Supplemental digital content is available in the text.
Key Words: Endometrial cancer, Patient-derived organoids (PDOs), Stemness
Abstract
Objective
Patient-derived organoids (PDOs), used in multiple tumor types, have allowed evaluation of tumor characteristics from individual patients. This study aimed to assess the feasibility of applying PDO in vitro culture for endocrine-based and drug sensitivity testing in endometrial cancer.
Methods
Endometrial cancer cells were enzymatically dissociated from tumors retrieved from fresh hysterectomy specimens and cultured within basement membrane extract in serum-free medium. An organoid growth assay was developed to assess the inhibitory effects of a variety of drugs including endocrine treatments. Organoid cultures were also prepared for histological and immunohistochemical comparison to the tumors of origin.
Results
Fifteen endometrial cancer specimens were successfully cultured as PDOs. Small spherical structures formed within 24 hours, and many continued to grow to larger, denser organoids, providing the basis for an organoid growth assay. The STAT3 transcription factor inhibitor, BBI608 (Napabucasin), strongly inhibited growth in almost all PDO cultures, suggesting that stemness programing is involved in organoid formation and/or growth. Inhibition by different growth factor receptor tyrosine kinase inhibitors was observed in several PDO specimens. Four cultures were inhibited by fulvestrant, implying the importance of estrogen-receptor signaling in some PDO cultures. Organoids closely resembled their tumors of origin in both histomorphology and immunohistochemical expression.
Conclusions
The use of endometrial cancer PDO cultures for development of drug sensitivity testing for individual patient tumors is feasible. The potential value of the PDO model for clinical decision making will require clinical trial evaluation.
Endometrial cancer is the most common gynecologic malignancy in the United States, with a growing incidence likely affected by changes in rates of obesity, median age, and progestin use.1 The most common histology is endometrioid adenocarcinoma, frequently expressing both estrogen receptor (ER) and progesterone receptor (PR). Treatment for most endometrial cancers includes surgery followed by adjuvant chemotherapy or radiation therapy, in presence of high-risk prognostic factors or metastatic disease. Endocrine-based treatments can be successfully used in patients who desire fertility or are not surgical candidates.2,3 However, even when ER and PR are expressed in such tumors, not all will respond to hormonal treatment, and currently there is no reliable way to identify patients for whom endocrine-based treatment will be effective.
Drug sensitivity outcomes have sometimes been linked to genomic factors, but even when the genomic landscape is known, predicting drug sensitivity is not always successful. Therefore, empiric drug sensitivity testing for individual patients could be valuable in clinical decision making, but such an approach has not yet become routinely feasible. Immortalized cancer cell lines have been used to model drug sensitivity but may not retain all of the unique and heterogeneous characteristics of the original tumor and are successfully derived in only a very small fraction of cases. Techniques for primary culture of endometrial cancer epithelial cells have been reported but with a success rate of 20%.4 Patient-derived xenograft tumors are more likely to represent the characteristics of the original tumor and can be repeatedly passaged, but the process is time consuming and expensive, and patient-derived xenograft lines are not successfully established in every case.5 Patient-derived organoid (PDO) cultures may represent a more efficient alternative for individual tumor culture. Patient-derived organoid cultures from several tumor types have been derived at a high success rate and shown to recapitulate characteristics of the tumors of origin such that genomic and functional studies could potentially allow for individualized treatment decisions.6
The 3-dimensional growth of malignant cells into spheroids in serum-free media is a property that may depend on a stem cell–like niche utilizing “stemness” signaling pathways.7 Cancer recurrence and treatment failure are believed to arise from treatment-resistant stem cell–like subpopulations.8 Consequently, the potential use of PDO cultures to model intrinsic resistance to traditional treatments, as well as susceptibility to novel therapies in individual tumor specimens, has great clinical relevance.
MATERIALS AND METHODS
Research subjects were recruited from the gynecologic oncology clinics at UC Davis Comprehensive Cancer Center in Sacramento, CA. All subjects gave written consent, and the process was approved by the UC Davis institutional review board. Only “leftover” tissues were utilized from hysterectomy specimens from women with endometrial endometrioid carcinoma. The specimens and associated clinical information were anonymized. Demographic and tumor characteristics of the enrolled subjects are shown in Table 1.
