile salts concentrations and slightly lower pH value in fed state, all tested samples demonstrated faster release in FeSSIF. For example, only 22.5% of drug from clofazimine powder was released in FaSSIF after 6 h whereas 49.5% release was achieved in FeSSIF. Although Lamprene demonstrated faster release than clofazimine powder, it was still not completed within even 6 h.
Figure 6.
Dissolution of clofazimine nanoparticles with different stabilizers (Lyo HPMCAS 126 NP, blue ●; Lyo lecithin NP, green ×; Lyo zein NP, ■; SD zein NP, brown ▼), compared to free clofazimine (Cfz, red ▲) powder and commercial product Lamprene (purple ◆) in FaSSIF (A) and FeSSIF (B).
Among the three stabilizers, HPMCAS 126 nanoparticles exhibited the slowest release. Interestingly, while lecithin samples appeared to have recrystallized more under gastric fluid conditions, lecithin nanoparticles exhibited comparable fast release profile to lyophilized zein formulations in both simulated intestinal fluids, which suggested that the redispersibility is not the sole factor that determines the release behaviors. It is likely that excess lecithin stabilizer modified the form of the clofazimine that recrystallized during the 30 min in gastric conditions, such that the drug was more susceptible to dissolution under the intestinal fluid conditions. For lyophilized lecithin and zein nanoparticles, roughly 30 min was required for complete release in fed state at intestine, while nearly 60 min was needed in fasted state. Compared with zein nanoparticles prepared by lyophilization, spray-dried zein nanoparticles displayed similar, rapid release behavior. Therefore, a dry powder process based on spray-drying is a viable route for commercialization. This fast-releasing behavior is highly desirable in the intestine as Cryptosporidium infections mostly reside in the intestine.4
Conclusion
To improve the dissolution characteristics of the poorly soluble drug clofazimine as a therapeutic for cryptosporidiosis, clofazimine nanoparticles were successfully developed with three stabilizers, HPMCAS, lecithin, and zein, through the FNP process with high encapsulation efficiency (>92%). Among three HPMCAS polymers with different substitution ratios of succinyl and acetyl groups, the most hydrophobic, HPMCAS 126, demonstrated the greatest particle size stability after being used to encapsulate clofazimine. Solidification of liquid colloidal nanoparticles was accomplished by lyophilization and spray-drying. For the lyophilization process, cryoprotectants were required for all formulations to produce a product cake with a short reconstitution time. Lyophilized HPMCAS 126 and lecithin nanoparticles showed some aggregation when redispersed with particle sizes of 1–2 μm. Zein exhibited extraordinary redispersibility to nanoscale size with only a slight size increase when used in combination with secondary stabilizer NaCas. Additionally, spray-dried zein particles demonstrated redispersbility comparable to lyophilized zein particles.
The dissolution behavior of clofazimine nanoparticles was evaluated by incubation in simulated stomach conditions, followed by a media swap into intestinal conditions. The newly developed clofazimine formulations in this work achieved significantly higher supersaturation levels (∼50–90×) in gastric fluid compared to clofazimine powder or the commercial product Lamprene. While the zein stabilizer maintained high levels of supersaturation during the 30 min gastric fluid test, the HPMCAS and lecithin nanoparticles showed significant decrease in supersaturation over the initial 15 min. The gastric fluid samples were then subjected to simulated fasted or feasted intestinal fluid (FaSSIF and FeSSIF) dissolution conditions. Neither the clofazimine API nor the commercial Lamprene showed complete dissolution during the 6 h release tests. In contrast, all of the FNP nanoparticle formulations showed complete release in intestinal conditions, despite the decrease in supersaturation they exhibited by the end of 30 min in gastric conditions as mentioned above.
These results demonstrate a route to economically produce clofazamine nanoparticle powders using FNP followed by spray-drying. A continuous, integrated process is clearly feasible, in which nanoparticles are produced continuously via FNP and fed in-line directly to a spray-drying unit. The low cost of goods is crucial to enable easy access of anticryptosporidium therapy to target patients in developing countries. However, there are still questions to be addressed, the chief of which is what stabilizer is optimal for the desired pediatric formulations for the treatment of cryptosporidiosis in infants. Zein, lecithin, and HPMCS stabilization provide fast dissolution and supersaturation, far superior to currently available formulations. Depending on the location of the Cryptosporidium in the GI tract, the slightly slower-releasing HPMCAS formulation may be desired. There is also the question of whether it is best to localize the drug in the intestines or to have it enter circulation to be systemically available. In addition, it is challenging to model the intestinal conditions during diarrhea in vitro. Clearly these questions will require future in vivo testing.
