Abstract
Background:
There is an increase in inflammatory and a reduction in anti-inflammatory cytokines in multiple sclerosis (MS). Considering the role of thyroid hormones in the development and regulation of both neural and immune systems, the aim of this study was to evaluate the effects of levothyroxine on serum concentrations of interleukin-10 (IL-10) and interferon gamma (IFN-γ) in animal models of MS.
Materials and Methods:
To induce demyelination in male Wistar rats, lysolecithin was injected into the optic chiasm. Then levothyroxine was injected intraperitoneally (20, 50, and 100 μg/kg) for 21 days. Serum levels of cytokines were measured by enzyme-linked immunosorbent assay at 7, 14, and 21 days after that.
Results:
The results showed that injection of lysolecithin to the optic chiasm only increased serum concentrations of IL-10 compared to the sham group (P < 0.05) at 7th day, but this increase was prevented by all doses of levothyroxine. IFN-γ was decreased significantly (P < 0.001) 21 days after. Comparing to the sham group at all sampling time and with respect to the MS group at the days 7 and 21, levothyroxine decreased serum concentrations of IFN-γ significantly.
Conclusion:
The results showed that thyroid hormones probably could produce protective effects against induced demyelination through affecting immune responses.
Keywords: Interferon-gamma, interleukin-10, levothyroxine, multiple sclerosis
Introduction
Multiple sclerosis (MS) is an inflammatory disease damaging the myelin in the central nervous system. The reason is not clear, but due to the infiltration of immune cells in the lesions, immune cells are implicated in its pathogenesis.[1] Following the demyelinating attacks, especially in the visual system, some levels of remyelination occur that depend on to both the proliferation and migration of progenitor cells.[2] However, this capacity is not sufficient and remains permanent lesions after each demyelination.[3] The only effective mechanism in the treatment of MS is the restoration of myelin that is performed by oligodendrocyte progenitor cells (OPC).[4] The proliferation and development of OPC to myelinating oligodendrocyte are affected by many factors including thyroid hormones. One of the possible reasons for cessation of remyelination is a lack of adequate response of OPC to the stimulating factors.[5] Thyroid hormones play an important role in the evolution of the nervous system from prebirth to adulthood.[6] Recent studies showed that the thyroid hormone plays an important role in the development of the nervous system in adults.[7] Thyroid hormones are essential for the development of oligodendrocytes as well as their migration into the demyelinated sites.[8]
Normally, the balance of the immune system is established by the balance between cytokines and when this balance is disturbed, leads to a disorder. In the MS disease, there is an increase in pro-inflammatory cytokines and a decrease in anti-inflammatory cytokines.[9] One of the involved cytokines is interferon gamma (IFN-γ) which increases the activity of macrophages and promotes inflammation and demyelination.[10,11] The other is interleukin 10 (IL-10) that as an anti-inflammatory cytokine can reduce the activity of inflammatory cells.[12] IL-10 has been shown that reduces progression of the MS.[13] One of the roles of thyroid hormones is a help to development and regulation of the immune system, and the balance between pro- and anti-inflammatory cytokines is also part of this balance. Accordingly, the aim of this study was to evaluate the effect of levothyroxine on the serum levels of IL-10 and IFN-γ in rat model of MS.
Materials and Methods
Subjects
The subjects were male Wistar rats (250–300 g) that were housed four per cage and maintained on a 12 h light-dark cycle in an air conditioned constant temperature (23 ± 1°C) room, with food and water made available ad libitum. The Ethic Committee for Animal Experiments at Isfahan University approved the study, and all experiments were conducted in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80–23) revised 1996. Experimental groups were the sham, lesion (MS), MS-levothyroxine (MS-Th) 20 μg/kg, MS-Th 50 μg/kg, and MS-Th 100 μg/kg (n = 12).
