Summary
Intact human skin surface is essential for protection against infection, preservation of body fluid homeostasis and thermoregulation. Burn injury compromises the skin barrier and enables bacterial infection, hence delaying burn wound healing. This study aimed to determine the microbial profile of burn wounds, and resistance patterns of microbes with respect to the source of the injured patient’s wound. Fifty wound swab samples were collected from fifty burn patients at the Korle-Bu Teaching Hospital, Accra (KBTH). Sterile swabs moistened with sterile saline were used to swab burn wounds. The swabs were plated on blood agar and MacConkey agar for 24 hrs at 37°C. Biochemical tests were carried out on the representative isolate on each plate, and antibacterial sensitivity pattern was determined using the Kirby-Bauer disc diffusion method. The study revealed that the main source of burns was gas flames (66%) and scalds (28%). Out of the 50 samples analysed, 86% were culture positive and 14% were culture negative for bacteria. The predominant organisms isolated were Pseudomonas sp. (30.2%) and Acinetobacter sp. (20.9%). Proteus mirabillis (2.3%) and Staphylococcus aureus (2.3%) were the least frequently isolated bacteria. Although Pseudomonas sp. showed varying resistance levels to gentamicin, cotrimoxazole and ciprofloxacin, all the Acinetobacter sp. were resistant to most of the tested antibiotics used. Resistant gram negative bacteria are the most common isolates associated with burn wounds in Accra, Ghana. Hence a careful selection of antibiotics to control the wound infection is required for proper management of burn wounds in order to help reduce morbidity and mortality.
Keywords: burn wounds, Accra, bacteria, antibiotic resistance, gas
Abstract
L’intégrité cutanée est essentielle à la protection contre les infections, l’homéostasie circulatoire et hydro-électrolytique ainsi qu’à la thermorégulation. La brûlure détruit la barrière cutanée et permet l’infection locale, qui obère la cicatrisation. Le but de cetteétude était d’évaluer le profil microbiologique des infections cutanées (bactéries et antibiogrammes). Cinquante écouvillons cutanés obtenus sur autant de patients du CTB du CHU Korle-Bu d’Accra. Les prélèvements étaient des écouvillonnages humides. Les échantillons ont été incubés sur gélose au sang et milieu de Mc Conkey pendant 24 h à 37°C. Les identifications bactériennes étaient réalisées par test biochimiques, les antibiogrammes par diffusion en milieu solide (méthode de Kirby-Bauer). Les 2/3 des brûlures étaient dues à du gaz enflammé, 28% étaient des ébouillantements. Quatre vingt six pour cents des cultures étaient positives, 14% négatives. P. æruginosa était retrouvé dans 30,2% des prélèvements, Acinetobacter 20,9%, P. mirabilis et S. aureus bien moins fréquemment (2,3% tous deux). Les résistances de Pseudomonas à la gentamicine, au cotrimoxazole et à la ciprofloxacine étaient variables, quand Acinetobacter était souvent multirésistant. Les BGN résistants sont les bactéries les plus souvent isolées des brûlures infectées à Accra, Ghana. De ce fait, un choix rigoureux des antibiotiques en cas d’infection de brûlure est nécessaire, afin de réduire la morbidité et la mortalité.