Acknowledgments
The work was supported by the Bill and Melinda Gates Foundation (BMGF, OPP1150755) and the National Science Foundation Graduate Research Fellowship (DGE-1656466) awarded to K.D.R. The authors thank Dr. Niya Bowers, Dr. Pius Tse, and Dr. Chih-Duen Tse for intellectual discussion. The authors also thank Dr. Kathryn Uhrich and Dr. Jason Hackenberg for access and assistance of DSC.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.molpharmaceut.7b00521.
Cfz structure, properties, and calibration curves, specifications of HPMCAS, PDI of Cfz NPs, appearance of Lyo and SD zein NPs, and NP release after redispersion in DI water (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Cholo M. C.; Steel H. C.; Fourie P. B.; Germishuizen W. A.; Anderson R. Clofazimine: current status and future prospects. J. Antimicrob. Chemother. 2012, 67 (2), 290–298. 10.1093/jac/dkr444. [DOI] [PubMed] [Google Scholar]
- O’Connor R.; O’Sullivan J. F.; O’Kennedy R. The Pharmacology, Metabolism, and Chemistry of Clofazimine. Drug Metab. Rev. 1995, 27 (4), 591–614. 10.3109/03602539508994208. [DOI] [PubMed] [Google Scholar]
- Schaad-Lanyi Z.; Dieterle W.; Dubois J.-P.; Theobald W.; Vischer W. Pharmacokinetics of clofazimine in healthy volunteers. Int. J. Lepr. Other Mycobact. Dis. 1987, 55 (1), 9–15. [PubMed] [Google Scholar]
- Love M. S.; Beasley F. C.; Jumani R. S.; Wright T. M.; Chatterjee A. K.; Huston C. D.; Schultz P. G.; McNamara C. W. A high-throughput phenotypic screen identifies clofazimine as a potential treatment for cryptosporidiosis. PLoS Neglected Trop. Dis. 2017, 11 (2), e0005373. 10.1371/journal.pntd.0005373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirley D.-A. T.; Moonah S. N.; Kotloff K. L. Burden of disease from Cryptosporidiosis. Curr. Opin. Infect. Dis. 2012, 25 (5), 555–563. 10.1097/QCO.0b013e328357e569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sow S. O.; Muhsen K.; Nasrin D.; Blackwelder W. C.; Wu Y.; Farag T. H.; Panchalingam S.; Sur D.; Zaidi A. K. M.; Faruque A. S. G.; Saha D.; Adegbola R.; Alonso P. L.; Breiman R. F.; Bassat Q.; Tamboura B.; Sanogo D.; Onwuchekwa U.; Manna B.; Ramamurthy T.; Kanungo S.; Ahmed S.; Qureshi S.; Quadri F.; Hossain A.; Das S. K.; Antonio M.; Hossain M. J.; Mandomando I.; Nhampossa T.; Acácio S.; Omore R.; Oundo J. O.; Ochieng J. B.; Mintz E. D.; O’Reilly C. E.; Berkeley L. Y.; Livio S.; Tennant S. M.; Sommerfelt H.; Nataro J. P.; Ziv-Baran T.; Robins-Browne R. M.; Mishcherkin V.; Zhang J.; Liu J.; Houpt E. R.; Kotloff K. L.; Levine M. M. The Burden of Cryptosporidium Diarrheal Disease among Children < 24 Months of Age in Moderate/High Mortality Regions of Sub-Saharan Africa and South Asia, Utilizing Data from the Global Enteric Multicenter Study (GEMS). PLoS Neglected Trop. Dis. 2016, 10 (5), e0004729. 10.1371/journal.pntd.0004729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huston C. D.; Spangenberg T.; Burrows J.; Willis P.; Wells T. N. C.; van Voorhis W. A Proposed Target Product Profile and Developmental Cascade for New Cryptosporidiosis Treatments. PLoS Neglected Trop. Dis. 2015, 9 (10), e0003987. 10.1371/journal.pntd.0003987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla G.; Nangia A. Clofazimine Mesylate: A High Solubility Stable Salt. Cryst. Growth Des. 2012, 12 (12), 6250–6259. 10.1021/cg301463z. [DOI] [Google Scholar]
