Abstract
Despite multimodal treatment that includes surgery, radiation and chemotherapy, virtually all glioblastomas (GBM) recur, indicating that these interventions are insufficient to eradicate all malignant cells. To identify potential new therapeutic targets in GBMs, we examined the expression and function of proteins that are associated with therapy resistance and cancer cell survival. We measured the expression of eight such proteins in 50 GBM samples by immunohistochemistry and analyzed patient survival. We report that GBM patients with high expression of ABCG2 (also called BCRP) or XIAP at the protein level had worse survival than those with low expression. The adjusted hazard ratio for ABCG2 was 2.35 and for XIAP was 2.65. Since glioma stem cells (GSCs) have been shown to be more resistant than bulk tumor cells to anti-cancer therapies and to express high levels of these proteins, we also sought to determine if ABCG2 and XIAP have functional roles in GSCs. We used small molecule inhibitors to treat patient-derived GBM tumorspheres in vitro and observed that inhibitors of ABCG2, Ko143 and fumitremorgin, significantly reduced self-renewal. These results suggest that ABCG2 and XIAP proteins may be useful indicators of patient survival and that inhibition of ABCG2 may be a promising therapeutic target in GBMs.
Keywords: Glioblastoma, ABCG2, XIAP, Ko143, glioma stem cells
INTRODUCTION
A particularly difficult challenge in the treatment of glioblastoma multiforme (GBM), the most common and lethal primary brain malignancy in adults, is the inability to effectively target all tumor cells. As a result, these tumors invariably recur. Despite multimodal therapy with surgery, radiation and chemotherapy, the median survival has not surpassed 15 months [1]. Tumor cells deploy three general strategies to evade anti-cancer treatments: 1) prevention of cell damage by extrusion of cytotoxic agents via ABC transporters, 2) repair of DNA damage induced by chemotherapy and radiation treatments, and 3) up-regulation of anti-apoptotic signals to circumvent apoptosis. These characteristics may be found in the majority of tumor cells, but they may be more active in glioma stem cells (GSCs) that are thought to be largely responsible for tumor recurrence. Cancer stem cells are a small subset of stem-like cancer cells that are capable of self-renewal and initiate tumors upon transplantation [2]. Although there is no definitive marker for GSCs that identify these cells in all GBM patient tumors, early studies have reported that GSCs are enriched in CD133+ [3, 4]. GSCs have been shown to be more resistant to radiation and chemotherapy than matched non-stem glioma cells [4–6]. Moreover, studies using experimental models have shown that tumor recurrence is fueled by residual glioma cells with stem cell like properties [7]. Taken together, these results suggest that GSCs are responsible for GBM recurrence and that a significant improvement in GBM patient outcome will require strategies that target these therapy resistant cells.
ABCG2 (ATP-binding cassette sub-family G member 2, also known as BCRP (Breast Cancer Resistance Protein), and XIAP (X-linked inhibitor of apoptosis) are two proteins whose activities mediate therapy resistance and apoptosis resistance, respectively [8–10]. The mRNAs of these two genes have been shown to be expressed at higher levels in CD133+ GBM cells than in CD133- cells [4]. ABCG2 is a member of the ABC transporters that use ATP to efflux endogenous small molecules and exogenous cytotoxic drugs [10, 11]. As such, ABCG2 is highly expressed in the blood-brain-barrier (BBB) and blocks penetrance of many cytotoxic therapies to brain and brain tumors [12–14]. In addition, ABCG2 is the main stem cell-associated ABC transporter whose activity has been associated with the “side-population (SP)” phenotype. SP cells extrude a fluorescent dye, Hoechst 33342, which enables the isolation and analysis of this stem-like cell population [15–17]. Moreover, we have shown that GSCs in a mouse model of malignant glioma is enriched in SP cells [18]. Consistently, SP cells have been shown to enrich for GSCs in human GBMs and other mouse models [19]. XIAP is a member of the family of inhibitors of apoptosis (IAPs) that mediate resistance to apoptosis, and has been recently pursued as a new therapeutic target in solid tumors [9, 20].
In this study, we report that protein levels of ABCG2 and XIAP are associated with poor survival among GBM patients. We also report that inhibition of ABCG2 with small molecule inhibitors result in reduced self-renewal of GBM tumorspheres, suggesting, that ABCG2 is not merely a marker of GSCs but also a promoter of GSC self-renewal.
METHODS
Annotated biospecimens
We analyzed paraffin-embedded pre-treatment tumor samples were obtained from GBM patients (n=50) who went on to received radiation and temozolomide (49 of 50) as part of their disease management between 2002 and 2008 and for whom follow-up data were available. All patients had WHO Grade IV, GBM tumors. The population was 58% male and had a mean age of 64 (range: 29–82) (Online Resource 1). All samples had been fixed in 10% buffered formalin. Clinical data included age, extent of resection and overall survival. This part of the study did not require informed consent and was approved by the Maine Medical Center Institutional Review Board (IRB#3202X). To isolate glioma tumorsphere lines, fresh tumor samples were obtained from patients who consented to tissue donation. This part of the study was also approved by the Maine Medical Center Institutional Review Board (IRB #3960).