Introduction
Globally, burns are considered devastating forms of trauma in patients with serious thermal injury.1,2 They can be caused by scalds, thermal, electrical, gas or chemical agents.1,4 Patients with serious burn injury require immediate specialized care in order to minimize bacterial infection, which is a major cause of morbidity and mortality in burn patients.5-8
Much progress has been made with respect to infection control and burn wound management, however, burn wound infection still poses a major clinical challenge in most developing countries, where wound site infections are a major source of post-operative illness and mortality among burn patients. 8 The consequential effect of burn wounds contaminated with pathogenic bacteria can delay wound healing, cause wound breakdown and herniation of the wound or complete wound dehiscence.9-10 Although in most cases the source of contamination is the patient’s normal flora or exogenous contamination from contaminated wound dressing devices in or from the hospital environment, various groups of microorganisms have been reported to be associated with wound infections. 10-11 A study carried out by Patil et al.11 in India revealed that Pseudomonas aeruginosa, Methylene Resistant Staphylococcus aureus (MRSA), Acinetobacter buamanni, Klebsiella pneumonia, Proteus mirabilis, Citrobacter sp., Coagulase negative Staphylococci, Enterobacter sp. and Escherichia coli were commonly associated with the wounds of burn patients. Whilst some studies have reported Staphylococcus aureus, Pseudomonas aeruginosa, Klebsiella sp. and Escherichia coli as predominant bacteria associated with burn wounds,12-14 the exact number of burn injuries is very difficult to estimate. Although some studies have shown that adult females and children (1-9 years) are at a greater risk of burn-related injuries than adult males,15-18 burn wound is an important cause of disability and mortality in all ages and in both developed and developing countries.19,20
Antibacterial susceptibility patterns for microorganisms isolated from hospitalized patients are continuously evolving, and this can pose a major challenge for clinicians treating burn wound victims.21,22 Therefore, the present study was conducted to determine the microbial profile of burn wounds, the antimicrobial susceptibility patterns of the microbes with respect to the source of wound, age, and sex among burn injured patients at the National Reconstructive Plastic Surgery and Burns Centre (NRPSBC) of the Korle-Bu Teaching Hospital (KBTH).
Methodology
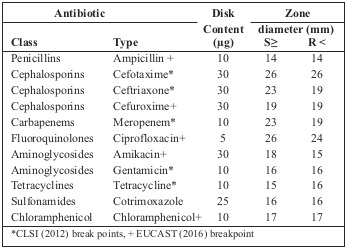
The investigation was a cross-sectional study carried out at the National Reconstructive Plastic Surgery and Burns Centre (NRPSBC) of the Korle–Bu Teaching Hospital (KBTH). KBTH is a leading national referral centre in Ghana and also serves as a referral centre for neighbouring countries.23All consenting burns patients admitted to the Burns Centre of KBTH from April – July, 2016 were recruited and included in the study. Sample collection A total of fifty wound swabs were collected, using a sterile cotton swab, from the wounds of burn patients admitted to the Burns Centre of the Korle-Bu Teaching Hospital (KBTH) from April to July, 2016. Without considering how long the patients were admitted for, all consenting patients were included in the study. Duration of patient admission ranged from 5 to 31 days. However, patients on antibiotic therapy for bacterial infection were excluded from the study. A single wound swab was taken from each patient prior to wound dressing with hydrocortisone. Swabs were taken from areas that appeared deep, with discharge, and the swabs were immediately transported to the Microbiology Unit of the School of Biomedical and Allied Health Sciences (SBAHS) for analysis. Data on age, gender and type of burn were also collected from the patients’ clinical folder. Laboratory analysis On arrival at the laboratory, the wound swabs were immediately cultured onto blood (Oxoid) and MacConkey agar (Oxoid), then incubated at 37°C for 18-24hrs. After 24 hrs, the colonial morphology of the colour, shape and general appearance of the individual colony on each of the plates was examined. 24 A representative single colony on the blood and MacConkey agar was gram stained and tested with indole and citrate, and Triple Sugar Ion test (TSI), urease and oxidase were performed to identify which bacteria species were present. Susceptibility testing Briefly, stored isolates were subcultured onto horse blood agar plates (37oC, 18 h) and individual colonies were suspended in saline to a turbidity equivalent of 0.5 McFarland standard.25 The suspensions obtained were then streaked on Mueller-Hinton agar plate using sterile swab sticks. The paper discs were gently but firmly placed on the inoculated plates using sterile forceps. The plates were incubated at 37oC for 24 hours after which zones of inhibition were measured and interpreted according to the Clinical and Laboratory Standard Institute.25 The reference strains used for the determination of MIC values were E. faecalisATCC 29212 and Staphylococcus aureus ATCC 29213. Clinical and Laboratory Standard Institute susceptibility break points were used when available, while other break points were sourced from EUCAST (European Society of Clinical Microbiology and Infectious Diseases) Break Points for Enterobacteriaceae 201626 (Table I). Each gram negative isolate was tested using eleven antibiotics: Ampicillin (10μg), Chloramphenicol (10μg), Cefotaxime (30μg), Tetracycline (10μg), Ceftriaxone (30μg), Gentamicin (10μg), Cefuroxime (30μg), Meropenem (10μg), Amikacin (30μg), Cotrimoxazole (25μg) and Ciprofloxacin (5μg), and each gram positive isolate was tested using Amikacin (30μg), Gentamicin (10μg), Cefuroxime (30μg) Cotrimoxazole (25μg), Erythromycin (15μg), Vacomycin (30μg) and Oxacillin (1μg). These antibiotics were used because they are the most commonly used in the treatment of bacterial infections in Ghana.27 Data analysis The data obtained from the study were analysed using descriptive statistics generated with the help of Microsoft Excel. The quantitative data generated from the study were coded and fed into Microsoft Excel and analyzed using GraphPad Prism software, version 6. In all cases, P-values less than 0.05 were considered statistically significant. Paired student t test was used to test for significance between prevalence of burns in the different sex and age groups. Ethical clearance The study was approved by the Ethics Committee of the School of Biomedical and Allied Health Sciences, University of Ghana: Ethics Identification Number SAHS/10403317/AA/ MLS/2015-2016. Participation by the patients was voluntary in accordance with the Ethics Committee’s guidelines.