TABLE 1.
Subject demographics and tumor characteristics
Fresh tumor (approximately 0.5–1 cm3) was placed in 20-mL transport medium composed of the following: Dulbecco modified Eagle medium (DMEM)/F12 (Gibco #11320-033), 100 μg/mL Primocin (broad-spectrum antimicrobial; InvivoGen), 10 mM nicotinamide (Sigma #N0636), 0.5 μM A 83-01 (transforming growth factor β kinase/ALK5 inhibitor; Sigma #SML0788), 3 μM SB 202190 (p38 MAP kinase inhibitor; Sigma #S70767), and 10 μM Y-27632 (rho kinase inhibitor; Selleckchem #S1049). The tumor was minced into fragments no larger than 1 mm and placed into a 50-mL polypropylene tube with 10 mL of fresh transport medium also containing 2.5 mg/mL collagenase type IV (Gibco #17104-019), 40 μg/mL hyaluronidase (Sigma #H3757), and 2 U/mL DNAase 1 (Worthington #6344). After rocking at 37°C for 2 hours, the suspension was passed through a 100-μm sieve (Fisher Scientific #22363549). The flow-through cells were recovered by centrifugation and resuspended in 1 mL cold phosphate-buffered saline. Four milliliters of cold ammonium chloride solution (Stemcell Technologies #07800) was added for red blood cell lysis. After 10 minutes on ice, the cells were recovered, resuspended in cold DMEM/F12, and counted in a hemocytometer. After 2 additional washings, the cell pellet was resuspended in cold basement membrane extract (RGF BME type 2; Trevigen #3533-005) in preparation for either the organoid growth assay or bulk organoid cultivation.
For bulk organoid culture, dissociated endometrial cancer cells, suspended in BME at 1 to 2 × 106 cells/mL, were deposited in droplets of approximately 10 μL into 6-well tissue culture plates (150 μL per well total) as previously described.9,10 The inverted plates were placed at 37°C with 5% CO2, and the droplets allowed to solidify for 20 minutes. The wells were then filled with 2 mL “complete medium” (CM, similar to that described by van de Wetering et al,11 with some modifications) composed of the following: DMEM/F12, 2% B27 supplement (Gibco #17504-044), 10 mM HEPES (Gibco #15630080), 1% Glutamax (Gibco #35050), 1.25 mM N-acetyl cysteine (Sigma #S7250), 100 μg/mL Primocin, 10 mM nicotinamide, 0.5 μM A 83-01, 3 μM SB 202190, 10 μM Y-27632, and 1 nM 17-β estradiol (E2; Sigma #E2758). The media was changed every 2 days, and after a cultivation period of 2 to 3 weeks, organoids were harvested for passage or organoid growth assays. The wells were washed with 2 mL cold phosphate-buffered saline 3 times, and the BME droplets dissolved with 2 mL/well Cell Recovery Solution (Corning #354253), as described by the manufacturer. Recovered organoids were then used for histological analysis or dissociated for passage or organoid growth assay. For dissociation, organoids were centrifuged and washed twice with cold DMEM/F12, then resuspended in 5 mL Accutase (Gibco #A11105-01) at 37°C with intermittent gentle mixing until the organoids were mostly dissociated into single cells or small clusters, usually 15 to 20 minutes. To this suspension 2.5 mg/mL bovine serum albumin was added, and the mixture was passed through a 100-μm sieve. The flow-through cells were then washed with cold DMEM/F12, counted, and suspended in cold BME for passage into new 6-well plates for use in the organoid growth assay.