Immunohistochemical analysis of patient samples
Formalin-fixed, paraffin-embedded tumor samples were cut in 4μm-thick sections. Consecutive sections were then treated with primary antibodies purchased from Abcam to detect ABCG2 (cat# ab3380), XIAP (cat# ab21278), MSH2 (cat# ab2353), and NESTIN (cat# ab5968), from Cell Signaling to detect phospho-AKT at Ser 473 (cat# 4060) and phospho-TP53 at Ser 33 (cat# 2526), from Chemicon (now EMD Millipore) to detect MGMT (Cat# Mab16200), and from Rockland Immunochemicals to detect phospho-ATM at Ser 1981 (cat# 200-301-500) according to the manufacturer’s instructions. Primary antibodies were used in the following dilutions: anti-ABCG2 at 1:100, anti-XIAP at 1:300, anti-MGMT at 1:400, anti-MSH2 at 1:50, anti-pATM at 1:75, anti-pTP53 at 1:500, anti-pAKT at 1:50 and anti-Nestin at 1:500. All primary antibodies were visualized with biotinylated secondary antibodies, streptavidin/HRP enzyme complex and DAB chromogen. The slides were counterstained with hematoxylin. Sections were evaluated by the study pathologist using light microscopy and scored based on a semi-quantitative approach of percentage of positive tumor cells (0–100%), multiplied by staining intensity (1= weak, 2=moderate, 3=strong). For tumors in which there was more than one block available for sectioning and staining (n=9), each section was stained and scored, and the final score for that tumor was the average between the scores in the set. In this manner, a total score range of 0–300 was generated for each marker for each tumor. Descriptive statistics, including median, range and box plots were conducted using the XLSTAT software package for EXCEL (Addinsoft). All raw and calculated scores are displayed in Supplementary Table 1. Clinical data were kept unavailable during the immunohistochemical analysis until all data were evaluated.
Survival analyses according to protein expression
Univariate analysis of survival according to protein expression was conducted using the Kaplan-Meier method. Patients were stratified into low-expression (samples scoring 50 or less) and high-expression (samples scoring above 50) for each of the markers analyzed. The expression categories were defined prior to analyses in order to reduce the probability of making a type I error, using the study pathologist’s experience with immunohistochemical analyses and what would be considered positive versus negative signals in a clinical pathology setting. Expression levels of all 8 proteins were categorized in the same manner. Overall survival was measured in months and only 4 patients had not had the event (death) at the time of the analyses and were censored. Multivariate analyses including age, extent of resection, and MGMT expression in addition to ABCG2 or XIAP protein expression, were conducted using the Cox regression method. For these analyses, patients were divided according to age into two groups using the median survival as the pivot point (<64 and >/=64), according to extent of resection (Total vs. less than total) and according to MGMT expression (High vs. low). Kaplan-Meier analyses were conducted using XLSTAT (Addinsoft) and Cox regression analyses were conducted using XLSTAT and SAS.
Survival analyses according to RNA expression
The TCGA GBM database (http://cancergenome.nih.gov/cancersselected/glioblastomamultiforme), which contains gene expression data on 580 individuals and linked phenotypic data, including mortality, were used to determine if XIAP and ABCG2 RNA levels were associated with survival. An analysis of covariance was run with a covariate of gene intensity and fixed effects of gender and treatment history. Kaplan-Meier plots were used to investigate the intensity effect of ABCG2 and XIAP on survival rate. Within the K-M plots, samples were classified as low-expression (below median gene intensity value) or high-expression (above median).
Tissue culture and secondary sphere formation assay
Primary GBM tumorsphere lines, MMC1, MMC10 and MMC11, were established from fresh clinical samples. Patient GBM tissues were coarsely homogenized with a blade and incubated with Accutase for 5–10 minutes at 37 °C. Dissociated single cells were cultured in neural stem cell medium (NSC medium: DMEM/F-12 1:1 with B27, hEGF (20ng/ml), hBFGF (20ng/ml), and Pencillin/Streptomycin) to establish stable tumorsphere lines. To test secondary sphere formation, tumorspheres were dissociated into single cells using Accutase, and 3000 cells were seeded in 3ml of NSC medium in 6 well plates in duplicates. Numbers of spheres (composed of >50 cells) in control or drug treated wells were counted 10 days later. U87MG cells were purchased from ATCC and routinely cultured in 10% FBS + pen/strep or in NSC medium for secondary sphere formation assays.
Chemicals
Embelin (Sigma) was dissolved in DMSO as a 27.2 mM stock solution and dissolved in 1:4 (vol/vol) DMSO/ethanol as 100μM working stock solution. Ko143 (Tocris) and fumitremorgin (Sigma) were dissolved in DMSO as 1 mM stock solution. Temozolomide (Temodar for IV) was dissolved in ddH20 as 0.5 M stock solution. Vehicle control (VEH) consisted of ddH20, DMSO or DMSO/ethanol equivalent to treatments.
Immunocytochemical analyses of tumorsphere cultures
Low passage GBM tumorsphere cells were seeded onto poly-D-Lysine-coated coverslips and fixed with 4% paraformaldehyde. For immunofluorescence analyses and FACS analyses, standard protocols were used. Antibodies were purchased from commercial sources: XIAP (1:600, Abcam) and ABCG2 (1:200, Abcam), NESTIN (1:600, BD Transduction laboratories), β-III-tubulin (1:300, Promega), GFAP (1:500, Chemicon), CD133-PE (Miltenyi), and CD15/SSEA1-FITC (BD Transduction laboratories). Goat Anti-Rabbit Alexa-488 (1:1000) and Goat Anti-mouse Alexa-594 (1:1000) secondary antibodies were purchased from Life Technologies. Nuclei were counter-stained with DAPI.
Mouse xenografts
To determine tumorigenic potential of MMC10 tumorsphere cells, 1×105 cells were injected into the striatum of NOD-SCID;Il2gr−/− (NSG) mouse brain using a stereotaxic device. When mice showed signs of brain tumor, they were euthanized to harvest tumor tissues for analysis.
Viability assays
GUAVA (EMD Millipore) and CellTiter-Blue (Promega) cell viability assays to measure viability were performed according to manufacturer’s recommendations.
Fluorescence-activated cell sorting (FACS)
Established tumorsphere cells were dissociated into single cells using Accutase and stained with antibodies against CD133 (PE) and CD15/SSEA1 (FITC) using a standard FACS protocol.