The rats were anesthetized with chloral hydrates (400 mg/kg, intraperitoneal [i.p.])[14] and their heads were fixed in a stereotaxic frame. A heating pad was used to maintain body temperature at 36.5 ± 0.5°C. The skull was exposed, and a small hole was drilled, and injection cannula was lowered into the optic chiasm (anteroposterior = −0.3 mm; mediolateral = 0 mm; dorsoventral = −8.9 mm).[15] Injection cannula was connected to a Hamilton syringe attached to a microinjector unit. The lesion groups received an injection of 2 μl of 1% lysolecithin (Sigma, St. Louis, USA) prepared in sterile 0.9% saline, pH 7.4, into the chiasm.[16] The sham groups underwent the same surgical procedures, but the same volume of saline was injected instead of lysolecithin.
From the 3rd day after surgery, rats in different treated groups were received i.p. injection of levothyroxine 20, 50, or 100 μg/kg (Sigma, St. Louis, USA)[17] for 21 days. Animals in the sham and the MS groups received the same volume of placebo.
After 7, 14, and 21 days from the start of treatments, four rats from each group were selected randomly and were anesthetized by i.p. injection of chloral hydrate and after decapitation by guillotine, the trunk blood was collected. After blood clotting, the samples were centrifuged (20 min, 6000 rpm), and serums were collected and were kept at −80°C. Serum levels of IL-10 and IFN-γ were determined by enzyme-linked immunosorbent assay (ELISA) using IL-10 rat ELISA kit and IFN-γ rat ELISA kit (Abcam; ab100765 and ab46107, respectively) according to the manufacturer's instructions. Each sample was double-checked and the average was reported.
Data were analyzed using the SPSS version 21 for Windows (SPSS, Chicago, IL, USA). The data were analyzed statistically by two-way analyze of variance followed by Tukey post hoc for between subjects’ differences and within effects. Adjustment for multiple comparisons was done by Bonferroni. The statistically significant level was considered P < 0.05. Results are expressed as mean ± standard error mean.
Results
Interleukin 10
As seen in Figure 1 and Table 1, analysis of within-subject effects showed no significant differences between the days (DAY effect, F [2,30] = 2.27, P = 0.121; Figure 1) and the pattern of changes across the days between the groups (GROUP * DAY effect interaction, F [8,30] =1.49, P = 0.2; Figure 1). Test of between-subject effects showed a significant difference between the groups (P = 0.001). Post hoc test showed a significant increase in serum concentration of IL-10 in the MS group (781.71 ± 51.23) with respect to the control (558.82 ± 51.23; P = 0.037), the MS-Th 20 μg/kg (488.15 ± 51.23; P = 0.008), the MS-Th 50 μg/kg (499.544 ± 51.23; P = 0.011), and MS-Th 100 μg/kg (349.371 ± 51.23; P = 0.001) [Figure 1]. There were no significant differences between the other groups.
Figure 1.
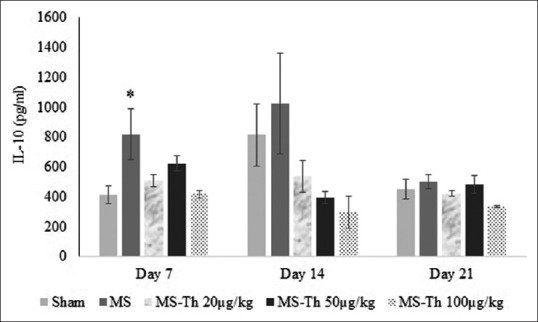
Effect of levothyroxine on serum levels of interleukin 10 after injection of lysolecithin in the optic chiasm of rats (MS is lesion group and Th is levothyroxine). Values are shown as mean ± standard error mean. *P < 0.05 with respect to the sham group (n = 12); MS: Multiple sclerosis
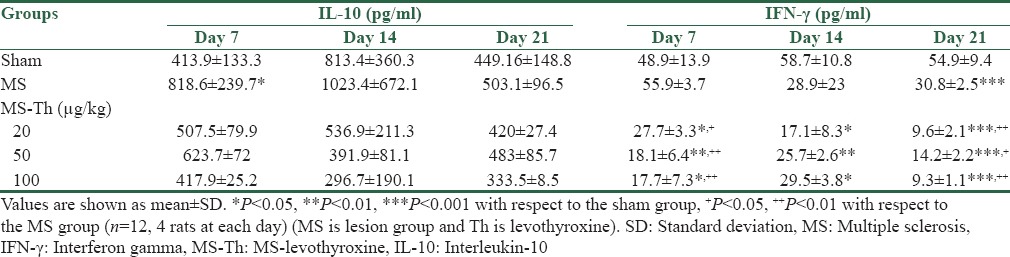
Table 1.