Table I. Guidelines for interpreting antimicrobial susceptibility results.
Results
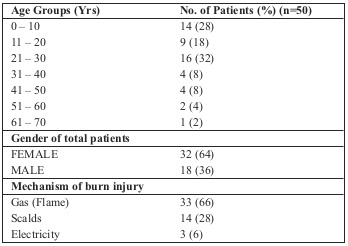
Out of a total of 50 samples collected from the burn patients, 18 (36%) were from males and 32 (64%) were from females (Table II). The majority of the patients were within the 21-30 age group (32%), followed by 0-10 (28%), 11-20 (18%), 31-40 (8%), 41-50 (8%), and 51-60 (4%), with the 61-70 age group having the least number of patients (2%). Gas (flame) was the cause of injury in 33 (66%) patients, scalds were responsible for 14 (28%) injuries and 3 (6%) patients had been injured by electricity.
Table II. Demographic characteristics of recruited burns patients.
Out of the 50 samples cultured, 43 (86%) were found to be positive for bacteria, while 7 (42%) showed no bacterial growth. In total, 9 different isolates were identified, with the predominant bacteria being Pseudomonas sp. (30.2%) (Table III). It was followed by Acinetobacter species (20.9%), Proteus mirabillis (16.3%), Enterobacter sp. (11.6%), Klebsiella sp. (7.0%), Citrobacter sp. (4.7%), Klebsiella oxytoca (4.7%), Proteus vulgaris (2.3%) and Staphylococcus aureus (2.3%).
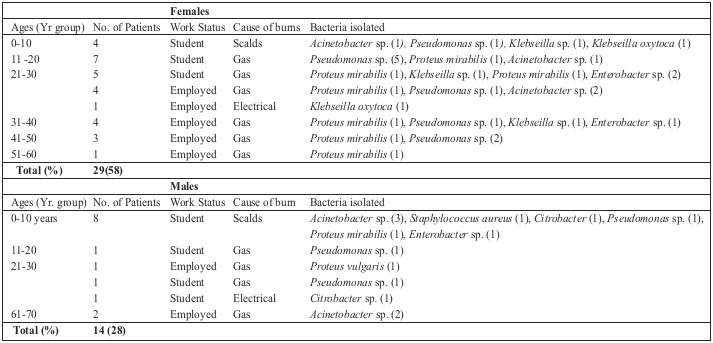
Most of the Pseudomonas sp. were found to be resistant to ampicillin, cotrimoxazole, cefuroxime and ceftriaxone (Table III). All of the Acinetobacter sp. were resistant to ampicillin, cotrimoxazole, cefuroxime, ceftriaxone and tetracycline. Whilst most of the Proteus mirabillis were resistant to ampicillin, cotrimoxazole, gentamicin, cefuroxime and ceftriaxone, all of the isolated Enterobacter sp. were also resistant to ampicillin, cotrimoxazole, gentamicin, cefuroxime and ceftriaxone. Klebsiella sp. and Klebsiella oxytoca were both found to be resistant to ampicillin, tetracyclin, cotrimoxazole and ciprofloxacin. One Staphylococcus aureus was isolated in this study. The isolate was found to be resistant to oxacillin, erythromycin, amikacin and gentamicin (data not included). Out of the 29 female burn wound patients, 16 were students and 13 were employed (Table IV). Whilst a lot of the female patients were found to have gas burns (12 students, 8 employed) often infected with Pseudomonas sp. or Proteus mirabillis, a few (4 students) had scalds as the source of injury. Paired student t test analysis of females with regards to type of burn and age group revealed that there was no significant association (p = 0.3361). The 14 male patients with burn injuries were students (11) or employed (3). In contrast to the females, males had most often suffered scalds (8 students), with gas (2 students, 3 employed) and electrical (1 student) burns the other sources of injury. Whilst the scald wounds were often infected with Acinetobacter sp., Staphylococcus aureus, and Citrobacter sp., a few gas wounds were infected with Pseudomonas sp., and Acinetobacter sp. Paired student t test analysis of males with regards to type of burn and age group revealed there was no significant association (p = 0.2893).