RESULTS
Mediators of therapy resistance are differentially expressed in GBM patient tumors
To determine the role of genes implicated in glioma therapy resistance and stem cells, we selected eight candidate proteins (ABCG2, XIAP, MGMT, MSH2, ATM, P53, AKT, and NESTIN) and performed immunohistochemical analyses on formalin-fixed, paraffin embedded tissue samples from 50 GBM patients (see Online Resource 1 for patient and tumor characteristics). Using antibodies specific to each protein, we compared the expression patterns of proteins involved in DNA repair (MGMT, MSH2, pATM), cell survival (XIAP, pTP53, pAKT), multidrug resistance (ABCG2), as well as, a stem cell marker (NESTIN). Each sample was scored by the study pathologist (HB) based on the extent and intensity of the signal (See Methods). All the raw and calculated scores are presented in Online Resource 2.
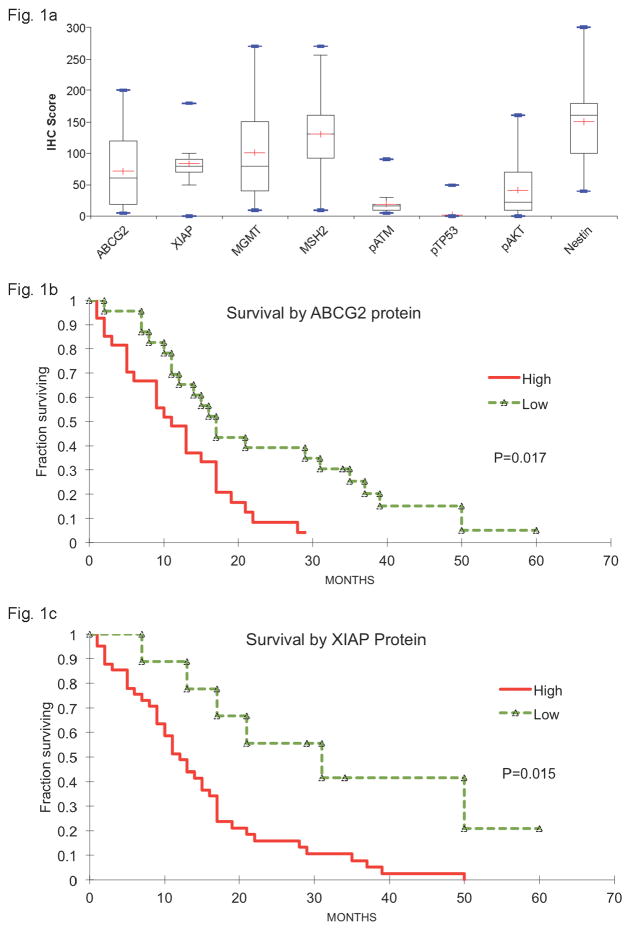
There was a wide range of protein expression patterns among GBM samples (Fig. 1a). Phospho-p53 (pTP53) was barely detectable in most samples, with a median score of 0, and phospho-ATM (pATM) was weakly detectable with a median score of 17. On the other hand, NESTIN and MSH2 appeared nearly ubiquitous with median scores of 160 and 130, respectively. The other proteins had a wider range of expression, from samples displaying undetectable levels to samples displaying robust levels, indicating high intra- and inter-tumoral heterogeneity. MGMT and phospho-AKT (pAKT) had median scores of 80 and 23, respectively. ABCG2 and XIAP had median scores of 60 and 80, respectively. Summary expression data are presented in Figure 1a.
Fig. 1. ABGC2 and XIAP protein expression levels are associated with GBM patient survival.
a) Box plot analyses of the immunohistochemical (IHC) scores of paraffin-embedded, formalin-fixed tumor samples from GBM patients after monoclonal antibodies were used to visualize the expression of 8 protein targets (ABCG2, XIAP, MGMT, MSH2, pATM, pTP53, pAKT, and NESTIN). Each box represents the IHC scores between the 1st and the 3rd quartile. Lines represent the median IHC score (
 −) and the maximum and minimum IHC scores (
−) and the maximum and minimum IHC scores (
 −). b,c) Kaplan-Meier analyses of overall survival in months among GBM patients whose tumor samples had low expression by IHC
−). b,c) Kaplan-Meier analyses of overall survival in months among GBM patients whose tumor samples had low expression by IHC
 (− −) versus high expression (
(− −) versus high expression (
 −−−) of ABCG2 (b) or XIAP (c) proteins.
−−−) of ABCG2 (b) or XIAP (c) proteins.
ABCG2 and XIAP protein expression levels are associated with GBM patient survival
To determine whether differential protein expression levels were associated with GBM patient survival, we analyzed the clinical outcome data of the 50 GBM patients. We first assigned tumors with expression score of 50 or less as “low expression” and those with scores above 50 as “high expression” based on the clinical judgment of the study pathologist (HB). Then we compared survival of patients with “high” and “low” expression scores for each protein. In agreement with previous results [21, 22], high expression of MGMT and pAKT was associated with shorter survival (Online Resource 3). In addition, we observed that high expression of ABCG2 and XIAP was similarly associated with significantly shorter survival (p=0.02 and 0.02, respectively, Fig. 1b and 1c). The crude hazard ratio for high-ABCG2 versus low-ABCG2 expression was 2.32 (Table 1, 95% confidence interval of 1.20–4.47) and for high-XIAP versus low-XIAP expression, it was 3.20 (Table 1, 95% confidence interval of 1.33–7.71). Since age, extent of resection and MGMT expression are known to affect survival, we sought to correct for these confounders. Table 1 displays the number and percentage of patients below age 64 (the median survival age for GBM patients) and those older, as well as patients who had a total gross resection of their tumor versus those who had a subtotal resection or a biopsy only. MGMT protein IHC score data were used as proxy for MGMT expression because the more commonly used MGMT promoter methylation data were not available for many of these patients. After taking into account these confounders, the adjusted hazard ratio for high ABCG2 versus low ABCG2 expression was 2.35 (95% confidence interval of 1.14–4.84) and for high versus low XIAP expression was 2.65 (95% confidence interval of 1.01–6.95). In contrast, the analysis of ABCG2 and XIAP RNA levels in the GBM dataset from The Cancer Genome Atlas (TCGA) did not reveal a significant association between XIAP RNA levels and patient survival, (Online Resource 3). Interestingly, patients with low ABCG2 RNA levels had worse survival (Online Resource 3, p= 0.03), suggesting that ABCG2 RNA and protein levels may not correlate in GBMs. These results indicate that ABCG2 and XIAP proteins, as detected by immunohistochemistry on formalin-fixed paraffin embedded tumor samples, may represent clinically useful independent prognostic indicators of GBM patient survival that warrant further analysis and validation.