- Narang A. S.; Srivastava A. K. Evaluation of Solid Dispersions of Clofazimine. Drug Dev. Ind. Pharm. 2002, 28 (8), 1001–1013. 10.1081/DDC-120006431. [DOI] [PubMed] [Google Scholar]
- Khadka P.; Ro J.; Kim H.; Kim I.; Kim J. T.; Kim H.; Cho J. M.; Yun G.; Lee J. Pharmaceutical particle technologies: An approach to improve drug solubility, dissolution and bioavailability. Asian J. Pharm. Sci. 2014, 9 (6), 304–316. 10.1016/j.ajps.2014.05.005. [DOI] [Google Scholar]
- Miller M. A.; DiNunzio J.; Matteucci M. E.; Ludher B. S.; Williams R. O.; Johnston K. P. Flocculated amorphous itraconazole nanoparticles for enhanced in vitro supersaturation and in vivo bioavailability. Drug Dev. Ind. Pharm. 2012, 38 (5), 557–570. 10.3109/03639045.2011.616513. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Chan J. W.; Moretti A.; Uhrich K. E. Designing polymers with sugar-based advantages for bioactive delivery applications. J. Controlled Release 2015, 219, 355–368. 10.1016/j.jconrel.2015.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dizaj S. M.; Vazifehasl Z.; Salatin S.; Adibkia K.; Javadzadeh Y. Nanosizing of drugs: Effect on dissolution rate. Res. Pharm. Sci. 2015, 10 (2), 95–108. [PMC free article] [PubMed] [Google Scholar]
- Kang C.; Sun Y.; Wang M.; Cheng X. Nanosized Camptothecin Conjugates for Single and Combined Drug Delivery. Eur. J. BioMed. Res. 2016, 2 (1), 8. 10.18088/ejbmr.2.1.2016.pp8-14. [DOI] [Google Scholar]
- Pustulka K. M.; Wohl A. R.; Lee H. S.; Michel A. R.; Han J.; Hoye T. R.; McCormick A. V.; Panyam J.; Macosko C. W. Flash Nanoprecipitation: Particle Structure and Stability. Mol. Pharmaceutics 2013, 10 (11), 4367–4377. 10.1021/mp400337f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson B. K.; Prud’homme R. K. Flash nanoprecipitation of organic actives and block copolymers using a confined impinging jets mixer. Aust. J. Chem. 2003, 56 (10), 1021–1024. 10.1071/CH03115. [DOI] [Google Scholar]
- Liu X.; Miller A. L. II; Yaszemski M. J.; Lu L. Biodegradable and crosslinkable PPF-PLGA-PEG self-assembled nanoparticles dual-decorated with folic acid ligands and Rhodamine B fluorescent probes for targeted cancer imaging. RSC Adv. 2015, 5 (42), 33275–33282. 10.1039/C5RA04096E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian F.; Wang J.; Hartley R.; Tao J.; Haddadin R.; Mathias N.; Hussain M. Solution Behavior of PVP-VA and HPMC-AS-Based Amorphous Solid Dispersions and Their Bioavailability Implications. Pharm. Res. 2012, 29 (10), 2766–2776. 10.1007/s11095-012-0695-7. [DOI] [PubMed] [Google Scholar]
- Tanno F.; Nishiyama Y.; Kokubo H.; Obara S. Evaluation of Hypromellose Acetate Succinate (HPMCAS) as a Carrier in Solid Dispersions. Drug Dev. Ind. Pharm. 2004, 30 (1), 9–17. 10.1081/DDC-120027506. [DOI] [PubMed] [Google Scholar]
- Friesen D. T.; Shanker R.; Crew M.; Smithey D. T.; Curatolo W. J.; Nightingale J. A. S. Hydroxypropyl Methylcellulose Acetate Succinate-Based Spray-Dried Dispersions: An Overview. Mol. Pharmaceutics 2008, 5 (6), 1003–1019. 10.1021/mp8000793. [DOI] [PubMed] [Google Scholar]
- Sarode A. L.; Obara S.; Tanno F. K.; Sandhu H.; Iyer R.; Shah N. Stability assessment of hypromellose acetate succinate (HPMCAS) NF for application in hot melt extrusion (HME). Carbohydr. Polym. 2014, 101, 146–153. 10.1016/j.carbpol.2013.09.017. [DOI] [PubMed] [Google Scholar]