An organoid growth assay was applied either to freshly dissociated tumor cells (initial culture designated as passage 0 or “P0”) or to Accutase-dissociated cells from cultivated organoids (eg, passage 1 or “P1”). Cells were suspended in cold BME at 1 × 106 cells/mL. The suspension was placed in a sterile reagent trough on ice and continuously mixed on a tube rocker. A multichannel pipetter was used to deposit 5000 cells/well (5 μL of the suspension) into wells on a 96-well tissue culture plate. Plates were inverted and incubated at 37°C for 20 minutes, then 100 μL CM-containing test components were added to each well. Media were changed every 2 days, and after 2 weeks, the numbers of organoids with a diameter of more than 100 μm were determined using video microscopy (Olympus IX81). An initial organoid growth assay with P0 cells was performed to determine the inhibitory capacity of a panel of drugs using a single concentration approximating the therapeutic plasma concentration for each drug as follows: 1 μM megestrol acetate (Sigma #M0513), 1 μM fulvestrant (Sigma #I4409), 1 μM letrozole (Sigma #L6545), 4.6 μM mifepristone (Sigma #M8046), 1 μM erlotinib (epidermal growth factor [EGF] receptor inhibitor; Selleckchem #S1023), 1 μM linsitinib (insulinlike growth factor 1 receptor inhibitor; Selleckchem #S1091), 1 μM BGJ-398 (fibroblast growth factor [FGF] receptor inhibitor; Selleckchem #S2183), 1 μM BBI608 (Napabucasin, STAT3 inhibitor; Selleckchem #S7977), 50 μM cisplatin (Sigma #232120), and 1 μM paclitaxel (Sigma #T7402). All test components were dissolved in dimethyl sulfoxide at 1000× concentrations. Dimethyl sulfoxide was also added to the control wells at 0.1%. E2 was omitted in wells with letrozole. After 2 weeks, the mean number of organoids (>100 μm) per well among 6 control well replicates was compared with that of 6 test well replicates. Because BBI608 resulted in marked inhibition of growth in initial growth assay with P0 cells, P1 organoids were subjected to retesting for drug concentration causing 50% inhibition (IC50) measurement. Four point dilutions were done to allow calculation of IC50 values.
Organoids obtained from bulk cultures were fixed in 3.7% paraformaldehyde at room temperature for 1 hour and processed as described by Kessler et al.12 All tissues were routinely processed and embedded in paraffin for tissue sectioning. Paraffin sections were examined by hematoxylin-eosin, and deparaffinized sections used for immunohistochemistry (IHC). Slides were incubated with antibodies for ER, PR, Ki-67, AE1/AE3, and CD10 (Dako), ALDH-1 (1:1000 dilution; BD Biosciences), and CD44 (1:50 dilution; Dako) and processed on a Dako Autostainer. Immunohistochemistry results were scored either negative (no reactivity) or positive (>1% reactivity). Ki67 was expressed on a semiquantitative scale using an Olympus BX46 microscope.