Table 1.
Multivariate adjusted hazard ratios and 95% confidence interval of death according to ABCG2 and XIAP high vs. low expression
| ABCG2 high | ABCG2 low | XIAP high | XIAP low | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| N | % | N | % | N | % | N | % | ||
| AGE | <64 | 12 | 44 | 16 | 79 | 19 | 46 | 9 | 100 |
| 64+ | 15 | 56 | 7 | 30 | 22 | 54 | 0 | 0 | |
|
|
|||||||||
| SURGERY | Total gross | 13 | 48 | 14 | 61 | 21 | 51 | 6 | 66 |
| Subtotal/biopsy | 14 | 52 | 9 | 39 | 20 | 49 | 3 | 33 | |
|
|
|||||||||
| MGMT | High expression | 21 | 78 | 9 | 39 | 27 | 66 | 2 | 22 |
| Low expression | 6 | 22 | 14 | 61 | 13 | 32 | 7 | 78 | |
|
|
|||||||||
| TOTAL | 27 | 23 | 41 | 9 | |||||
| CRUDE HR | ADJUSTED HR for age, extent of surgery and MGMT expression |
|
|---|---|---|
|
|
||
| ABCG2 | 2.32 (95% CI 1.20–4.47) | 2.35 (95% CI 1.14–4.84) |
| XIAP | 3.20 (95% CI 1.33–7.71) | 2.65 (95% CI 1.01–6.95) |
Primary GBM tumorsphere cells express XIAP and ABCG2
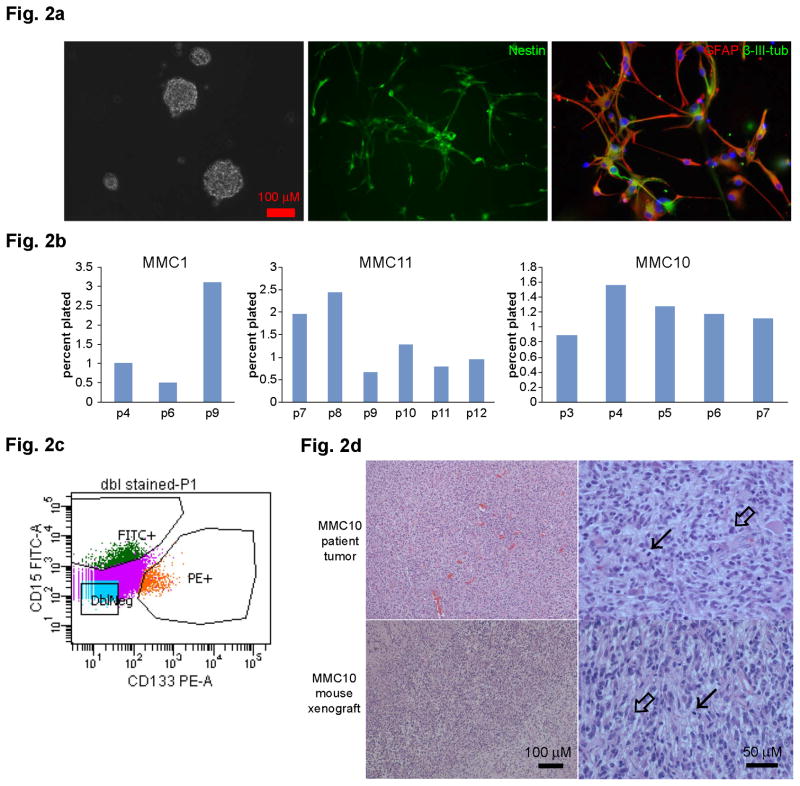
Since patient tumor analyses revealed that poor survival is associated with high protein levels of XIAP and ABCG2 and these genes were reported to be higher expressed in CD133+ cells compared to CD133- cells [4], we tested whether ABCG2 and XIAP are functionally important in GSCs. To do this, we isolated and established tumorsphere lines from multiple fresh GBM patient tumors resected at Maine Medical Center. These GBM tumorsphere lines expressed NESTIN, a neural stem cell marker, as well as markers of neuronal (β-III-tubulin) and glial (GFAP) lineages upon differentiation in vitro (Fig. 2a), indicating multi-lineage differentiation potential in these tumorsphere lines. Furthermore, they can self-renew in serum-free neural stem cell (NSC) medium for > 20 passages in vitro. The percentage of secondary sphere forming cells varied from 0.5 to 3.1 % in different tumorsphere lines (Fig. 2b). The tumorsphere lines also expressed varying combinations of cell surface GSC markers, CD133 and CD15/SSEA1 (Fig. 2c). In addition, when injected into the striatum of NOD-SCID;Il2gr−/− (NSG) mice, they formed highly invasive tumors that resemble the original patient tumor (Fig. 2d). For example, dense cellularity, atypical nuclei and high mitotic activity can be observed in both the original MMC10 patient tumor and the corresponding xenografted tumor (Fig. 2d).
Fig. 2. Isolation of primary GBM tumorspheres that are self-renewing, multipotential, and tumorigenic.
a) Tumorspheres derived from patient samples express a neural stem cell marker (NESTIN). They can be differentiated to express a neuronal marker (β-III-tubulin) and a glial marker (GFAP). b) Three independent patient tumorsphere lines, MMC1, MMC10, and MMC11, were tested for secondary sphere formation in vitro at a clonal density for multiple passages. Approximately 0.5–3% of tumorsphere cells self-renew long term and can be passaged for >20 passages (not shown). The number of passages is denoted below each graph. c) Tumorsphere cells express GSC markers, CD133 and CD15/SSEA1, as determined by FACS analyses. d) Microscopic images of tissue sections stained with hematoxylin and eosin at high and low magnifications. Solid arrows point to mitoses and open arrows point to atypical cells.
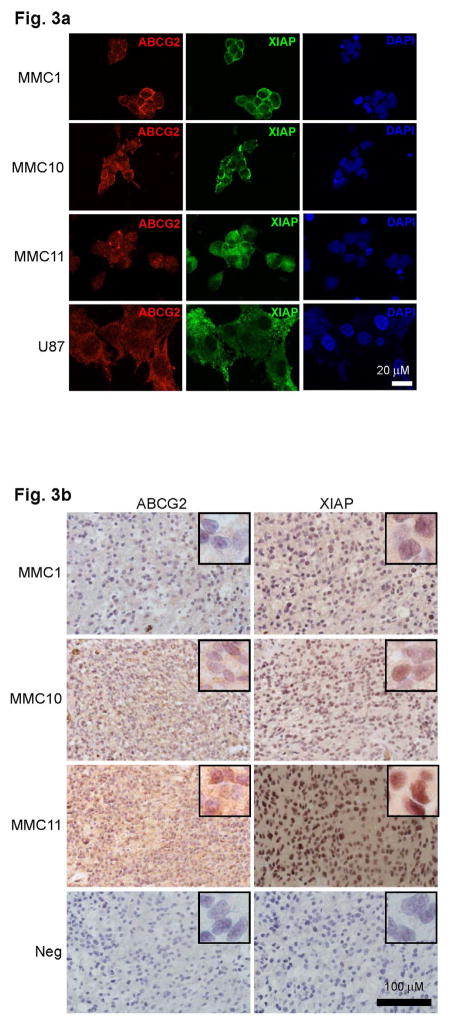
Using three independent primary GBM tumorsphere lines (MMC1, MMC10, MMC11), we analyzed expression patterns of the ABCG2 and XIAP proteins. XIAP and ABCG2 were both expressed in the three tumorsphere lines (Fig. 3a), in agreement with the matching patient tumors (Fig. 3b).
Fig. 3. ABCG2 and XIAP expression in matching patient tumors and tumorsphere lines.
a) Immunofluoresence analyses of patient-derived tumorsphere lines MMC1, MMC10 and MMC11 and U87 cells stained with antibodies against ABCG2 (red), and XIAP (green). All nuclei are stained with DAPI (blue). b) immunohistochemistry (IHC) images of MMC1, MMC10, and MMC11 patient tumors using antibodies against ABCG2 and XIAP. IHC scores for MMC1, MMC10 and MMC11 were 40, 160 and 180 respectively for ABCG2, and 100, 140 and 100 respectively for XIAP.
ABCG2 inhibition suppresses GBM tumorsphere self-renewal
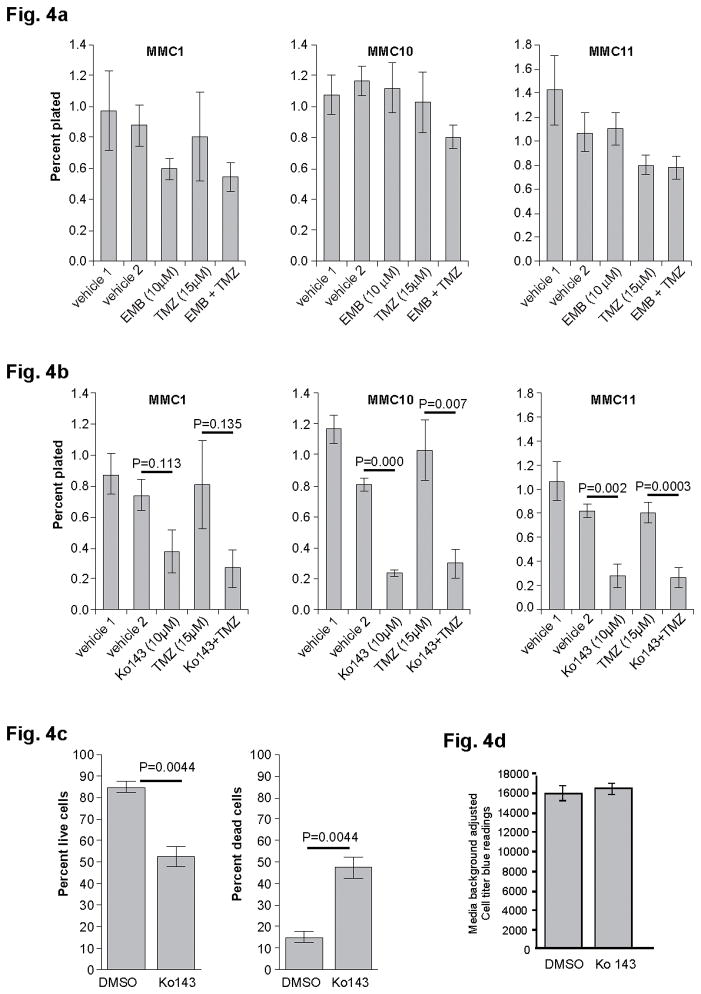
To determine whether XIAP and ABCG2 have a role in GSC self-renewal or survival, we performed secondary sphere formation assays in the presence or absence of small molecule inhibitors of XIAP (Embelin) or ABCG2 (Ko143 and Fumitremorgin (Ftc)). Dissociated, low passage GBM tumorsphere cells from MMC1, MMC10, and MMC11 lines were plated at a clonal density (1cell/μl) in NSC medium. GBM cells were treated with either 10μM Embelin (EMB), Ko143, or Ftc, with corresponding vehicle alone as controls. This concentration was chosen to achieve complete inhibition of the three targets as previously reported [[23, 24]]. In addition, EMB and Ko143 were tested in combination with Temozolomide (TMZ, 15 μM), the primary chemotherapeutic agent used to treat GBMs. Treatment of GBM tumorspheres with EMB, alone or in combination with TMZ, had little to no effect on self-renewal (Fig. 4a). In contrast, Ko143 treatment reduced self-renewal in the three MMC lines tested. This reduction was statistically significant in MMC10 and MMC11 (p=0.0001 and p=0.002, respectively) where overall ABCG2 expression was high, but not MMC1 cells (p<0.11, Fig. 4b) where ABCG2 expression was low (Fig. 3b). Consistent results were obtained when cells were treated with a different ABCG2 inhibitor, Ftc (Online Resource 4 and not shown). To determine whether Ko143 specifically affected self-renewal, we measured percentages of live and dead cells post treatment. Viability analysis using the MMC11 line showed that 10μM treatment of Ko143 increased the percentage of dead cells and decreased percentage of live cells (p=0.004, Fig. 4c), indicating that increased cell death contributes to decreased self-renewal. To test whether ABCG2 inhibition affects GBM cell survival in general, we treated U87MG cells, an established human GBM cell line, with Ko143. Unlike primary GBM tumorsphere cells, U87MG cell viability did not change with Ko143 treatment (Fig. 4d), suggesting that 10μM K0143 is not generally toxic but that GSCs in tumorspheres are sensitive to Ko143 treatment. Together, these results suggest that inhibition of ABCG2 function suppresses self-renewal of GBM cells in a subset of patients.