Table III. Prevalence of isolated bacteria and the antibiotic resistant profiles of gram negative bacteria from the burn wound patients.
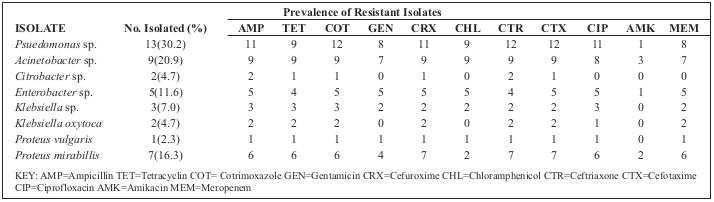
Table IV. Prevalence of isolated bacteria with respect to the age groups, sex, work status and causes of burns in patients.
Discussion
This study reports for the first time prevalence of burn injury and associated resistant bacteria in patients in a teaching hospital in Accra, Ghana. In this present study, the incidence of burn injuries was higher in females: 32 (64%) compared to 18 males (36%). This is in conformity with a study conducted in India by Rao et al.6 which also reported a higher incidence of burn injuries in females (56.9%) than in males (43.1%). The relatively higher number of cases of burns in females may be due to their greater participation in kitchen activities. However, our findings are in contrast to a previous study by Ekrami and Kalantar28 from India, which showed a higher prevalence (59.3%) of burn injuries in males than in females (40.6%). The studied patients ranged from 1 to 62 years of age, the 21-30 age group being the most affected age group - which correlates with the study by Chaudhary et al.29 This could be due to the fact that the 21-30 group is the most active group, and most involved in outdoor activities.
Among the culture positive samples, 29 (58%) were from female patients and 14 (28%) were from male patients. Thirtythree (66%) of the studied patients had flame injury caused by gas, while 14 (28%) had scald injury and 3 (6%) had electrical injuries. This correlates with a study conducted by Shahzad et al.30 in Pakistan, which reported the predominant burn agent to be gas flame (76%), followed by scald (14%), contact (6%), electrical (3%) and chemical (1%). This study revealed Pseudomonas sp., Acinetobacter spp, Proteus mirabillis, Enterobacter spp, Klebsiella sp., Citrobacter sp., Klebsiella oxytoca and Proteus vulgaris to be the most common gram negative bacteria species isolated from burn wounds, with Staphylococcus aureus being the only gram positive bacteria sp. isolated. This is similar to a study carried out by Patil et al.,11 which also revealed Pseudomonas sp, Acinetobacter sp., Proteus mirabillis, Klebsiella sp., Citrobacter sp, Enterobacter sp and Escherichia coli as the most common gram negative bacteria associated with burn wounds. This study showed a high incidence of gram negative organisms compared to gram positive organisms as previously reported in the study by Shahzad et al.30 from Pakistan.