Percent inhibition in the organoid growth assay was calculated as follows: percent inhibition = 100 − 100 (D / C), where C = average number of organoids per well among 6 control replicates, and D = average number of organoids per well among 6 drug replicates. Student t tests were used to compare the means of 6 control well organoid counts with mean organoid counts from the set of 6 wells for each test component. Two-tailed significance was set at P < 0.05. The IC50 calculations were derived by nonlinear regression analysis using GraphPad Prism software.
RESULTS
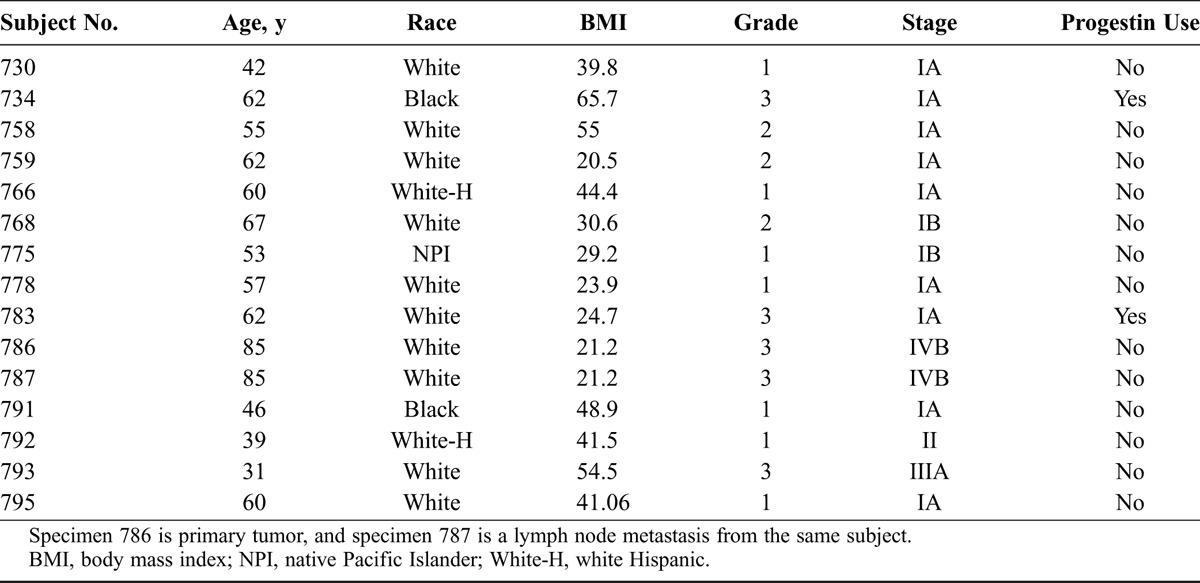
Fifteen endometrial cancer PDO primary cultures were successfully established from 14 endometrial and 1 metastatic tumors. Within the initial 12 hours of culture, small spheroids with well-defined margins formed in all cultures, most measuring less than 30 μm in diameter. Over the next 2 weeks, a subset of these spheroids progressively grew into larger, denser organoid structures (Supplementary Fig. S1, http://links.lww.com/IGC/A512). An example of the growth of a single organoid from specimen 778 is shown in Figure 1A.
FIGURE 1.
A, An example of progressive growth of a single organoid from specimen 778 (day 1: <30 μm, day 5: 45 μm; day 9: 95 μm; day 15: 240 μm). B, An example of stromal elements growing in PDO cultures (P1 culture 791). C and D, Varied growth of individual organoids of PDO culture 778 over 15 days; organoids of more than 100 μm continued to grow (C), organoids of less than 100 μm halted growth (D).
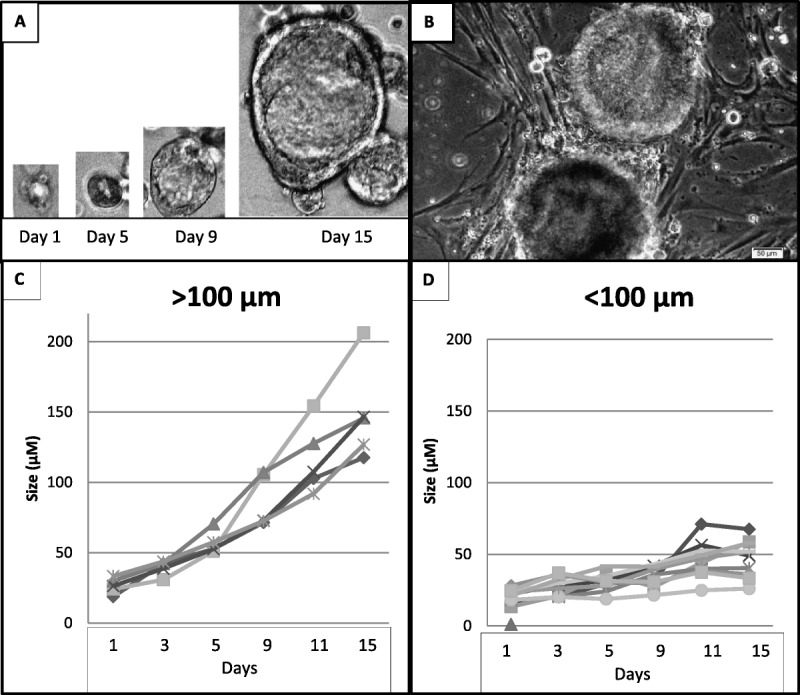
Individual organoids grew at varied rates, and some halted growth (Figs. 1C, D). Serial observation during 2 weeks of culture showed that individual organoids that eventually attained a diameter greater than 100 μm had continued to grow, whereas organoids of lesser diameter had mostly stopped growing. Figure 2 shows the distribution of organoid sizes for specimen 791 at days 0 and 11, showing that 23.2% of the total organoid population demonstrated progressive growth to greater than 100-μm diameter structures by day 11. Similar pattern organoid growth was seen in the other PDO cultures. Among 13 endometrial cancer specimens assayed as P0 untreated control cultures, seeded with 5000 cell/well (each with 6 replicate wells in 96-well format), the mean number of progressively growing organoids (>100 μm at 2 weeks) was 20.6/well (range, 6.5–38.8/well). The average coefficient of variation for this mean was 18.6% (range, 10.7%–33.1%).
FIGURE 2.
Distribution of organoid sizes at days 0, 6, and 11 of PDO culture 791, showing that 23.2% of the total organoid population demonstrated progressive growth to more than 100-μm-diameter structures by day 11.
In addition to the organoid forms, all P0 cultures contained a variable number of other cellular elements with fibroblast-like morphology (Fig. 1B). These nonepithelial elements were present in all P0 cultures; therefore, enumeration of the number of progressively growing organoids was chosen over ATP-based viability measurement as the end point of the organoid growth assay. Furthermore, the selection of organoids of more than 100 μm in diameter eliminated many structures that had spontaneously undergone growth arrest. In initial exploratory experiments, the growth of P0 cultures from specimens 730 and 734 was observed in the presence and absence of the following growth factors and ligands: 50 ng/mL EGF, 10 ng/mL FGF 2, 10 ng/mL FGF 10, 10 nM prostaglandin E2, 100 ng/mL R-spondin-1, 50 ng/mL Noggin, and 10 ng/mL Wnt3A. Because the number of organoids (>100 μm at 2 weeks of culture) was not significantly different than control cultures lacking these supplements (not shown) in P0 cultures, they were subsequently excluded from the growth medium. Several cultures were routinely passaged and showed progressive growth.
Almost all PDO cultures were dramatically inhibited by the 1 μM STAT3 inhibitor BBI608 (Napabucasin, US patent 8,877,803), suggesting that stemness programing is involved in organoid formation and/or growth. Eleven (78.6%) of 14 PDO cultures were significantly inhibited by 1 μM paclitaxel, suggesting a role for microtubule dynamics in organoid formation or growth. No PDO cultures were strongly inhibited by 50 μM cisplatin. Patient-derived organoid growth was significantly reduced in some cultures by different growth factor tyrosine kinase inhibitors, which may implicate the importance, in some cases, of autocrine or paracrine sources of growth factor signaling for organoid growth. Four (57.1%) of 7 grade 1 PDO cultures were inhibited by 1 μM fulvestrant, implying the importance of ER signaling in organoid formation and/or growth in some cases. None of the PDO cultures were inhibited by 1 μM megestrol acetate. Three PDO cultures were tested for inhibition by other progestins including 1 μM medroxyprogesterone acetate and 5 μM levonorgestrel, but none had significant inhibitory effects (not shown). Nine PDO cultures were tested with 4.65 μM mifepristone, and none were significantly inhibited. Patient-derived organoid cultures from primary tumor site (786) and lymph node metastasis (787) showed the same inhibition pattern in the growth assay (Supplementary Fig. S2, http://links.lww.com/IGC/A513). Complete assay results are summarized in Supplementary Table S3, http://links.lww.com/IGC/A516.
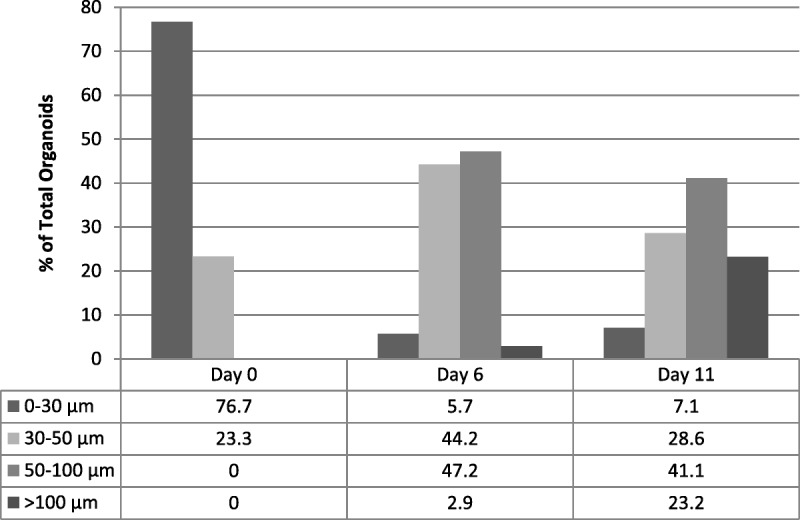
The IC50 values for BBI608 were obtained for 10 endometrial cancer PDO cultures (Table 2). The mean IC50 for BBI608 was 0.328 μM (range, 0.02–0.948 μM). An example of IC50 plot for BBI is shown in Supplementary Figure S5, http://links.lww.com/IGC/A514.
TABLE 2.
IC50 values for BBI608 for 10 endometrial cancer PDO cultures
Morphologically, organoids closely resembled their tumors of origin (Fig. 3). Patient-derived organoid and patient tumor had similar IHC staining patterns with few exceptions (Fig. 3 and Supplementary Table S4, http://links.lww.com/IGC/A515). All grade 1 endometrioid carcinomas expressed ER/PR in both PDO cultures and the original tumors. All endometrioid carcinoma (regardless of grade) expressed AE1/AE3 (epithelial marker) and lacked CD10 (stromal marker), which was expressed only in the benign stromal component of the original patient tumor samples. Ki-67 proliferation index was identical for PDO and patient tumor in 4 cases, but lower in 9 PDO cultures when compared with the original tumors. ALDH1 and CD44 have been reported to be associated with endometrial cancer stem cell–like cells.10,13 ALDH1 was positive in both PDO and patient tumor in all but 2 patients, which were both negative. However, CD44 expression showed more variability. Six cases showed CD44 positivity in both PDO and patient tumor, whereas the others were either negative in both (n = 3) or discordant (n = 4).
FIGURE 3.
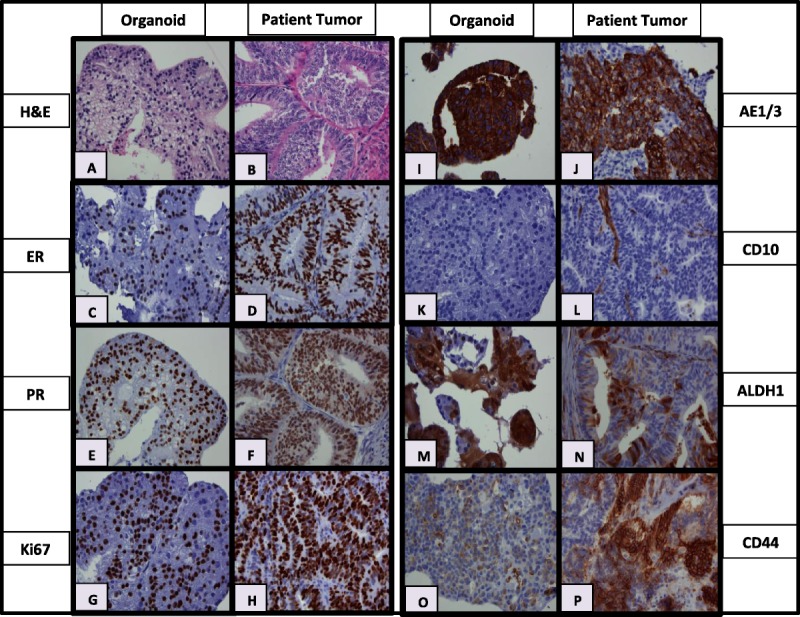
Hematoxylin-eosin and IHC results for representative PDO cultures (left) and their corresponding tumors of origin (right). A and B, Hematoxylin-eosin from specimen 730, (C, D) ER from 775, (E, F) PR from 759, (G, H) Ki67 from 730, (I, J) AE1/AE3 from 768, (K, L) CD10 from 759, (M, N) ALDH1 from 766, and (O, P) CD44 from 794.
DISCUSSION
Endometrial cancer PDO cultures have been grown successfully, and primary cultures have been utilized in individualized endocrine-based and drug sensitivity testing. Of 15 attempts, using the methods described here, PDO cultures were successfully obtained in 15 cases of endometrial cancer. This work confirms the reported results showing successful generation and drug testing of “cancer tumor-originated spheroids” from endometrial cancers, using similar methods and serum-free conditions.14 However, our current study evaluates drug sensitivity in primary organoid cultures, without use of xenografts. Using the methods described here, 2 weeks were required for initial screening assay results and approximately 2 weeks for IC50 assays. The organoid growth assays were done mostly with P0 cultures rather than multiply passaged cultures; therefore, the results more likely reflect the characteristics of the original tumor. Because P0 cultures were reconstituted from virtually all of the cellular elements within the tumor, preserved nonepithelial elements within the cultures could affect the growth of epithelial organoids.
Unlike reports for other tumor organoid systems,9,11,12,15 no exogenous growth factors (EGF, FGF2, FGF10), prostaglandins, or receptor ligands (R-spondin 1, Noggin, Wnt3A) were needed for primary endometrial cancer PDO generation and limited passage culture. This raises the possibility that autocrine or paracrine phenomena within the cultures support organoid growth during P0 organoid culture and possibly could reflect mechanisms active in the in vivo tumor microenvironment. Individual PDO cultures had distinct patterns of inhibition by the 3 receptor tyrosine kinase inhibitors tested in this study, suggesting a varied pattern of growth factor signaling among different PDO cultures.
The regenerative potential of the endometrium suggests existence of cellular subpopulations with stem cell characteristics, and endometrial neoplasms may deploy such mechanisms. Organoid formation in serum-free medium and STAT3 inhibitor BBI608 inhibition suggest that cells with stem cell–like characteristics are critical for organoid formation and growth.8 Further study will be required to clarify the characteristics of the cells needed to form organoids and in vivo testing done to confirm the value of this drug in endometrial cancer. Further study will also be required to determine if exogenous growth factors or other ligands are needed for long-term organoid passage. Characterization of genomic heterogeneity and transcriptome patterns between PDOs and their tumors of origin will expand the understanding of the organoid phenomenon in endometrial cancer. Future studies comparing xenograft and PDO models would further strengthen results of the current study.
The reproducibility of organoid growth in primary in vitro culture makes application of endocrine-based and drug testing feasible for personalized tumor characterization. Organoid growth dependence on E2 or ER signaling is evaluable using the organoid growth assay, but because PR signaling is not required for organoid growth, the PDO growth assay as described is not useful to test for progestin sensitivity. Because some endometrioid endometrial cancers respond to progestin treatment in vivo, this finding was surprising. Murine models suggest progestin effects in endometrial epithelium are mediated by paracrine signaling from endometrial stromal elements.16 Stromal elements necessary for producing progestin effects may be lacking in the PDO growth model described here. Alternatively, PDO formation and growth may derive from progestin-independent steps in tumor formation.
The organoid growth assay has additional limitations. The detection of large inhibitory effects on organoid formation/growth is feasible using this assay, but further work will be required to validate the reproducibility and robustness of the assay. The progressively growing fraction of organoids was chosen as the study parameter in the growth assay, but smaller structures (<100 μm) could reflect important steps in organoid formation independent of progressive growth. More sophisticated imaging techniques would be required to evaluate all of the characteristics of organoids of smaller size that did not demonstrate progressive growth; however, this was beyond the scope of this feasibility study. Similarly, while we made an assumption that the mesenchymal elements in the PDO cultures were derived from stromal cells within the tumors of origin, further studies will be required to evaluate the possibility that some cells with a mesenchymal appearance may result from epithelial-to-mesenchymal transitions. In addition, growth of organoids in BME and under serum-free conditions may derive from a narrow stem cell–like subpopulation of the total patient tumor cell population. Consequently, results of the organoid growth assay may not represent some characteristics of the original tumor in vivo, such as the differentiating fraction of the tumor that may not grow well in serum-free conditions. However, substantial evidence suggests that cancer stem cell–like elements may be involved in cancer treatment failure and recurrence.8 Patient-derived organoid cultures may permit the evaluation of drug sensitivities among such cancer stem cell–like subpopulations. Ultimately, clinical trials comparing drug sensitivity profiles derived from in vitro PDO growth assays to actual clinical outcomes in patients undergoing treatment would be required to validate the usefulness of the PDO assay for individualized clinical decision making.
CONCLUSIONS
Patient-derived organoid cultures from endometrial cancer specimens were successfully grown and passaged in serum-free conditions. Patient-derived organoids and their tumors of origin had similar morphologic and IHC characteristics. This in vitro model represents an efficient alternative to study endocrine profiles and drug susceptibilities in individual tumors. STAT3 inhibitor BBI608 demonstrated an almost uniform inhibition in PDO cultures and has potential as a novel targeted therapeutic agent in endometrial cancer. Clinical studies will be needed to correlate PDO assay results with patient outcomes.
Supplementary Material
Footnotes
Support was provided through research funds from the Department of Obstetrics and Gynecology at UC Davis.
The authors declare no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citation appears in the printed text and is provided in the HTML and PDF versions of this article on the journal’s Web site (www.ijgc.net).
REFERENCES
- 1.Wartko P, Sherman ME, Yang HP, et al. Recent changes in endometrial cancer trends among menopausal-age U.S. women. Cancer Epidemiol. 2013;37:374–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rose PG, Brunetto VL, VanLe L, et al. A phase II trial of anastrozole in advanced recurrent or persistent endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2000;78:212–216. [DOI] [PubMed] [Google Scholar]
- 3.Baker J, Obermair A, Gebski V, et al. Efficacy of oral or intrauterine device-delivered progestin in patients with complex endometrial hyperplasia with atypia or early endometrial adenocarcinoma: a meta-analysis and systematic review of the literature. Gynecol Oncol. 2012;125:263–270. [DOI] [PubMed] [Google Scholar]
- 4.Schrauwen S, Coenegrachts L, Depreeuw J, et al. Microsatellite instable and microsatellite stable primary endometrial carcinoma cells and their subcutaneous and orthotopic xenografts recapitulate the characteristics of the corresponding primary tumor. Int J Gynecol Cancer. 2015;25:363–371. [DOI] [PubMed] [Google Scholar]
- 5.Depreeuw J, Hermans E, Schrauwen S, et al. Characterization of patient-derived tumor xenograft models of endometrial cancer for preclinical evaluation of targeted therapies. Gynecol Oncol. 2015;139:118–126. [DOI] [PubMed] [Google Scholar]
- 6.Neal JT, Kuo CJ. Organoids as models for neoplastic transformation. Annu Rev Pathol. 2016;11:199–220. [DOI] [PubMed] [Google Scholar]
- 7.Chen SF, Chang YC, Nieh S, et al. Nonadhesive culture system as a model of rapid sphere formation with cancer stem cell properties. PLoS One. 2012;7:e31864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y, Rogoff HA, Keates S, et al. Suppression of cancer relapse and metastasis by inhibiting cancer stemness. Proc Natl Acad Sci U S A. 2015;112:1839–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drost J, Karthaus WR, Gao D, et al. Organoid culture systems for prostate epithelial and cancer tissue. Nat Protoc. 2016;11:347–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Zee M, Sacchetti A, Cansoy M, et al. IL6/JAK1/STAT3 signaling blockade in endometrial cancer affects the ALDHhi/CD126+ stem-like component and reduces tumor burden. Cancer Res. 2015;75:3608–3622. [DOI] [PubMed] [Google Scholar]
- 11.van de Wetering M, Francies HE, Francis JM, et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell. 2015;161:933–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kessler M, Hoffmann K, Brinkmann V, et al. The Notch and Wnt pathways regulate stemness and differentiation in human fallopian tube organoids. Nat Commun. 2015;6:8989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carvalho MJ, Laranjo M, Abrantes AM, et al. Clinical translation for endometrial cancer stem cells hypothesis. Cancer Metastasis Rev. 2015;34:401–416. [DOI] [PubMed] [Google Scholar]
- 14.Kiyohara Y, Yoshino K, Kubota S, et al. Drug screening and grouping by sensitivity with a panel of primary cultured cancer spheroids derived from endometrial cancer. Cancer Sci. 2016;107:452–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sato T, Stange DE, Ferrante M, et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterol. 2011;141:1762–1772. [DOI] [PubMed] [Google Scholar]
- 16.Janzen DM, Rosales MA, Paik DY, et al. Progesterone receptor signaling in the microenvironment of endometrial cancer influences its response to hormonal therapy. Cancer Res. 2013;73:4697–4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