Effect of levothyroxine on serum levels of interleukin 10 and interferon gamma after injection of lysolecithin in the optic chiasm of rats
Interferon-gamma
As seen in Figure 2 and Table 1, analysis of within-subject effects showed a significant decrease in serum concentration of IFN-γ across the days (DAY effect, F [2,30] =5.75, P = 0.008; Figure 2) and the pattern of decreases across the days between the groups (GROUP * DAY effect interaction, F [8,30] =3.5, P = 0.006; Figure 2). Test of between-subject effects showed a significant difference between the groups (P = 0.001).
Figure 2.
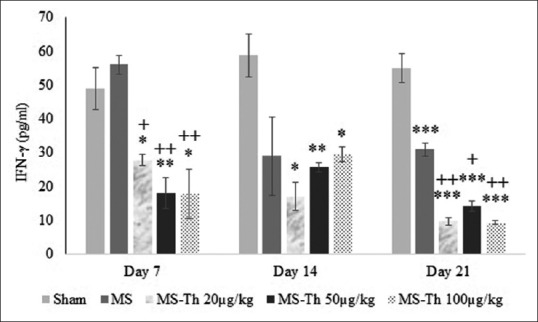
Effect of levothyroxine on serum levels of interferon gamma after injection of lysolecithin in the optic chiasm of rats (MS is lesion group and Th is levothyroxine). Values are shown as mean ± standard error mean. *P < 0.05, **P < 0.01 and ***P < 0.001 with respect to the sham group; +P < 0.05 and ++P < 0.01 with respect to the multiple sclerosis group (n = 12); MS: Multiple sclerosis
With respect to the control group (54.2 ± 2.31) serum concentration of IFN-γ was decreased significantly in the MS group (38.61 ± 2.31; P = 0.001), the MS-Th 20 μg/kg (18.17 ± 2.31; P = 0.001), the MS-Th 50 μg/kg (19.34 ± 2.31; P = 0.001) and MS-Th 100 μg/kg (18.89 ± 2.31; P = 0.001) [Figure 2]. These decrements were more significant in the MS-Th 20 μg/kg (P = 0.001), the MS-Th 50 μg/kg (P = 0.001) and MS-Th 100 μg/kg (P = 0.001) with respect to the MS group [Figure 2].
Discussion
The result showed that 7 days after injection of lysolecithin in the optic chiasm serum levels of IL-10 was significantly increased, but it was reduced on the following days and came to the sham group. Serum concentrations of IFN-γ were same to the sham group on the 7th day, however, it was reduced during the following days and on day 21 was significantly reduced.
Long as an antigen enters the body will bring cellular immune responses that followed by a peak in the levels of inflammatory cytokines during the early days. Then, if removal of the antigen, immune response and the levels of cytokines decline to the normal levels. IFN-γ is a pro-inflammatory cytokine and has been specified that increases in the MS patients.[18] It has been observed that the use of IFN-γ in patients with MS worsen the symptoms.[19] Nevertheless, several studies have suggested that IFN-γ has beneficial effects in animal models of MS.[20] Anyway, because IFN-γ has an important role in immune responses, different functions from it have shown in diseases.[18] IFN-γ through affecting the immune cells induces secretion of different cytokines that their abnormal expression causes autoimmune disorders.[21] In this study, immune and inflammatory responses were induced by injection of lysolecithin into the optic chiasm that was probably accompanied by an increase in IFN-γ during the first few days. This model of demyelination is reversible, and lysolecithin is quickly decomposed and removed, and repair of myelin occurs over several days.[16] Thus, by eliminating the primary cause of damage, probably immune responses began to decline and reached to the normal at the day 7.