- Schubert M. A.; Müller-Goymann C. C. Characterisation of surface-modified solid lipid nanoparticles (SLN): Influence of lecithin and nonionic emulsifier. Eur. J. Pharm. Biopharm. 2005, 61 (1–2), 77–86. 10.1016/j.ejpb.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Shi F.; Zhao J.-H.; Liu Y.; Wang Z.; Zhang Y.-T.; Feng N.-P. Preparation and characterization of solid lipid nanoparticles loaded with frankincense and myrrh oil. Int. J. Nanomed. 2012, 7, 2033–2043. 10.2147/IJN.S30085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H.; Yue Y.; Liu G.; Li Y.; Zhang J.; Gong Q.; Yan Z.; Duan M. Preparation and Characterization of a Lecithin Nanoemulsion as a Topical Delivery System. Nanoscale Res. Lett. 2010, 5 (1), 224–230. 10.1007/s11671-009-9469-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y.; Wang Q. Zein-based micro- and nano-particles for drug and nutrient delivery: A review. J. Appl. Polym. Sci. 2014, 10.1002/app.40696. [DOI] [Google Scholar]
- Podaralla S.; Perumal O. Influence of Formulation Factors on the Preparation of Zein Nanoparticles. AAPS PharmSciTech 2012, 13 (3), 919–927. 10.1208/s12249-012-9816-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaji J.; Patole V. Protein and Peptide Drug Delivery: Oral Approaches. Indian Journal of Pharmaceutical Sciences 2008, 70 (3), 269–277. 10.4103/0250-474X.42967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A.; Shi Y.; Yan Z.; Hao H.; Zhang Y.; Zhong J.; Hou H. Dosage form developments of nanosuspension drug delivery system for oral administration route. Curr. Pharm. Des. 2015, 21 (29), 4355–4365. 10.2174/1381612821666150901105026. [DOI] [PubMed] [Google Scholar]
- Lopez F. L.; Ernest T. B.; Tuleu C.; Gul M. O. Formulation approaches to pediatric oral drug delivery: benefits and limitations of current platforms. Expert Opin. Drug Delivery 2015, 12 (11), 1727–1740. 10.1517/17425247.2015.1060218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkerton N. M.; Grandeury A.; Fisch A.; Brozio J.; Riebesehl B. U.; Prud’homme R. K. Formation of Stable Nanocarriers by in Situ Ion Pairing during Block-Copolymer-Directed Rapid Precipitation. Mol. Pharmaceutics 2013, 10 (1), 319–328. 10.1021/mp300452g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissmueller N. T.; Lu H. D.; Hurley A.; Prud’homme R. K. Nanocarriers from GRAS Zein Proteins to Encapsulate Hydrophobic Actives. Biomacromolecules 2016, 17 (11), 3828–3837. 10.1021/acs.biomac.6b01440. [DOI] [PubMed] [Google Scholar]
- Frisken B. J. Revisiting the method of cumulants for the analysis of dynamic light-scattering data. Appl. Opt. 2001, 40 (24), 4087–4091. 10.1364/AO.40.004087. [DOI] [PubMed] [Google Scholar]
- Del Gaudio P.; Russo P.; Rodriguez Dorado R.; Sansone F.; Mencherini T.; Gasparri F.; Aquino R. P. Submicrometric hypromellose acetate succinate particles as carrier for soy isoflavones extract with improved skin penetration performance. Carbohydr. Polym. 2017, 165, 22–29. 10.1016/j.carbpol.2017.02.025. [DOI] [PubMed] [Google Scholar]
- Hafner A.; Lovrić J.; Pepić I.; Filipović-Grčić J. Lecithin/chitosan nanoparticles for transdermal delivery of melatonin. J. Microencapsulation 2011, 28 (8), 807–815. 10.3109/02652048.2011.622053. [DOI] [PubMed] [Google Scholar]