Fig. 4. Inhibition of ABCG2 suppresses GBM tumorsphere self-renewal.
Secondary sphere formation assay with low passage (p<10) MMC1, MMC10, and MMC11 cells in the presence of (a) 10μM Embelin (XIAP inhibitor) or (b) 10μM Ko143 (ABCG2 inhibitor) alone or, in combination with 15μM Temozolomide (TMZ). Error bars: SEM. c) GUAVA viability measurement of MMC11 cells showing live/dead cells at 72 hours post 10μM Ko143 treatment. Error bars= STME. d) Viability of U87 cells treated with control (DMSO) or 10μM Ko143. Error bars: STME.
DISCUSSION
In this study, we show that high levels of ABCG2 and XIAP protein expression are associated with worse GBM patient survival and that ABCG2 inhibition decreases survival and self-renewal of GSCs in a subset of GBMs. Although ABCG2 has been implicated in modulating migration/invasion of glioma cells independent of its role as a membrane transporter [25], this is the first report of its role in promoting GSC self-renewal/survival (in the absence of chemotherapy treatment) to our knowledge. Since GSCs are implicated in GBM recurrence, our data support further exploration of the mechanism of ABCG2’s effect on glioma cell self-renewal since it may represent an unexplored therapeutic avenue against this disease.
Interestingly, RNA and protein level analyses of ABCG2 expression levels and patient survival showed conflicting results. While protein level analyses showed poorer survival for patients with high levels of expression, RNA level analyses of the TCGA data suggested better survival for patients with high ABCG2 RNA levels. A possible explanation for this discrepancy is that ABCG2 is post- transcriptionally and/or -translationally regulated in GBM cells such that RNA and protein levels do not correlate. Previous studies have reported that ABCG2 is post-transcriptionally regulated [26, 27]. In addition, others have reported that ABCG2 activity was predicted by its protein abundance and not by its mRNA expression [28]. Our observation is consistent with these reports and suggests that protein level analyses of ABCG2 expression must be included in future studies.
ABCG2 has received much attention as a potential therapeutic target for treating malignant cancers for two principal reasons. First, it is expressed in drug-resistant cancer cells and contributes to therapy resistance by extruding chemotherapies, including Doxorubicin, Ironotecan, Methotrexate, Topotecan, Imatinib and others [10, 29]. For example, a recent study using a mouse medulloblastoma model showed that combining ABCG2 inhibition by Ko143 treatment significantly enhanced therapeutic effect of topotecan, compared to topotecan treatment alone [12]. Consistently, dual ABCG2/Pgp inhibitors, such as Elacridar, have been tested in a limited number of human clinical trials where it has shown that co-administration of Elacridar increased the oral bioavailability of topotecan [30, 31]. Second, it is expressed in the endothelial cells of the blood-brain barrier where it is part of the protective mechanism that restricts entry of exogenous compounds, including small molecule chemotherapeutics into the brain [13, 32]. In this study, we propose a third reason for pursuing ABCG2 as a potential target in GBMs: its role in promoting survival and self-renewal of GSCs.