Out of nine different bacterial species isolated from 43 patients, Pseudomonas sp. was the most predominant bacteria associated with the burn wounds. The high prevalence of Pseudomonas sp. in this study may be due to the fact that the organism thrives well in a moist environment.31 Our findings correlate with the study by Lakshmi et al.32 from India, which also reported Pseudomonas sp. as the most common isolate in burn wounds with a prevalence of 33.6%. This study, however, is in contrast to studies conducted by Chaudhary et al.29 in Nepal and Rao et al.6 in India, which reported Staphylococcus aureus as the most common isolate associated with burn wounds with a prevalence of 28.71% and 42% respectively. Another study by Srinivasan et al.33 conducted in India also reported Klebsiella sp. (33.91%) to be the predominant isolate associated with burn wounds. The differences in isolated bacterial isolates in burn wounds may be due to a variation in treatment practices in the different geographical locations of burn victims.34
The antimicrobial susceptibility pattern of the different gram negative isolates from the burn patients revealed that Pseudomonas sp. was resistant to amikacin, ceftriaxone, ciprofloxacin and gentamicin. Findings from this study are similar to those of Shahzad et al.,30 who reported varying resistance levels to amikacin (35%), ceftriaxone (85%), ciprofloxacin (70%) and gentamicin (97%). This study also revealed a resistance to cefotaxime and tetracyclin in Pseudomonas sp. Our findings are similar to those of Rao et al.,7 who reported a resistance to cefotaxime (34.9%), and Mehedi et al.,35 who revealed a resistance to tetracycline (65.57%) in Pseudomonas sp.. Acinetobacter sp. showed 100% resistance to both ceftriaxone and cefuroxime, which is similar to a study by Nahar et al.,36 from Bangladesh, who also reported a high resistance of Acinetobacter species to ceftriaxone (100%) and cefuroxime (100%). Resistance of Acinetobacter sp. to gentamicin in this study is similar to that found in a study by Moradi et al.37 in Iran, who also reported a resistance of Acinetobacter to gentamicin (78%). Moradi et al.37 also reported resistance to cefotaxime (95%), which does not correlate with our findings of 100% resistance of Acinetobacter sp. to cefotaxime. Proteus mirabillis were resistant to tetracycline (100%), which is similar to a study by Mordi and Momoh38 in Benin, who also reported a 100% resistance of Proteus mirabillis to tetracycline. Enterobacter sp. was resistant to cefotaxime (100%), which is in contrast to findings by Otta et al.39 in India, who reported moderate resistance (50%) of Enterobacter sp. to cefotaxime.
Conclusion
Findings of the study suggest that multidrug resistant gram negative organisms are the most common isolates from burn wounds. Hence a careful selection of antibiotics to treat burn wound infection is required for proper management of these wounds in order to help reduce morbidity and mortality associated with multi-resistant bacteria.
Acknowledgments
Conflict of interest.The authors declare that there is no conflict of interest regarding the publication of this paper.
Acknowledgments.The authors wish to express their sincere gratitude to all staff and patients of the National Reconstructive Plastic Surgery and Burns Centre (NRPSBC) in the Korle–Bu Teaching Hospital (KBTH) who contributed to the success of the study in one way or the other.
Availability of data and materials.The datasets used and/or analysed during this study are available from the corresponding author upon reasonable request.
References
- 1.World Health Organisation. [10/01/2017];Burn prevention: success stories and lessons learned. 2011 Source: Source:http://www.who.int/violence_injury_prevention/publications/other_injury/burn_success_stories/en/ [Google Scholar]
- 2.Santucci S.G., Gobara S., Santos C.R., Fontana C., Levin A.S. Infections in a burn intensive care unit: experience of seven years. J Hosp Infect. 2003;56:6–13. doi: 10.1053/jhin.2002.1340. [DOI] [PubMed] [Google Scholar]
- 3.Enoch S., Roshan A., Shah M. Emergency and early management of burns and scalds. British Medical Journal. 2009;8:338. doi: 10.1136/bmj.b1037. [DOI] [PubMed] [Google Scholar]