Another cytokine that is produced in the immunological reactions is IL-10. It is an anti-inflammatory cytokine that affects regulation of immune system and inflammation and can suppress production of pro-inflammatory cytokines such as TNFα and IFN-γ.[22] Studies have shown that the levels of IL-10 are very low in the patients with MS[23] and by reducing IL-10, TNFα can rise and cause neuronal demyelination.[24] According to the present results, the serum levels of IL-10 was significantly increased on the 7th day. Thus, the reduction of IFN-γ at this day can be a result of the enhancement of IL-10. However, with the passing of time and eliminating the cause of immune response, IL-10 levels began to decline to the normal levels. Nevertheless, as long as the levels of IL-10 was high, it had continued its suppressive effects on the production of IFN-γ, and the reduced levels of IFN-γ can be resulted from this suppression, especially on day 21. Although the triggering factor has been removed from the body, return to normal levels of cytokines takes time.
As a secondary observation, our study demonstrates that levothyroxine prevented the alterations in the levels of IL-10, and also decreased serum levels of IFN-γ in the MS rats. It has demonstrated that thyroid hormones have modulatory effects on immune responses.[25] Studies have shown that both hyperthyroidism and hypothyroidism affects the immune system.[26] In hyperthyroidism, that the thyroid hormones are increased, the levels of pro-inflammatory markers are reduced.[27] In a study that has been evaluated the effects of thyroxine on the expression of cytokines by T cells, it has been demonstrated that thyroxine decreased the cytokine production by T cells and reduced serum levels of IFN-γ and IL-10 both in vitro and in vivo studies.[28] Our results showed that chronic use of levothyroxine suppresses the production of IFN-γ. Hence, we can expect that the thyroid hormones by modulating the immune response affect inflammatory processes and may reduce its complications.
Conclusion
In the present results, we have shown that following lysolecithin-induced demyelination in the optic chiasm, the serum levels of inflammatory cytokines are changed. However, levothyroxine with doses that produce hyperthyroidism suppressed these changes. Therefore, thyroid hormones may produce protective effects against induced demyelination through affecting immune responses.
Financial support and sponsorship
Financially supported by Isfahan University of Medical Sciences, Isfahan, Iran, grant number was 193105.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- 2.Franklin RJ. Why does remyelination fail in multiple sclerosis? Nat Rev Neurosci. 2002;3:705–14. doi: 10.1038/nrn917. [DOI] [PubMed] [Google Scholar]
- 3.Stangel M, Hartung HP. Remyelinating strategies for the treatment of multiple sclerosis. Prog Neurobiol. 2002;68:361–76. doi: 10.1016/s0301-0082(02)00105-3. [DOI] [PubMed] [Google Scholar]
- 4.Calzà L, Fernandez M, Giardino L. Cellular approaches to central nervous system remyelination stimulation: Thyroid hormone to promote myelin repair via endogenous stem and precursor cells. J Mol Endocrinol. 2010;44:13–23. doi: 10.1677/JME-09-0067. [DOI] [PubMed] [Google Scholar]
- 5.Lachapelle F, Avellana-Adalid V, Nait-Oumesmar B, Baron-Van Evercooren A. Fibroblast growth factor-2 (FGF-2) and platelet-derived growth factor AB (PDGF AB) promote adult SVZ-derived oligodendrogenesis in vivo. Mol Cell Neurosci. 2002;20:390–403. doi: 10.1006/mcne.2002.1124. [DOI] [PubMed] [Google Scholar]
- 6.Préau L, Fini JB, Morvan-Dubois G, Demeneix B. Thyroid hormone signaling during early neurogenesis and its significance as a vulnerable window for endocrine disruption. Biochim Biophys Acta. 2015;1849:112–21. doi: 10.1016/j.bbagrm.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 7.Remaud S, Gothié JD, Morvan-Dubois G, Demeneix BA. Thyroid hormone signaling and adult neurogenesis in mammals. Front Endocrinol (Lausanne) 2014;5:62. doi: 10.3389/fendo.2014.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dugas JC, Ibrahim A, Barres BA. The T3-induced gene KLF9 regulates oligodendrocyte differentiation and myelin regeneration. Mol Cell Neurosci. 2012;50:45–57. doi: 10.1016/j.mcn.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Høglund RA, Maghazachi AA. Multiple sclerosis and the role of immune cells. World J Exp Med. 2014;4:27–37. doi: 10.5493/wjem.v4.i3.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poyraz T, Idiman E, Uysal S, Iyilikci L, Ozakbas S, Coskuner Poyraz E, et al. The cooling effect on proinflammatory cytokines interferon-gamma, tumor necrosis factor-alpha, and nitric oxide in patients with multiple sclerosis. ISRN Neurol 2013. 2013 doi: 10.1155/2013/964572. 964572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langer-Gould A, Gupta R, Huang S, Hagan A, Atkuri K, Leimpeter AD, et al. Interferon-gamma-producing T cells, pregnancy, and postpartum relapses of multiple sclerosis. Arch Neurol. 2010;67:51–7. doi: 10.1001/archneurol.2009.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ireland SJ, Monson NL, Davis LS. Seeking balance: Potentiation and inhibition of multiple sclerosis autoimmune responses by IL-6 and IL-10. Cytokine. 2015;73:236–44. doi: 10.1016/j.cyto.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niino M, Fukazawa T, Miyazaki Y, Takahashi E, Minami N, Amino I, et al. Suppression of IL-10 production by calcitriol in patients with multiple sclerosis. J Neuroimmunol. 2014;270:86–94. doi: 10.1016/j.jneuroim.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 14.Moghaddasi M, Javanmard SH, Reisi P, Tajadini M, Taati M. The effect of regular exercise on antioxidant enzyme activities and lipid peroxidation levels in both hippocampi after occluding one carotid in rat. J Physiol Sci. 2014;64:325–32. doi: 10.1007/s12576-014-0322-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 5th ed. San Diego: Academic Press; 2005. p. 36. [Google Scholar]
- 16.Asghari AA, Azarnia M, Mirnajafi-Zadeh J, Javan M. Adenosine A1 receptor agonist, N6-cyclohexyladenosine, protects myelin and induces remyelination in an experimental model of rat optic chiasm demyelination; electrophysiological and histopathological studies. J Neurol Sci. 2013;325:22–8. doi: 10.1016/j.jns.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 17.Wu CY, Liu B, Wang HL, Ruan DY. Levothyroxine rescues the lead-induced hypothyroidism and impairment of long-term potentiation in hippocampal CA1 region of the developmental rats. Toxicol Appl Pharmacol. 2011;256:191–7. doi: 10.1016/j.taap.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 18.Lees JR, Cross AH. A little stress is good: IFN-gamma, demyelination, and multiple sclerosis. J Clin Invest. 2007;117:297–9. doi: 10.1172/JCI31254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Panitch HS, Hirsch RL, Schindler J, Johnson KP. Treatment of multiple sclerosis with gamma interferon: Exacerbations associated with activation of the immune system. Neurology. 1987;37:1097–102. doi: 10.1212/wnl.37.7.1097. [DOI] [PubMed] [Google Scholar]
- 20.Pusic AD, Kraig RP. Phasic treatment with interferon gamma stimulates release of exosomes that protect against spreading depression. J Interferon Cytokine Res. 2015;35:795–807. doi: 10.1089/jir.2015.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen W, Ren X, Zhu J, Xu Y, Lin J, Li Y, et al. Discovery of a new structural class of competitive hDHODH inhibitors with in vitro and in vivo anti-inflammatory, immunosuppressive effects. Eur J Pharmacol. 2016;791:205–12. doi: 10.1016/j.ejphar.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Marchant A, Bruyns C, Vandenabeele P, Ducarme M, Gérard C, Delvaux A, et al. Interleukin-10 controls interferon-gamma and tumor necrosis factor production during experimental endotoxemia. Eur J Immunol. 1994;24:1167–71. doi: 10.1002/eji.1830240524. [DOI] [PubMed] [Google Scholar]
- 23.Ozenci V, Kouwenhoven M, Huang YM, Xiao B, Kivisäkk P, Fredrikson S, et al. