Abstract
Background
Among central and peripheral factors contributing to exercise intolerance (EI) in heart failure (HF), the extent to which skeletal muscle (SM) energy metabolic abnormalities occur and contribute to EI and increased fatigability in HF patients with reduced or preserved ejection fraction (HFrEF and HFpEF, respectively) are not known. An energetic plantar flexion exercise fatigability test and magnetic resonance spectroscopy were used to probe the mechanistic in vivo relationships between SM high-energy phosphate concentrations, mitochondrial function and EI in HFrEF and HFpEF patients and in healthy controls.
Methods and Results
Resting SM high-energy phosphate concentrations and ATP flux rates were normal in HFrEF and HFpEF patients. Fatigue occurred at similar SM energetic levels in all subjects, consistent with a common SM “energetic limit”. Importantly, HFrEF NYHA class II–III patients with EI and high fatigability exhibited significantly faster rates of exercise-induced high-energy phosphate decline than did HFrEF patients with low fatigability (NYHA class I), despite similar left ventricular ejection fractions. HFpEF patients exhibited severe EI, the most rapid rates of high-energy phosphate depletion during exercise, and impaired maximal oxidative capacity.
Conclusions
Symptomatic fatigue during plantar flexion exercise occurs at a common energetic limit in all subjects. HFrEF and HFpEF patients with EI and increased fatigability manifest early, rapid exercise-induced declines in SM high-energy phosphates and reduced oxidative capacity as compared to healthy and low fatigability HF patients, suggesting SM metabolism is a potentially important target for future HF treatment strategies.
Subject codes: Metabolism, Heart Failure, Magnetic Resonance Imaging, Exercise Testing
Exercise intolerance (EI) and exertional fatigue are hallmark symptoms of heart failure (HF) and are associated with increased disability and mortality1. HF is a multi-dimensional problem with both central (i.e. cardiac) and peripheral (i.e. skeletal muscle (SM), neurologic) factors contributing to EI. Observations that EI can persist for months after normalization of cardiac output following cardiac transplantation and that exercise training improves exercise tolerance in HF patients without improving exercise cardiac output both suggest that peripheral factors may be important contributors to EI and increased fatigability in HF2–4.
Fatigue is often conceptualized as an energy-deficient state5 and this is certainly the case in skeletal muscle, where ATP is absolutely required to fuel muscle contractile function. Skeletal muscle fatigue in isolated preparations is related to high-energy phosphate depletion, reduced energy release from ATP hydrolysis (ΔG~ATP), and accumulation of inorganic phosphate (Pi) from ATP degradation6. Although impaired ATP metabolism and energy deprivation play an important role in muscle fatigue and weakness in metabolic myopathies7–9 and muscular dystrophies7, 10, 11, the role of skeletal muscle energy deprivation in HF is not well characterized. Phosphorous (31P) magnetic resonance spectroscopy (MRS) studies enable repeated assessments of ATP and creatine phosphate (PCr) concentrations and turnover rates during exercise that are not possible with muscle biopsy specimens. However to date, 31P MRS studies in patients with heart failure and a reduced ejection fraction (HFrEF) have produced conflicting results with some,12–14 but not others,15–17 reporting reduced SM high-energy phosphate ratios during sub-maximal exercise. In heart failure with preserved ejection fraction (HFpEF) reduced skeletal muscle type 1 fibres and mitochondrial content have been observed18–21 and a single prior 31P MRS skeletal muscle study included only two patients22.
To evaluate the role that SM metabolism plays in HF exercise intolerance, it is important to recognize that fatigue may occur at markedly different exercise durations or intensities. The term “fatigability” was introduced to relate the symptom of tiredness or fatigue to the level, duration or intensity of the exercise that induced the symptom5. Thus a complete SM metabolic profile during exercise requires assessments at rest, the rate of change of those parameters during exercise, measures at a common duration and/or intensity, and again at final fatigue. Furthermore, SM parameters recorded during treadmill or bicycle exercise are likely influenced by central-mediated hemodynamic shifts, making it difficult to determine whether any changes in the SM parameters are secondary to such global factors or to intrinsic, primary SM-associated metabolic abnormalities. Finally, important metabolic information should not only include the ratios of high-energy phosphate compounds as reported previously, but also their absolute concentrations and rates of synthesis.
In the present study we exploit an energetic fatigability test that combines graded plantar (small muscle) flexion exercise (PFE) performed in conjunction with repeated non-invasive 31P MRS measures of muscle high-energy phosphates, inorganic phosphate and intracellular pH at rest and during progressive exercise stages performed to fatigue. Four subject cohorts were studied: (1) those with HFrEF and EI as defined by New York Heart Association (NYHA) class 2 or 3; (2) those with HFrEF without EI as defined by NYHA class 1, but with left ventricular ejection fractions (EF) matched to those in group 1; (3) patients with HFpEF; and (4) healthy subjects. The findings are the first to quantify SM metabolism in HFpEF patients and are consistent with the hypothesis that SM metabolic abnormalities play an important mechanistic role in EI in HFrEF and HFpEF patients.
Methods
The Johns Hopkins Institutional Review Board approved all human studies. All subjects gave informed written consent after explanation of the study and protocol.
Subjects
Eleven healthy subjects (6 women, age 51±7 years) with no history of hypertension, diabetes mellitus, or of heart or vascular disease served as controls. HF patients had a clinical diagnosis of chronic HF and included 20 subjects with HFrEF (EF≤40%) 7 of whom had NYHA class I symptoms (2 women, age 43±14 years) and 13 with NYHA class II or III symptoms (7 women, age 52±11 years). Twelve other patients had chronic HFpEF (8 women, age 62±11), as defined by Framingham criteria with EF≥50%23. (See Supplemental Materials for additional details).
Study protocol
All subjects underwent conventional magnetic resonance imaging (MRI) (for determination of muscle and fat content) and 31P MRS (for energetics) at rest, during graded multi-stage PFE (Supplemental Materials Fig 1), and during the post exercise recovery period in a clinical 3 Tesla MRI system. Relative and absolute concentrations of high-energy phosphates were measured as were ATP kinetics with 31P magnetization transfer techniques. Fatigue symptoms were recorded at each stage using an 11-point Borg scale. Exercise was terminated when the subject was unable to exercise further. In addition, an upright bicycle ergometry cardiopulmonary exercise test to exhaustion to measure peak VO2 and a six-minute walk (6MW) were performed. (See Supplemental Materials for details of protocol and statistical analysis).
Results
Patient Characteristics
HFpEF patients were older than NYHA class I HFrEF patients (Table 1). Symptomatic HF patients (HFrEF NYHA class II–III and HFpEF) were more obese than healthy subjects. As expected, symptomatic HF patients (HFrEF NYHA class II–III and HFpEF) had significant EI with reduced mean 6MW distances and peak VO2 as compared to healthy subjects (Table 1). However, NYHA class I HFrEF patients had nearly normal functional measures with 6MW and peak VO2 similar to those of healthy subjects despite their significantly lower EF.
Table 1.
Demographics
| Healthy (n=11) |
HFrEF I (n=7) |
HFrEF II–III (n=13) |
HFpEF (n=12) |
ANOVA p-value |
|
|---|---|---|---|---|---|
|
| |||||
| Age (years) | 51±7 | 43±13# | 52±11 | 62±11 | =0.005 |
|
| |||||
| Gender, n (%) | |||||
| Women | 6 (55) | 2 (29) | 7 (54) | 8 (67) | |
|
| |||||
| NYHA Class, n (%) | |||||
| I | 7 (100) | 0 (0) | 0 (0) | ||
| II | 11 (85) | 4 (33) | |||
| III | 2 (15) | 8 (67) | |||
| IV | 0 (0) | 0 (0) | |||
|
| |||||
| Race, n (%) | |||||
| African American | 2 (18) | 3 (43) | 6 (46) | 7 (58) | |
| White | 8 (73) | 4 (57) | 6 (46) | 5 (42) | |
| Other | 1 (9) | 1 (8) | |||
|
| |||||
| Medications, n (%) | |||||
| ACE/ARB | 0 (0) | 7 (100) | 13 (100) | 5 (42) | |
| Beta-blockers | 0 (0) | 7 (100) | 12 (92) | 4 (33) | |
| Diuretic | 0 (0) | 6 (86) | 13 (100) | 12 (100) | |
| Digoxin | 0 (0) | 0 (0) | 3 (23) | 0 (0) | |
| ASA | 0 (0) | 3 (43) | 6 (46) | 6 (50) | |
| Statin | 1 (9) | 3 (43) | 6 (46) | 8 (67) | |
|
| |||||
| BMI | 23.7±3.2 | 31.8±5.1# | 33.0±7.0*,# | 41.0±8.9§§§ | <0.001 |
|
| |||||
| LVEF (%) | 24±11# | 27±11# | 62±5 | <0.001 | |
|
| |||||
| 6MW (m) | 622±105 | 503±149# | 394±88§§§ | 288±109§§§ | <0.001 |
|
| |||||
| Peak VO2 (ml/kg/min) | 34.5±10.2 | 23.3±7.7# | 16.4±5.8** | 10.8±3.7§§§ | <0.001 |
|
| |||||
| Peak VO2 (ml/min) | 2428±876 | 2231±709 | 1710±613*,# | 1109±354§§§ | <0.01 |
|
| |||||
| RER | 1.09±0.07 | 1.00±0.06 | 1.03±0.11 | 0.97±0.10 | |
Data are means±SD. NYHA, New York Heart Association; BMI, body mass index; LVEF, left ventricular ejection fraction; 6MW, six minute walk; Peak VO2, peak oxygen consumption on CPET testing; RER, respiratory exchange ratio.
p<0.05 vs. HFpEF,
p<0.05 vs. Healthy,
p<0.02 vs. Healthy,
p<0.001 vs. Healthy,.
Plantar Flexion Exercise Performance and Established Functional Parameters
Mean PFE time, maximum exercise weight, and total work were reduced in HFpEF and HFrEF NYHA class II–III patients compared to healthy subjects and HFrEF patients with NYHA class I symptoms (Fig 1A). HFpEF patients exhibited the most EI during PFE with HFpEF and HFrEF NYHA class II–III patients able to perform only one-third to one-half of the total work performed by healthy subjects (Fig 1A). Of note, indices of PFE performance (exercise time, maximum exercise weight, and total work) correlated significantly with the more established, global functional indices of 6MW and peak VO2 (Fig 1B). Thus PFE performance parallels accepted functional measures used in many HF studies. Similar correlations are observed when corrected for ideal body weight (Supplemental Materials Fig 2), rather than true body weight, suggesting that the observed differences are not attributable to obesity.
Figure 1.
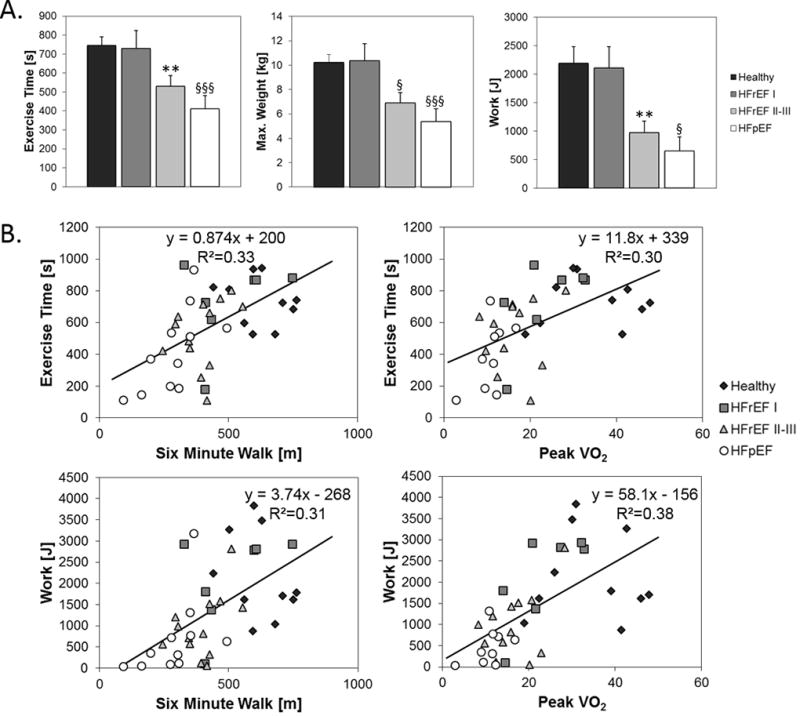
A.) Mean PFE time, maximum PFE exercise weight, and total PFE work of healthy subjects, HFrEF NYHA class I (HFrEF I), HFrEF NYHA class II–III (HFrEF II–III), and HFpEF patients. All three indices are reduced in HFpEF and HFrEF NYHA class II–III patients as compared to healthy subjects and HFrEF patients with NYHA class I symptoms. B.) Correlations between indices of PFE performance (exercise time, maximum exercise weight, and total work) and more established, functional indices of 6MW and peak VO2. Comparisons vs healthy subjects,, ** p<0.02, § p<0.005, §§§ p<0.001.
31P MRS Energetic Fatigability Test
Representative images and spectra from a 31P MRS/MRI energetic fatigability test are shown in Fig 2A–C. During exercise there is depletion of PCr and accumulation of Pi, with intracellular acidification reflected by the change in chemical shift of the Pi resonance (Fig 2C). ATP synthesis through CK and that from Pi were calculated from 31P magnetization transfer spectra at rest (Fig 2D–G). The time course of energetic changes and fatigue symptoms are shown for a healthy subject with low fatigability (Fig 3A) and a HF patient with high fatigability (Fig 3B). Note that SM energetics can be quantified with a high temporal resolution (2s), PCr is progressively depleted during staged-exercise while ATP is preserved, and, critically, the rate of PCr decline is more rapid in the subject with high fatigability. Note that both subjects reach a similar subjective level of fatigue measured by the Borg scale but at different workloads and exercise durations.
Figure 2.
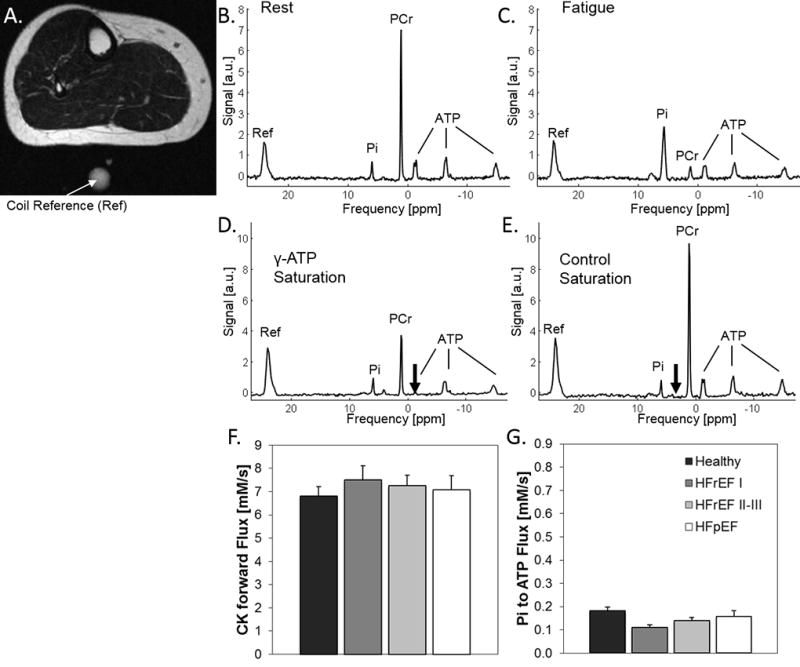
A.) Representative MRI of the calf with coil reference (indicated by white arrow). 31P spectrum from the calf of the same subject at rest (B) at the time point of fatigue (C). Note the depletion of PCr and accumulation of Pi at fatigue. 31P MRS spectra at rest wherein the reduction in the PCr peak during ATP saturation (D, saturation indicated by black arrow), as compared to PCr peak during control saturation (E, saturation indicated by black arrow), is proportional to the ATP synthesis rate through CK. F.) Summary ATP synthesis rates from PCr through CK. G.) Summary ATP synthesis rates from Pi. There are no significant differences among the four groups at baseline resting conditions.
Figure 3.
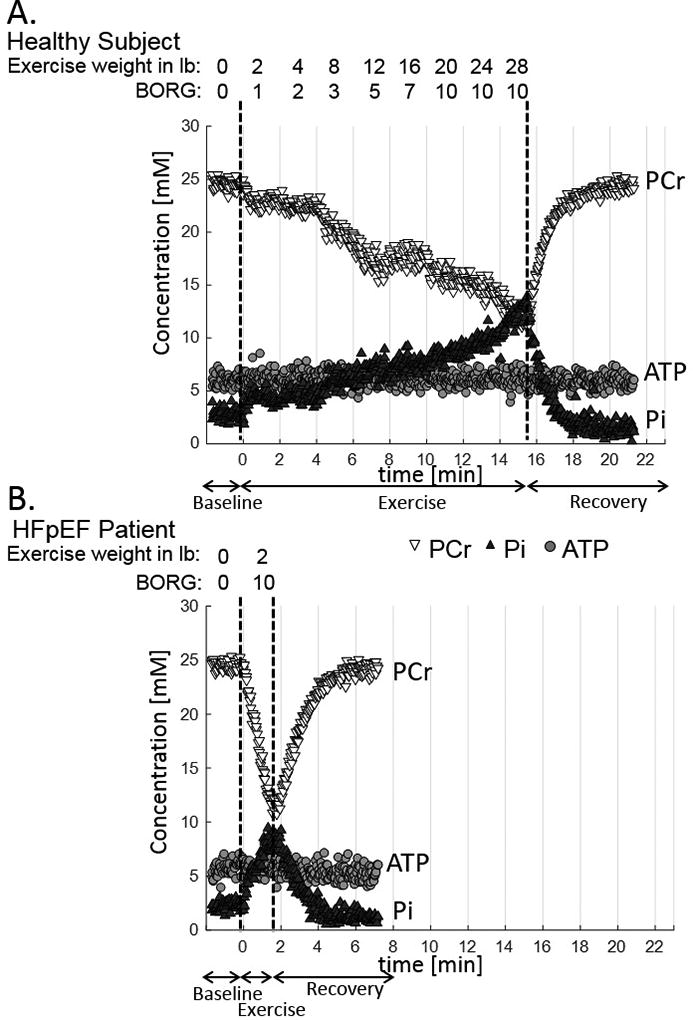
Time course of energetic changes and fatigue symptoms for a healthy subject with low fatigability (A) and for a HF patient with high fatigability (B). In both cases PCr is progressively depleted and Pi accumulates during staged-exercise while ATP is preserved. The rates of PCr decrease and Pi increase are more rapid in the subject with high fatigability. Both subjects reached a similar subjective level of fatigue (Borg scale) and PCr depletion but at different workloads and exercise durations.
Skeletal Muscle Energetics at Rest, Exercise and Fatigue
During baseline resting conditions there were no significant differences in ATP synthesis rates from PCr through CK and from Pi (Fig 2FG) or other SM energetic parameters among the four groups (Fig 4A–F). Thus resting SM high-energy phosphates and energetics are normal in HF patients. However, phosphodiesters (PDE), a byproduct of phospholipid catabolism, are increased. Despite differences in exercise time and work, energetic changes are similar at the point of fatigue in all groups (Fig 4G–K) with comparable PCr depletion, Pi and ADP accumulation, intracellular acidosis, and change in ΔG~ATP. Thus the SM energetic profile is similar in healthy subjects and HF patients before exercise and at the point of fatigue (Fig 4), though, again, the times to fatigue significantly differ (Fig 1).
Figure 4.
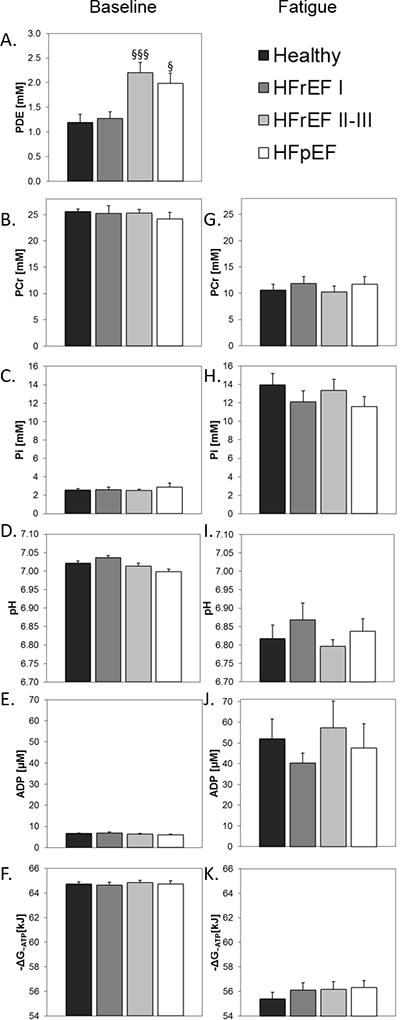
Skeletal muscle energetic parameters (PCr, Pi, pH, ADP, ΔG~ATP) and phosphodiester (PDE) during baseline resting conditions (A–F, left column) and at the point of fatigue (G–K, right column). There are no significant differences in the SM energetic parameters among the four groups at rest or at the point of fatigue (although PDE differed at baseline). Comparisons vs healthy subjects, § p<0.005, §§§ p<0.001.
Rate of High-energy Phosphate Decline During Exercise and Post-exercise Rate of Recovery
Although SM high-energy phosphates are comparable at rest and at the time of fatigue in the four groups, the rates of PCr decline and Pi accumulation differ significantly. Specifically, the normalized rates of PCr decline during PFE were greater in HFpEF (p<0.001) and HFrEF NYHA class II–III patients (p<0.005) than in healthy subjects (Fig 5A). Because the rate averaged over all of exercise could be impacted by reduced cardiac-derived SM perfusion in HF patients at peak exercise, we also measured high-energy phosphate decline during the earliest 4 minutes of low-level exercise (Fig 5B). The initial rates of high-energy phosphate decline were still significantly greater in HFrEF II–III (p<0.05) and HFpEF patients (p<0.001) than in healthy subjects. There is a strong correlation between the average rate of PCr decline during PFE and the maximum exercise time (Fig 5C, R2=0.83, p<0.001), indicating that the accelerated exercise-induced SM high-energy phosphate loss in symptomatic HFrEF and HFpEF patients is closely related to their exercise intolerance.
Figure 5.
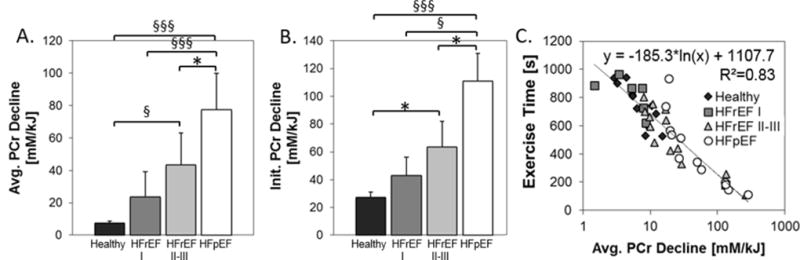
A) Normalized rate of PCr decline during PFE. B) Initial normalized rate of PCr decline during the first 4 minutes of PFE, C) Correlation of maximal PFE time and rate of PCr decline. Significant differences are indicated: * p < 0.05, § p < 0.005. §§§ p < 0.001
The rate of PCr recovery following exercise directly reflects oxidative re-phosphorylation of creatine and is related to maximal mitochondrial oxidative capacity24. The time for PCr recovery was significantly delayed (Fig 6A) and maximum oxidative capacity reduced (Fig 6B) in HFpEF and HFrEF II–III patients as compared to healthy subjects, indicating impaired mitochondrial function in the former two HF groups. In addition, maximum oxidative capacity in HFrEF patients with EI (NYHA Class II–III) was half that of the NYHA Class I HFrEF patients despite comparable mean EFs. This indicates that impaired SM mitochondrial function is more closely related to EI and HF symptoms than is EF in HFrEF patients. Thus symptomatic HFrEF patients with EI and HFpEF patients exhibit an accelerated loss of SM high-energy phosphates commencing during early exercise, before we expect blood flow to be limiting during small muscle exercise. These patients also exhibited impaired mitochondrial oxidative capacity as compared to healthy subjects and to HFrEF patients with nearly normal exercise tolerance.
Figure 6.
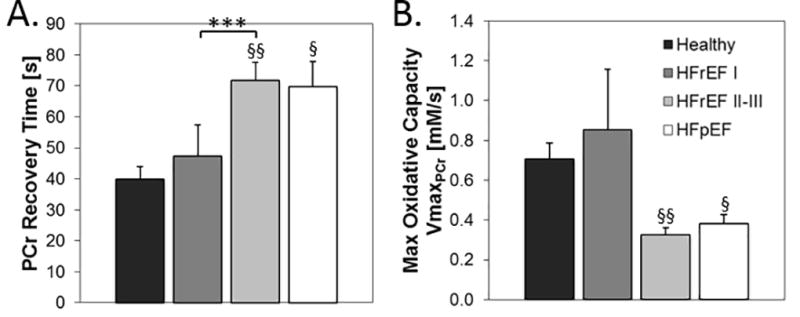
A) The rate of PCr recovery following PFE is significantly delayed in HFpEF and HFrEF II–III patients as compared to HFrEF class I and healthy subjects. B) Indices of maximal oxidative capacity (VmaxPCr) are lower in NYHA class II-II HFrEF and HFpEF patients than in the other two groups without EI. Comparisons vs healthy subjects, § p<0.005, §§ p<0.002. Comparisons vs HFrEF NYHA class I patients, *** p<0.01.
Skeletal Muscle Fat Content
Because obesity is associated with the development of both HFpEF and HFrEF and because obesity-associated lipid accumulation and lipotoxicity are thought to adversely affect cardiac muscle function25, we also measured fat content in SM. Muscle fat fraction is nonsignifcantly increased in HFrEF patients compared to that of healthy subjects and did not differ between HFrEF NYHA class I and class II–III patients (Fig 7). In contrast, SM fat fraction is increased almost three-fold in HFpEF patients as compared to healthy subjects (Fig 7). However, no correlations between muscle fat content or muscle mass and high-energy phosphate decline during exercise or during post exercise recovery are observed (Supplemental Materials Fig 4).
Figure 7.
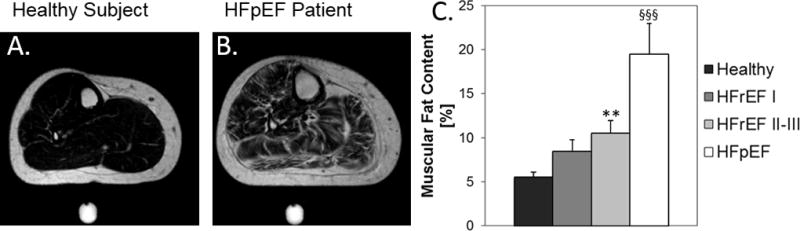
Anatomical MRI showing fat distribution in the calf of a healthy subject (A) and in the calf of a HFpEF patient (B). C) Muscular fat content in the four groups. Comparisons vs. healthy subjects, ** p<0.02, §§§ p<0.001.
Discussion
SM high-energy phosphate metabolism and exercise intolerance in HF
We evaluated SM fatigability in HF patients with and without EI using an approach that allowed simultaneous assessment of fatigue symptoms, lower extremity exercise capacity, and SM energetics at rest, matched workloads and fatigue. Exercise duration and work performed with this energetic fatigability stress test correlated with conventional functional measures of 6MW and exercise peak VO2 (Fig 1). The novel findings are that all subjects fatigue at a common SM energetic level but that a faster rate of energetic decline distinguishes HF patients with EI from those with normal exercise tolerance. Of the cohorts studied, HFpEF patients exhibit the most dramatic SM energetic changes (Figs 2,5–6).
Reduced skeletal muscle PCr/ATP and PCr/Pi ratios were previously observed with 31P MRS in HFrEF patients during submaximal steady-state exercise in most,12–14 but not all, prior studies15–17. To our knowledge the present study is the first to noninvasively quantify absolute concentrations of skeletal muscle ATP and PCr in these patient populations and to do so throughout a graded exercise regimen performed to fatigue. Importantly, because the high-energy phosphate ratio of PCr/ATP may not change with concomitant depletion in both PCr and ATP, the present absolute HEP results demonstrate that SM ATP and PCr are not reduced in HFrEF or HFpEF patients at rest. Because the CK reaction is the primary muscle energy reserve reaction and because ATP flux through myocardial CK is reduced in human HF and predicts future clinical HF events26, we also tested whether reduced skeletal muscle CK ATP synthesis is present in HF and contributes to EI. However, skeletal muscle ATP synthesis through CK at rest is not reduced in HF patients (Fig 2). Thus resting skeletal muscle high-energy phosphate stores and CK reserve are not reduced and cannot account for EI in HFrEF or in HFpEF patients.
Framework for energetic fatigability in HF
While “fatigue” is typically defined as a subjective sense of tiredness, “fatigability” is an arguably more useful term because it relates the symptom of tiredness to the level, duration and/or intensity of the activity that induced the symptom5. Fatigability is an important concept in HF because it can distinguish individuals who experience identical levels of symptomatic fatigue after very different amounts of activity (e.g. walking 5m vs. running a 10k race).
As originally described by Eldadah5, a healthy subject with low fatigability (Fig 8A, blue line) achieves a higher level, duration and/or intensity of work than does a subject with high fatigability (Fig 8A, red line) who experiences the same level of fatigue symptoms (“fatigue limit”) at less activity. Here we expand the fatigability construct to include an energetic dimension (Fig 8B). If there was no energetic basis for increased fatigability in HF, then fatigue would be independent of SM high-energy phosphate stores (eg, less depletion, Fig 8B red line #1 if skeletal muscle energetics are not limiting). However if an energetic component to HF fatigue exists, then those patients with high fatigability (Fig 8B red lines #2 and #3) and those with low fatigability (Fig 8B blue line) would fatigue at a comparable “energetic limit” or metabolic index. The current findings of a common energetic limit in HF patients and healthy subjects (Fig 4) that is reached more rapidly in HF patients with increased fatigability because of a more rapid rate of SM energetic depletion (Fig 5, Fig 8B, red line #2) support the hypothesis that high-energy phosphate energetics is an important contributor to HF fatigability. In theory, a significant increase in [ADP], [Pi] or change in ΔG~ATP could impair myofilament function before ATP is fully depleted, as observed here. Note that the present observation of a common energetic limit involves exercise of a relatively small muscle group not likely limited by central hemodynamics in subjects studied, and suggests the possibility that new interventions to delay or slow the SM energetic decline during exercise could reduce EI in both HFrEF and HFpEF.
Figure 8.
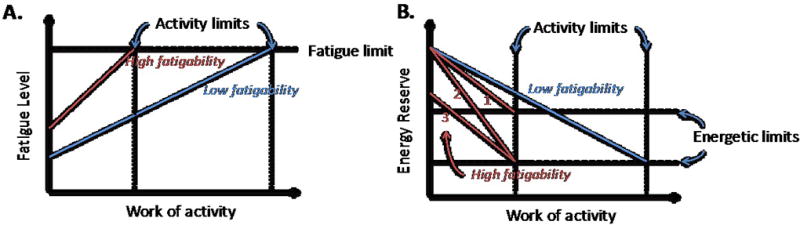
A.) Fatigability construct showing the relationship between fatigue symptoms and activity (adapted from Eldadah5). Fatigue and work levels are compared in a healthy subject with low fatigability (blue line) and a subject with high fatigability (red line). Both experience the same level of fatigue symptoms (“fatigue limit”) but at different levels of activity. B.) The fatigability construct is expanded to include energetic contributions (y-axis). If there is no energetic basis for fatigue in HF, then fatigue could occur with residual (less depleted) energy stores in individuals with high fatigability (red line #1) than in those with low fatigability (blue line). However if an energetic component to fatigue in HF is the limiting factor, one would expect subjects with high (red lines #2 and #3) and low (blue line) fatigability to fatigue at the same “energetic limit” albeit at different activity levels. If that is the case, the earlier time to energy depletion in those with high fatigability could be due to either lower resting, baseline energy reserve (red line 3) and/or more rapid decline during exercise (red line 2).
Furthermore, the rates of high-energy phosphate decline during exercise are significantly faster in HFrEF patients with EI and high fatigability (NYHA class II–III) than are the rates in HFrEF patients with low fatigability (NYHA class I), despite similarly low EFs (Fig 5). HFpEF patients with severe EI, exhibit the most rapid rates of high-energy phosphate depletion during exercise. Their maximal oxidative capacity, reflected in delayed high-energy phosphate recovery, is comparable to that of HFrEF NYHA class II–III patients (Fig. 6). Indeed the rate of high-energy phosphate decline during PFE is inversely related to exercise duration (Fig 5C). All of these observations are consistent with normal baseline energetics but a faster exercise-induced decline to a common energetic limit in symptomatic HF (Fig 8B, red line #2).
Potential mechanistic factors underlying impaired SM energetics in HF
The more rapid decline of high-energy phosphate during exercise in HF patients with EI could be due to reduced ATP production and/or increased ATP utilization during exercise27. Reduced SM mitochondrial number and enzyme activity were previously reported in HFrEF28 and HFpEF18–21. We observed reduced maximal oxidative capacity in HFrEF and HFpEF patients (Fig 6), consistent with most16, 27, 29 but not all15 prior reports in HFrEF patients. We believe these to be the first findings of significantly reduced maximal oxidative capacity in HFpEF. Although increased ATP consumption during exercise could occur at a matched workload in HF due to reduced muscle mass or inefficiency, we did not observe significant reductions in muscle mass in these patients (Supplemental Materials Fig 3).
Reduced SM blood flow in HF patients could contribute to the more rapid energetic decline, catabolite accumulation and mitochondrial dysfunction during exercise in HF patients. Although early studies showed reduced blood flow in HFrEF30–32, subsequent studies during upper or lower extremity exercise in HFrEF patients did not demonstrate reductions in extremity blood flow, tissue oxygenation, or myoglobin desaturation during exercise sufficient to cause SM energetic abnormalities12,13,33,34. Such observations reduce the likelihood of deficiencies in oxygen delivery as an underlying cause, and suggest instead, a SM mitochondrial metabolic abnormality in HF. This is consistent with the present observation of a rapid PCr decline during the earliest minutes of small muscle group exercise when the workload is very low (2–4 lbs) (Fig 3,5B) and blood flow may not be limiting. Intrinsic SM energetic abnormalities that are closely related to EI in HF patients are consistent with the hypothesis that the myopathy of HF may not be driven so much by hemodynamic abnormalities, but rather by changes in SM mitochondrial function, perhaps related to the milieu of neurohormonal factors, metabolites and cytokines that “bathe” the skeletal muscle35. Alternatively, if HF patients become sedentary due to reduced cardiac reserve36, the inactivity, per se, may contribute to EI and SM mitochondrial abnormalities.
While these are the first 31P MRS findings showing statistically significant skeletal muscle energetic abnormalities during exercise in HFpEF patients, Bhella and collaborators described delayed PCr recovery in two HFpEF patients22, a sample size that precluded quantitative comparisons. Although cardiac dysfunction is a significant contributor to EI in HFpEF patients37,38, there is also evidence that peripheral factors contribute22,39,40. Recently, reduced peripheral oxygen extraction was identified as a major determinant of upright bicycle exercise capacity in HFpEF patients39. This was attributed to limited diffusive oxygen transport, possibly due to greater diffusion distances or heterogeneity in the matching of regional flow to metabolic demand39. However, the deficit in SM maximal oxidative capacity observed here in HFpEF patients could also contribute to reduced peripheral oxygen extraction during exercise. A meta-analysis of six randomized trials indicated that exercise training in HFpEF patients improves cardiorespiratory fitness but this occurs without measurable changes in systolic or diastolic function41. In one randomized trial, exercise training improved peak VO2 in HFpEF patients with only a minimal effect on cardiac output but with a significant increase in peak arterial-venous oxygen difference. Those findings indicate that peripheral factors contribute to improved exercise capacity with exercise training in HFpEF and, by inference that peripheral mechanisms contribute to EI in HFpEF3. All of these studies are consistent with the concept that HFpEF is a systemic syndrome affecting multiple organs, including SM42.
The in vivo SM energetic abnormalities in HFpEF patients (i.e. accelerated rate of PCr decline and of Pi and H+ accumulation during exercise in Fig 5, and reduced in vivo maximal oxidative capacity in Fig 6) are similar or more marked in the HFpEF, than in the HFrEF, group. Remarkably, the intermuscular (not subcutaneous) fat was increased an average 3–4 fold over that in healthy subjects (Fig 7), possibly reflecting common HFpEF co-morbidities of obesity, diabetes and dyslipidemia. An earlier study reported that increased intermuscular fat in HFpEF patients correlated with reduced peak VO220.
Limitations
The cohort size was small and derived from patients referred to a tertiary care center, and so may not represent the more general population. Nonetheless highly significant differences in SM energetics between those HF patients with, and without, EI were detected. The 31P MRS PFE fatigability exam is not intended to replace standard 6MW or peak VO2 testing but instead to provide insight into the role of SM energetic fatigue in HF patients. The correlation of these plantar flexion findings with conventional measures of total body exercise capacity is of interest because it cannot be assumed that capacity of a small muscle mass and total body exercise are necessarily limited by the same processes. Lower extremity blood flow, endothelial function43, and muscle perfusion were not measured with MRI during PFE due to motion artifacts and systemic lactate measures were not obtained. Thus we cannot exclude the possibility that reduced perfusion during exercise contributed to the observed metabolic declines during exercise in HF subjects with EI. It seems unlikely, though, that reduced perfusion alone is responsible for energetic decline during the first minutes of very low-level exercise or for reduced maximal oxidative capacity (VmaxPCr) during recovery. HFpEF patients in many studies including this one tend to be older, more obese and have greater NYHA symptoms and exercise intolerance. These data do not demonstrate whether the more dramatic energetic changes in HFpEF patients are due to HFpEF per se or to these other factors. It will be important in future studies to study older, obese subjects without heart failure, asymptomatic HFpEF patients, and to match NYHA symptoms and exercise tolerance in HFpEF and HFrEF populations.
Conclusions
To evaluate whether SM energetic abnormalities exist and contribute to EI in HF patients we exploited a SM energetic fatigability test that is noninvasive, correlates with established functional measures (6MW and peakVO2), and allows studies of SM energetics at matched workloads and at fatigue. HFrEF patients with increased fatigability and EI exhibit more rapid exercise-induced declines in SM high-energy phosphates than do HFrEF patients with no EI, despite matched left ventricular ejection fractions. HFpEF patients in this study exhibit the most profound EI, energetic abnormalities, and rapid PCr depletion during exercise as well as increased muscle lipids. On average, all subjects fatigue at the same mean SM energetic state, suggesting a common “energetic limit”. Because of the relatively high plasticity and remodelling potential of skeletal muscle to respond to multiple stimuli, as compared to cardiac muscle, these studies offer a new approach to quantify SM energetics in HF and suggest that interventions that augment SM metabolism may offer another treatment target for the EI and disability that markedly impair quality of life for both HFrEF and HFpEF patients.
Supplementary Material
Clinical Perspective.
What’s new?
-
◦
Skeletal muscle energetics were studied with 31P MRS during plantar flexion exercise (PFE) in healthy subjects and HF patients with and without exercise intolerance (EI).
-
◦
PFE performance correlated with separate six-minute walk and peak VO2 testing. Skeletal muscle energetic levels in HFrEF and HFpEF patients were normal at rest.
-
◦
HFrEF NYHA class II–III patients with EI and high fatigability exhibited significantly faster rates of exercise-induced high-energy phosphate decline than did HFrEF patients with low fatigability (NYHA class I).
-
◦
HFpEF patients exhibited severe EI, the most rapid rates of high-energy phosphate depletion during exercise, and impaired maximal oxidative capacity.
What are the clinical implications?
-
◦
Exercise intolerance (EI) and exertional fatigue are hallmark symptoms of heart failure (HF) and peripheral factors may contribute to EI in HF.
-
◦
This study showed that symptomatic fatigue during plantar flexion exercise occurs at a common energetic limit in healthy and heart failure subjects.
-
◦
HFrEF and HFpEF patients with EI and increased fatigability manifest early, rapid exercise-induced declines in SM high-energy phosphates and reduced oxidative capacity as compared to healthy and low fatigability HF patients.
-
◦
These observations suggest that skeletal muscle metabolism may be a potentially important target for future HFrEF and HFpEF treatment strategies.
Acknowledgments
Sources of Funding: NIH: HL61912, HL63030, AG045634.
Footnotes
Disclosures: After the study was completed KW became an employee of Philips Healthcare at which time he performed data analysis and manuscript preparation. No financial support from Philips Healthcare was received for the study.
References
- 1.Pina IL, Apstein CS, Balady GJ, Belardinelli R, Chaitman BR, Duscha BD, Fletcher BJ, Fleg JL, Myers JN, Sullivan MJ. Exercise and heart failure: A statement from the American Heart Association Committee on exercise, rehabilitation, and prevention. Circulation. 2003;107:1210–1225. doi: 10.1161/01.cir.0000055013.92097.40. [DOI] [PubMed] [Google Scholar]
- 2.Stevenson LW, Sietsema K, Tillisch JH, Lem V, Walden J, Kobashigawa JA, Moriguchi J. Exercise capacity for survivors of cardiac transplantation or sustained medical therapy for stable heart failure. Circulation. 1990;81:78–85. doi: 10.1161/01.cir.81.1.78. [DOI] [PubMed] [Google Scholar]
- 3.Haykowsky MJ, Brubaker PH, Stewart KP, Morgan TM, Eggebeen J, Kitzman DW. Effect of endurance training on the determinants of peak exercise oxygen consumption in elderly patients with stable compensated heart failure and preserved ejection fraction. J Am Coll Cardiol. 2012;60:120–128. doi: 10.1016/j.jacc.2012.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kao AC, Van Trigt P, 3rd, Shaeffer-McCall GS, Shaw JP, Kuzil BB, Page RD, Higginbotham MB. Central and peripheral limitations to upright exercise in untrained cardiac transplant recipients. Circulation. 1994;89:2605–2615. doi: 10.1161/01.cir.89.6.2605. [DOI] [PubMed] [Google Scholar]
- 5.Eldadah BA. Fatigue and fatigability in older adults. PM R. 2010;2:406–413. doi: 10.1016/j.pmrj.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 6.Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev. 2008;88:287–332. doi: 10.1152/physrev.00015.2007. [DOI] [PubMed] [Google Scholar]
- 7.Radda GK. The use of NMR spectroscopy for the understanding of disease. Science. 1986;233:640–5. doi: 10.1126/science.3726553. [DOI] [PubMed] [Google Scholar]
- 8.Das AM, Steuerwald U, Illsinger S. Inborn errors of energy metabolism associated with myopathies. J Biomed Biotechnol. 2010;2010:340849. doi: 10.1155/2010/340849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wortmann RL. Metabolic myopathies. Curr Opin Rheumatol. 1991;3:925–33. doi: 10.1097/00002281-199112000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Barnes PR, Kemp GJ, Taylor DJ, Radda GK. Skeletal muscle metabolism in myotonic dystrophy A 31P magnetic resonance spectroscopy study. Brain. 1997;120(Pt 10):1699–1711. doi: 10.1093/brain/120.10.1699. [DOI] [PubMed] [Google Scholar]
- 11.Younkin DP, Berman P, Sladky J, Chee C, Bank W, Chance B. 31P NMR studies in Duchenne muscular dystrophy: age-related metabolic changes. Neurology. 1987;37:165–169. doi: 10.1212/wnl.37.1.165. [DOI] [PubMed] [Google Scholar]
- 12.Massie B, Conway M, Yonge R, Frostick S, Ledingham J, Sleight P, Radda G, Rajagopalan B. Skeletal muscle metabolism in patients with congestive heart failure: relation to clinical severity and blood flow. Circulation. 1987;76:1009–1019. doi: 10.1161/01.cir.76.5.1009. [DOI] [PubMed] [Google Scholar]
- 13.Wiener DH, Fink LI, Maris J, Jones RA, Chance B, Wilson JR. Abnormal skeletal muscle bioenergetics during exercise in patients with heart failure: role of reduced muscle blood flow. Circulation. 1986;73:1127–1136. doi: 10.1161/01.cir.73.6.1127. [DOI] [PubMed] [Google Scholar]
- 14.Mancini DM, Coyle E, Coggan A, Beltz J, Ferraro N, Montain S, Wilson JR. Contribution of intrinsic skeletal muscle changes to 31P NMR skeletal muscle metabolic abnormalities in patients with chronic heart failure. Circulation. 1989;80:1338–1346. doi: 10.1161/01.cir.80.5.1338. [DOI] [PubMed] [Google Scholar]
- 15.Marie PY, Escanye JM, Brunotte F, Robin B, Walker P, Zannad F, Robert J, Gilgenkrantz JM. Skeletal muscle metabolism in the leg during exercise in patients with congestive heart failure. Clin Sci (Lond) 1990;78:515–519. doi: 10.1042/cs0780515. [DOI] [PubMed] [Google Scholar]
- 16.Mancini DM, Walter G, Reichek N, Lenkinski R, McCully KK, Mullen JL, Wilson JR. Contribution of skeletal muscle atrophy to exercise intolerance and altered muscle metabolism in heart failure. Circulation. 1992;85:1364–1373. doi: 10.1161/01.cir.85.4.1364. [DOI] [PubMed] [Google Scholar]
- 17.Nakae I, Mitsunami K, Matsuo S, Inubushi T, Morikawa S, Koh T, Horie M. Detection of calf muscle alterations in patients with chronic heart failure by P magnetic resonance spectroscopy: Impaired adaptation to continuous exercise. Experiment Clin Cardiol. 2005;10:4–8. [PMC free article] [PubMed] [Google Scholar]
- 18.Molina AJ, Bharadwaj MS, Van Horn C, Nicklas BJ, Lyles MF, Eggebeen J, Haykowsky MJ, Brubaker PH, Kitzman DW. Skeletal Muscle Mitochondrial Content, Oxidative Capacity, and Mfn2 Expression Are Reduced in Older Patients With Heart Failure and Preserved Ejection Fraction and Are Related to Exercise Intolerance. JACC Heart Fail. 2016;4:636–645. doi: 10.1016/j.jchf.2016.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kitzman DW, Nicklas B, Kraus WE, Lyles MF, Eggebeen J, Morgan TM, Haykowsky M. Skeletal muscle abnormalities and exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Physiol Heart Circ Physiol. 2014;306:H1364–1370. doi: 10.1152/ajpheart.00004.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haykowsky MJ, Kouba EJ, Brubaker PH, Nicklas BJ, Eggebeen J, Kitzman DW. Skeletal muscle composition and its relation to exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Cardiol. 2014;113:1211–6. doi: 10.1016/j.amjcard.2013.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bowen TS, Rolim NP, Fischer T, Baekkerud FH, Medeiros A, Werner S, Bronstad E, Rognmo O, Mangner N, Linke A, Schuler G, Silva GJ, Wisloff U, Adams V Optimex Study G. Heart failure with preserved ejection fraction induces molecular, mitochondrial, histological, and functional alterations in rat respiratory and limb skeletal muscle. Eur J Heart Fail. 2015;17:263–272. doi: 10.1002/ejhf.239. [DOI] [PubMed] [Google Scholar]
- 22.Bhella PS, Prasad A, Heinicke K, Hastings JL, Arbab-Zadeh A, Adams-Huet B, Pacini EL, Shibata S, Palmer MD, Newcomer BR, Levine BD. Abnormal haemodynamic response to exercise in heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13:1296–1304. doi: 10.1093/eurjhf/hfr133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borlaug BA, Melenovsky V, Russell SD, Kessler K, Pacak K, Becker LC, Kass DA. Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation. 2006;114:2138–2147. doi: 10.1161/CIRCULATIONAHA.106.632745. [DOI] [PubMed] [Google Scholar]
- 24.Lanza IR, Bhagra S, Nair KS, Port JD. Measurement of human skeletal muscle oxidative capacity by 31P-MR spectroscopy: a cross-validation with in vitro measurements. J Magn Reson Imaging. 2011;34:1143–50. doi: 10.1002/jmri.22733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldberg IJ, Trent CM, Schulze PC. Lipid metabolism and toxicity in the heart. Cell Metabol. 2012;15:805–812. doi: 10.1016/j.cmet.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bottomley PA, Panjrath GS, Lai S, Hirsch GA, Wu K, Najjar SS, Steinberg A, Gerstenblith G, Weiss RG. Metabolic rates of ATP transfer through creatine kinase (CK Flux) predict clinical heart failure events and death. Sci Transl Med. 2013;5:215re3. doi: 10.1126/scitranslmed.3007328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kemp GJ, Thompson CH, Stratton JR, Brunotte F, Conway M, Adamopoulos S, Arnolda L, Radda GK, Rajagopalan B. Abnormalities in exercising skeletal muscle in congestive heart failure can be explained in terms of decreased mitochondrial ATP synthesis, reduced metabolic efficiency, and increased glycogenolysis. Heart. 1996;76:35–41. doi: 10.1136/hrt.76.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drexler H, Riede U, Munzel T, Konig H, Funke E, Just H. Alterations of skeletal muscle in chronic heart failure. Circulation. 1992;85:1751–1759. doi: 10.1161/01.cir.85.5.1751. [DOI] [PubMed] [Google Scholar]
- 29.Cohen-Solal A, Laperche T, Morvan D, Geneves M, Caviezel B, Gourgon R. Prolonged kinetics of recovery of oxygen consumption after maximal graded exercise in patients with chronic heart failure. Analysis with gas exchange measurements and NMR spectroscopy. Circulation. 1995;91:2924–2932. doi: 10.1161/01.cir.91.12.2924. [DOI] [PubMed] [Google Scholar]
- 30.Zelis R, Mason DT, Braunwald E. A comparison of the effects of vasodilator stimuli on peripheral resistance vessels in normal subjects and in patients with congestive heart failure. J Clin Invest. 1968;47:960–970. doi: 10.1172/JCI105788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zelis R, Longhurst J, Capone RJ, Mason DT. A comparison of regional blood flow and oxygen utilization during dynamic forearm exercise in normal subjects and patients with congestive heart failure. Circulation. 1974;50:137–143. doi: 10.1161/01.cir.50.1.137. [DOI] [PubMed] [Google Scholar]
- 32.Sullivan MJ, Knight JD, Higginbotham MB, Cobb FR. Relation between central and peripheral hemodynamics during exercise in patients with chronic heart failure. Muscle blood flow is reduced with maintenance of arterial perfusion pressure. Circulation. 1989;80:769–781. doi: 10.1161/01.cir.80.4.769. [DOI] [PubMed] [Google Scholar]
- 33.Wilson JR, Fink L, Maris J, Ferraro N, Power-Vanwart J, Eleff S, Chance B. Evaluation of energy metabolism in skeletal muscle of patients with heart failure with gated phosphorus-31 nuclear magnetic resonance. Circulation. 1985;71:57–62. doi: 10.1161/01.cir.71.1.57. [DOI] [PubMed] [Google Scholar]
- 34.Mancini DM, Wilson JR, Bolinger L, Li H, Kendrick K, Chance B, Leigh JS. In vivo magnetic resonance spectroscopy measurement of deoxymyoglobin during exercise in patients with heart failure. Demonstration of abnormal muscle metabolism despite adequate oxygenation. Circulation. 1994;90:500–8. doi: 10.1161/01.cir.90.1.500. [DOI] [PubMed] [Google Scholar]
- 35.Nilsson KR, Duscha BD, Hranitzky PM, Kraus WE. Chronic heart failure and exercise intolerance: the hemodynamic paradox. Curr Cardiol Rev. 2008;4:92–100. doi: 10.2174/157340308784245757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borlaug BA, Kane GC, Melenovsky V, Olson TP. Abnormal right ventricular-pulmonary artery coupling with exercise in heart failure with preserved ejection fraction. Eur Heart J. 2016;37:3293–3302. doi: 10.1093/eurheartj/ehw241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abudiab MM, Redfield MM, Melenovsky V, Olson TP, Kass DA, Johnson BD, Borlaug BA. Cardiac output response to exercise in relation to metabolic demand in heart failure with preserved ejection fraction. Eur J Heart Fail. 2013;15:776–785. doi: 10.1093/eurjhf/hft026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Santos M, Opotowsky AR, Shah AM, Tracy J, Waxman AB, Systrom DM. Central cardiac limit to aerobic capacity in patients with exertional pulmonary venous hypertension: implications for heart failure with preserved ejection fraction. Circ Heart Fail. 2015;8:278–285. doi: 10.1161/CIRCHEARTFAILURE.114.001551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dhakal BP, Malhotra R, Murphy RM, Pappagianopoulos PP, Baggish AL, Weiner RB, Houstis NE, Eisman AS, Hough SS, Lewis GD. Mechanisms of exercise intolerance in heart failure with preserved ejection fraction: the role of abnormal peripheral oxygen extraction. Circ Heart Fail. 2015;8:286–294. doi: 10.1161/CIRCHEARTFAILURE.114.001825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haykowsky MJ, Brubaker PH, John JM, Stewart KP, Morgan TM, Kitzman DW. Determinants of exercise intolerance in elderly heart failure patients with preserved ejection fraction. J Am Coll Cardiol. 2011;58:265–274. doi: 10.1016/j.jacc.2011.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pandey A, Parashar A, Kumbhani DJ, Agarwal S, Garg J, Kitzman D, Levine BD, Drazner M, Berry JD. Exercise training in patients with heart failure and preserved ejection fraction: meta-analysis of randomized control trials. Circ Heart Fail. 2015;8:33–40. doi: 10.1161/CIRCHEARTFAILURE.114.001615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shah SJ, Kitzman DW, Borlaug BA, van Heerebeek L, Zile MR, Kass DA, Paulus WJ. Phenotype-Specific Treatment of Heart Failure With Preserved Ejection Fraction: A Multiorgan Roadmap. Circulation. 2016;134:73–90. doi: 10.1161/CIRCULATIONAHA.116.021884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Borlaug BA, Olson TP, Lam CS, Flood KS, Lerman A, Johnson BD, Redfield MM. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2010;56:845–854. doi: 10.1016/j.jacc.2010.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