- Sonvico F.; Cagnani A.; Rossi A.; Motta S.; Di Bari M. T.; Cavatorta F.; Alonso M. J.; Deriu A.; Colombo P. Formation of self-organized nanoparticles by lecithin/chitosan ionic interaction. Int. J. Pharm. 2006, 324 (1), 67–73. 10.1016/j.ijpharm.2006.06.036. [DOI] [PubMed] [Google Scholar]
- Zhong Q.; Jin M. Zein nanoparticles produced by liquid–liquid dispersion. Food Hydrocolloids 2009, 23 (8), 2380–2387. 10.1016/j.foodhyd.2009.06.015. [DOI] [Google Scholar]
- Zhang Y.; Niu Y.; Luo Y.; Ge M.; Yang T.; Yu L.; Wang Q. Fabrication, characterization and antimicrobial activities of thymol-loaded zein nanoparticles stabilized by sodium caseinate–chitosan hydrochloride double layers. Food Chem. 2014, 142, 269–275. 10.1016/j.foodchem.2013.07.058. [DOI] [PubMed] [Google Scholar]
- Chen H.; Zhong Q. Processes improving the dispersibility of spray-dried zein nanoparticles using sodium caseinate. Food Hydrocolloids 2014, 35, 358–366. 10.1016/j.foodhyd.2013.06.012. [DOI] [Google Scholar]
- Zhu Z. Flash Nanoprecipitation: Prediction and Enhancement of Particle Stability via Drug Structure. Mol. Pharmaceutics 2014, 11 (3), 776–786. 10.1021/mp500025e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelwahed W.; Degobert G.; Stainmesse S.; Fessi H. Freeze-drying of nanoparticles: Formulation, process and storage considerations. Adv. Drug Delivery Rev. 2006, 58 (15), 1688–1713. 10.1016/j.addr.2006.09.017. [DOI] [PubMed] [Google Scholar]
- Guan T.; Miao Y.; Xu L.; Yang S.; Wang J.; He H.; Tang X.; Cai C.; Xu H. Injectable nimodipine-loaded nanoliposomes: Preparation, lyophilization and characteristics. Int. J. Pharm. 2011, 410 (1–2), 180–187. 10.1016/j.ijpharm.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Matteucci M. E.; Paguio J. C.; Miller M. A.; Williams R. O. III; Johnston K. P. Flocculated Amorphous Nanoparticles for Highly Supersaturated Solutions. Pharm. Res. 2008, 25 (11), 2477–2487. 10.1007/s11095-008-9659-3. [DOI] [PubMed] [Google Scholar]
- Sanna V.; Caria G.; Mariani A. Effect of lipid nanoparticles containing fatty alcohols having different chain length on the ex vivo skin permeability of Econazole nitrate. Powder Technol. 2010, 201 (1), 32–36. 10.1016/j.powtec.2010.02.035. [DOI] [Google Scholar]
- Zhang W.; Gong X.; Liu C.; Piao Y.; Sun Y.; Diao G. Water-soluble inclusion complex of fullerene with [gamma]-cyclodextrin polymer for photodynamic therapy. J. Mater. Chem. B 2014, 2 (31), 5107–5115. 10.1039/C4TB00560K. [DOI] [PubMed] [Google Scholar]
- Hu F.-Q.; Jiang S.-P.; Du Y.-Z.; Yuan H.; Ye Y.-Q.; Zeng S. Preparation and characteristics of monostearin nanostructured lipid carriers. Int. J. Pharm. 2006, 314 (1), 83–89. 10.1016/j.ijpharm.2006.01.040. [DOI] [PubMed] [Google Scholar]
- Müller V.; Piai J. F.; Fajardo A. R.; Fávaro S. L.; Rubira A. F.; Muniz E. C. Preparation and characterization of zein and zein-chitosan microspheres with great prospective of application in controlled drug release. J. Nanomater. 2011, 10.1155/2011/928728. [DOI] [Google Scholar]
- Moghimipour E.; Ameri A.; Handali S. Absorption-Enhancing Effects of Bile Salts. Molecules 2015, 20 (8), 14451. 10.3390/molecules200814451. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.