Our findings in this report are consistent with multiple studies implicating ABCG2 in stem cell maintenance. Others and we have shown that SP cells are enriched with GSCs in mouse models of malignant glioma and in U87 cells [18, 19]. In addition, ABCG2 was reported to be a direct target of Notch and HIF2a, key regulators of self-renewal in normal neural stem cells and GSCs [33–35]. Furthermore, Ko143 was shown to reduce self-renewal of prostate stem and progenitor cells of the mouse ventral prostate [36]. Newer and more specific inhibitors of ABCG2 should be tested in the clinic.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Maine Medical Center Institutional Review Board and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Supplementary Material
Acknowledgments
We thank Dr. Lee Lucas for her guidance with the statistical methods and Drs. Tom Gridley, Wendy Craig and Christine Lu-Emerson for their critical review of the manuscript. We also thank Jesse Hammer at the Jackson Laboratory’s Multimedia Department for his expert assistance in preparing the figures. We would like to acknowledge the Maine Medical Center BioBank, for their assistance in procuring biospecimens in accordance with IRB guidelines, and the Maine Medical Center Histology Core Facility (grants P30GM103392 (R. Friesel, P.I.) and P30GM103465 (D. Wojchowski, P.I.), by the National Institute of General Medical Sciences) for their assistance with the immunohistochemistry staining. This work was funded by unrestricted research grants from the Maine Medical Center Neuroscience Institute, the Northern New England Clinical Oncology Society, the Schering-Plough Corporation (grant ID XX-3903), and the Donaldson Charitable Trust.
Footnotes
Conflict of Interest: This work was funded by unrestricted research grants from the Maine Medical Center Neuroscience Institute, the Northern New England Clinical Oncology Society, and the Schering-Plough Corporation (grant ID XX-3903). These sponsors had no involvement in study design, data collection, analysis or interpretation, manuscript writing, or the decision to submit the article for publication. The authors report no conflicts of interest beyond the direct research support detailed above.
References
- 1.Johnson DR, O’Neill BP. Glioblastoma survival in the United States before and during the temozolomide era. J Neurooncol. 2012;107:359–364. doi: 10.1007/s11060-011-0749-4. [DOI] [PubMed] [Google Scholar]
- 2.Visvader JE, Lindeman GJ. Cancer stem cells: current status and evolving complexities. Cell stem Cell. 2012;10:717–728. doi: 10.1016/j.stem.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 3.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 4.Liu G, Yuan X, Zeng Z, Tunici P, Ng H, Abdulkadir IR, Lu L, Irvin D, Black KL, Yu JS. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer. 2006;5:67. doi: 10.1186/1476-4598-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 6.Hambardzumyan D, Becher OJ, Holland EC. Cancer stem cells and survival pathways. Cell Cycle. 2008;7:1371–1378. doi: 10.4161/cc.7.10.5954. d[pii] [DOI] [PubMed] [Google Scholar]
- 7.Chen J, Li Y, Yu T-S, McKay RM, Burns DK, Kernie SG, Parada LF. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488:522–526. doi: 10.1038/nature11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirschmann-Jax C, Foster AE, Wulf GG, Nuchtern JG, Jax TW, Gobel U, Goodell MA, Brenner MK. A distinct “side population” of cells with high drug efflux capacity in human tumor cells. Proc Natl Acad Sci U S A. 2004;101:14228–14233. doi: 10.1073/pnas.0400067101. 0400067101 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vellanki SH, Grabrucker A, Liebau S, Proepper C, Eramo A, Braun V, Boeckers T, Debatin KM, Fulda S. Small-molecule XIAP inhibitors enhance gamma-irradiation-induced apoptosis in glioblastoma. Neoplasia. 2009;11:743–752. doi: 10.1593/neo.09436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noguchi K, Katayama K, Sugimoto Y. Human ABC transporter ABCG2/BCRP expression in chemoresistance: basic and clinical perspectives for molecular cancer therapeutics. Pharmgenomics Pers Med. 2014;7:53–64. doi: 10.2147/PGPM.S38295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ricci JW, Lovato D, Larson RS. ABCG2 Inhibitors: Will They Find Clinical Relevance? J Develop Drugs. 2015:4. doi: 10.4172/2329-6631.1000138. [DOI] [Google Scholar]
- 12.Morfouace M, Cheepala S, Jackson S, Fukuda Y, Patel YT, Fatima S, Kawauchi D, Shelat AA, Stewart CF, Sorrentino BP, Schuetz JD, Roussel MF. ABCG2 Transporter Expression Impacts Group 3 Medulloblastoma Response to Chemotherapy. Cancer Res. 2015;75:3879–3889. doi: 10.1158/0008-5472.CAN-15-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robey RW, Ierano C, Zhan Z, Bates SE. The challenge of exploiting ABCG2 in the clinic. Curr Pharm Biotechnol. 2011;12:595–608. doi: 10.2174/138920111795163913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–284. doi: 10.1038/nrc1590. nrc1590 [pii] [DOI] [PubMed] [Google Scholar]
- 15.Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M, Nakauchi H, Sorrentino BP. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med. 2001;7:1028–1034. doi: 10.1038/nm0901-1028. [DOI] [PubMed] [Google Scholar]
- 16.Goodell MA, Rosenzweig M, Kim H, Marks DF, DeMaria M, Paradis G, Grupp SA, Sieff CA, Mulligan RC, Johnson RP. Dye efflux studies suggest that hematopoietic stem cells expressing low or undetectable levels of CD34 antigen exist in multiple species. Nat Med. 1997;3:1337–1345. doi: 10.1038/nm1297-1337. [DOI] [PubMed] [Google Scholar]
- 17.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris MA, Yang H, Low BE, Mukherje J, Guha A, Bronson RT, Shultz LD, Israel MA, Yun K. Cancer stem cells are enriched in the side population cells in a mouse model of glioma. Cancer Res. 2008;68:10051–10059. doi: 10.1158/0008-5472.CAN-08-0786. 68/24/10051 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bleau AM, Hambardzumyan D, Ozawa T, Fomchenko EI, Huse JT, Brennan CW, Holland EC. PTEN/PI3K/Akt pathway regulates the side population phenotype and ABCG2 activity in glioma tumor stem-like cells. Cell stem Cell. 2009;4:226–235. doi: 10.1016/j.stem.2009.01.007. S1934-5909(09)00010-1 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee FA, Zee BC, Cheung FY, Kwong P, Chiang CL, Leung KC, Siu SW, Lee C, Lai M, Kwok C, Chong M, Jolivet J, Tung S. Randomized Phase II Study of the X-linked Inhibitor of Apoptosis (XIAP) Antisense AEG35156 in Combination With Sorafenib in Patients With Advanced Hepatocellular Carcinoma (HCC) Am J Clin Oncol. 2014 doi: 10.1097/COC.0000000000000099. [DOI] [PubMed]
- 21.Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, Bromberg JE, Hau P, Mirimanoff RO, Cairncross JG, Janzer RC, Stupp R. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki Y, Shirai K, Oka K, Mobaraki A, Yoshida Y, Noda SE, Okamoto M, Suzuki Y, Itoh J, Itoh H, Ishiuchi S, Nakano T. Higher pAkt expression predicts a significant worse prognosis in glioblastomas. J Radiat Res. 2010;51:343–348. doi: 10.1269/jrr.09109. [DOI] [PubMed] [Google Scholar]
- 23.Allen JD, van Loevezijn A, Lakhai JM, van der Valk M, van Tellingen O, Reid G, Schellens JH, Koomen GJ, Schinkel AH. Potent and specific inhibition of the breast cancer resistance protein multidrug transporter in vitro and in mouse intestine by a novel analogue of fumitremorgin C. Mol Cancer Ther. 2002;1:417–425. [PubMed] [Google Scholar]
- 24.Nikolovska-Coleska Z, Xu L, Hu Z, Tomita Y, Li P, Roller PP, Wang R, Fang X, Guo R, Zhang M, Lippman ME, Yang D, Wang S. Discovery of embelin as a cell-permeable, small-molecular weight inhibitor of XIAP through structure-based computational screening of a traditional herbal medicine three-dimensional structure database. J Med Chem. 2004;47:2430–2440. doi: 10.1021/jm030420+. [DOI] [PubMed] [Google Scholar]
- 25.Shi L, Wang Z, Sun G, Wan Y, Guo J, Fu X. miR-145 inhibits migration and invasion of glioma stem cells by targeting ABCG2. Neuromolecular Med. 2014;16:517–528. doi: 10.1007/s12017-014-8305-y. [DOI] [PubMed] [Google Scholar]
- 26.Ogura J, Kobayashi M, Itagaki S, Hirano T, Iseki K. Post-transcriptional regulation of breast cancer resistance protein after intestinal ischemia-reperfusion. Biol Pharm Bull. 2008;31:1032–1035. doi: 10.1248/bpb.31.1032. [DOI] [PubMed] [Google Scholar]
- 27.Sandor S, Jordanidisz T, Schamberger A, Varady G, Erdei Z, Apati A, Sarkadi B, Orban TI. Functional characterization of the ABCG2 5′ non-coding exon variants: Stem cell specificity, translation efficiency and the influence of drug selection. Biochim Biophys Acta. 2016;1859:943–951. doi: 10.1016/j.bbagrm.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 28.Harwood MD, Neuhoff S, Rostami-Hodjegan A, Warhurst G. Breast Cancer Resistance Protein Abundance, but Not mRNA Expression, Correlates With Estrone-3-Sulfate Transport in Caco-2. J Pharm Sci. 2016;105:1370–1375. doi: 10.1016/j.xphs.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 29.Krishnamurthy P, Schuetz JD. Role of ABCG2/BCRP in biology and medicine. Annu Rev Pharmacol Toxicol. 2006;46:381–410. doi: 10.1146/annurev.pharmtox.46.120604.141238. [DOI] [PubMed] [Google Scholar]
- 30.Kuppens IE, Witteveen EO, Jewell RC, Radema SA, Paul EM, Mangum SG, Beijnen JH, Voest EE, Schellens JH. A phase I, randomized, open-label, parallel-cohort, dose-finding study of elacridar (GF120918) and oral topotecan in cancer patients. Clin Cancer Res. 2007;13:3276–3285. doi: 10.1158/1078-0432.CCR-06-2414. [DOI] [PubMed] [Google Scholar]
- 31.Kruijtzer CM, Beijnen JH, Rosing H, ten Bokkel Huinink WW, Schot M, Jewell RC, Paul EM, Schellens JH. Increased oral bioavailability of topotecan in combination with the breast cancer resistance protein and P-glycoprotein inhibitor GF120918. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002;20:2943–2950. doi: 10.1200/JCO.2002.12.116. [DOI] [PubMed] [Google Scholar]
- 32.Lagas JS, van Waterschoot RA, van Tilburg VA, Hillebrand MJ, Lankheet N, Rosing H, Beijnen JH, Schinkel AH. Brain accumulation of dasatinib is restricted by P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) and can be enhanced by elacridar treatment. Clin Cancer Res. 2009;15:2344–2351. doi: 10.1158/1078-0432.CCR-08-2253. [DOI] [PubMed] [Google Scholar]
- 33.Bleau AM, Huse JT, Holland EC. The ABCG2 resistance network of glioblastoma. Cell Cycle. 2009;8:2936–2944. [PubMed] [Google Scholar]
- 34.Bhattacharya S, Das A, Mallya K, Ahmad I. Maintenance of retinal stem cells by Abcg2 is regulated by notch signaling. J Cell Sci. 2007;120:2652–2662. doi: 10.1242/jcs.008417. jcs.008417 [pii] [DOI] [PubMed] [Google Scholar]
- 35.Wang Z, Li Y, Banerjee S, Sarkar FH. Emerging role of Notch in stem cells and cancer. Cancer letters. 2009;279:8–12. doi: 10.1016/j.canlet.2008.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Samant MD, Jackson CM, Felix CL, Jones AJ, Goodrich DW, Foster BA, Huss WJ. Multi-Drug Resistance ABC Transporter Inhibition Enhances Murine Ventral Prostate Stem/Progenitor Cell Differentiation. Stem Cells Dev. 2015;24:1236–1251. doi: 10.1089/scd.2014.0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.