- 4.Mirmohammadi S.J., Mehrparvar A.H., Kazemeini K., Mostaghaci M. Epidemiology characteristics of occupational burns in Yazd, Iran. International Journal of Preventive Medicine. 2013;4(6):723–727. [PMC free article] [PubMed] [Google Scholar]
- 5.Neriman A., Nursen G., Sevinç Y., Ramazan K. Burn wound infections in a medical hospital burn unit in Bursa, Turkey. International Journal of Caring Sciences. 2014;7(3):776. [Google Scholar]
- 6.Rao S.R., Lakshmi L.J., Pavani S., Kawle V., Prakash S.J. Bacteriological profile, Antibiogram of burn wound isolates and detection of MRSA and ESBL production at tertiary care hospital, Hyderabad. World Journal of Pharmacy and Pharmaceutical Sci. 2014;3(10):1691–1698. [Google Scholar]
- 7.Gowri S., Vijaya A.N., Rajesh P. Epidemiological study of burn injuries admitted in two hospitals in North Karnataka. Indian Journal Community Medicine. 2010;35(4):507–512. doi: 10.4103/0970-0218.74363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Macedo J.L.S., Santos J.B. Bacterial and fungal colonisation of burn wound. Memorial Institute of Oswaldo Cruz. 2005;100(5):535–539. doi: 10.1590/s0074-02762005000500014. [DOI] [PubMed] [Google Scholar]
- 9.Kaye K.S., Schmit K., Pieper C., Sloan R. The effect of increasing age on the risk of surgical site infection. Journal of Infectious Diseases. 2005;191(7):1052–1062. doi: 10.1086/428626. [DOI] [PubMed] [Google Scholar]
- 10.Shittu A., Kolawole D., Ear O.A. A study of wound infections in two health institutions in ILe-Ife, Nigeria. African Journal of Biomedical Research. 2002;5:97–102. [Google Scholar]
- 11.Patil P., Joshi S., Bharadwaj R. Aerobic bacterial infections in a burns unit of Sassoon General Hospital, Pune. International Journal of Healthcare and Biomedical Research, 2015;3(3):106–112. [Google Scholar]
- 12.Kehinde A.O., Ademola S.A., Okeshola O.A., Oluwatosin O.M., Bakare R.A. Pattern of bacterial pathogens in burn wound infections in Ibadan Nigeria. Ann Burns Fire Disasters. 2004;17(1):9–16. [Google Scholar]
- 13.Motayo B.O., Akinbo J.A., Ogiogwa I.J., Idowu A.A. Bacteria colonisation and antibiotic susceptibility pattern of wound infections in a hospital in Abeokuta. Frontiers in Science. 2013;3(1):43–48. [Google Scholar]
- 14.Mahammedaman M., Alemseged A., Tsegaye S. Antimicrobial Susceptibility pattern of bacterial isolates from wound infection and their sensitivity to alternative topical agents at Jimma University Specialized Hospital, South-West Ethopia. Annals of Clinical Microbiology and Antimicrobials. 2014;13:14. doi: 10.1186/1476-0711-13-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peck M.D. Epidemiology of burns throughout the world. Part I: Distribution and risk factors. Burns. 2011;37(7):1087–1100. doi: 10.1016/j.burns.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 16.Grivna M., Eid H.O., Abu-Zidan F.M. Epidemiology of burns in the United Arab Emirates: Lessons for prevention. Burns. 2014;40(3):500–505. doi: 10.1016/j.burns.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 17.Hemeda M., Maher A., Mabrouk A. Epidemiology of burns admitted to Ain Shams University Burns Unit, Cairo, Egypt. Burns. 2003;29(4):353–358. doi: 10.1016/s0305-4179(03)00044-5. [DOI] [PubMed] [Google Scholar]
- 18.Aldemir M., Kara I.H., Girgin S., Guloglu C. Factors affecting mortality and epidemiological data in patients hospitalised with burns in Diyarbakir, Turkey. South African Journal of Surgery. 2005;43(4):159–162. [PubMed] [Google Scholar]
- 19.Mohammadi A.A., Amini M., Mehrabani D., Kiani Z., Seddigh A. A survey on 30 months electrical burns in Shiraz University of Medical Sciences Burn Hospital. Burns. 2008;34:111–113. doi: 10.1016/j.burns.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 20.Pasalar M., Mohammadi A.A., Rajaeefard A.R., Neghab M. Epidemiology of burns during pregnancy in southern Iran: Effect on maternal and fetal outcomes. World Appl Sci J. 2013;28:153–158. [Google Scholar]
- 21.Coban Y.K. Infection control in severely burned patients. World Journal Critical Care Medicine. 2012;1(4):94–101. doi: 10.5492/wjccm.v1.i4.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Branski L.K., Al-Mousawi A., Rivero H., Jeschke M.G. Emerging infections in burns patients. Surgical Infections (Larchmt), 2009;10(5):389–397. doi: 10.1089/sur.2009.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. [24/01/2017];National Reconstructive Plastic Surgery and Burns Centre (NRPSBC) of the Korle-Bu Teaching Hospital (KBTH) Source: http://www.nrpsghana.org/ [Google Scholar]
- 24.Cheesbrough M. District Laboratory Practice in Tropical Countries. Part 1. Cambridge (The Pitt Building, Trumpinton Street, Cambridge CB2 IRP): The press syndicate of the University of Cambridge. 2005 [Google Scholar]
- 25.CLSI. Performance Stardards for Antimicrobial Disk Diffusion Tests. Approved Stardard - 12th Ed. CLSI documents MO2 – 12, Wayne, PA: Clinical and Laboratory Stardards Institutes. 2015 [Google Scholar]
- 26.EUCAST (European Society of Clinical Microbiology and Infectious Diseases) Break points for Enterobacteriaceae, 2016. [10/11/2016]; [Google Scholar]
- 27.Opintan J.A., Newman M.J., Arhin R.E., Donkor E.S., Gyansa-Lutterodt M., Mills-Pappoe W. Laboratory-based nationwide surveillance of antimicrobial resistance in Ghana. Infection and Drug Resistance. 2015;8:379–389. doi: 10.2147/IDR.S88725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ekrami A., Kalantar E. Bacterial infections in burn patients at a burn hospital in India. Indian Journal of Medical Research. 2007;126(6):541–544. [PubMed] [Google Scholar]
- 29.Chaudhary P., Shakya C., Pokhrel S.R., Shrestha B. Prospective study on bacterial isolates with their antibiotic susceptibility pattern from pus (wound) sample in Kathmandu Model Hospital. International Journal of Medicine and Biological Sciences. 2015;1(1):15–22. [Google Scholar]
- 30.Shahzad M.N., Ahmed N., Khan I.H., Mirza A.B., Waheed F. Bacterial profile of burn wound infections in burn patients. Annals 2012. 8(1):54–57. [Google Scholar]
- 31.Atoyebi O.A., Sowemimo G.A., Odugbemi T. Bacterial flora of burn wounds in Lagos, Nigeria: a prospective study. Burns. 1992;18(6):448–451. doi: 10.1016/0305-4179(92)90175-t. [DOI] [PubMed] [Google Scholar]
- 32.Lakshmi N., Ramalakshni K., Perala K.B., Jayalaxmi M. Bacteriological profile and antibiogram of burn wound infections in a tertiary care hospital. Journal of Dental and Medical Sciences. 2015;14(10):1–4. [Google Scholar]
- 33.Srinivasan S., Vartak A.M., Patil A., Saldanha J. Bacteriology of the burn wound at the Bai Jerbal Hospital for children, Mumbai, India. Indian Journal of Plastic Surgery. 2009;42(2):213–218. doi: 10.4103/0970-0358.59284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ananthakrishanan A., Kanungo R., Kumar K., Badrinath S. Detection of extended spectrum beta lactamase producers among surgical wound infections and burns patients in JIPMER. Indian Journal of Medical Microbiology. 2002;18:160–165. [Google Scholar]
- 35.Mehedi H.M., Arongozed Golam M.K., Zakaria A. Isolation and identification of different bacteria from different types of burn wound infections and their antimicrobial sensitivity pattern. International Journal of Research in Applied, Natural and Social Sciences. 2013;1(3):125–132. [Google Scholar]
- 36.Nahar A., Anwar S., Saleh A.A., Miah A.R. Isolation of Acinetobacter species and their antimicrobial resistance pattern in an Intensive Care Unit (ICU) of a tertiary care hospital in Dhaka, Bangladesh. Bangladesh Journal of Medical Microbiology. 2012;6(1):3–6. [Google Scholar]
- 37.Moradi J., Hashemi B.F., Bahador A. Antibiotic resistance of Acinetobacter buaumanii in Iran: A systematic review of the published literature. Osong Public Health and Research Perpectives. 2015;6(2):79–86. doi: 10.1016/j.phrp.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mordi M.R., Momoh I.M. Incidence of Proteus species in wound infections and their sensitivity pattern in the University of Benin Teaching Hospital. African Journal of Biotechnology. 2009;8(5):725–730. [Google Scholar]
- 39.Otta S., Dash J.K., Swain B. Aerobic bacteriology of burn wound infections. CHRISMED Journal of Health and Research. 2015;2(4):337–341. [Google Scholar]


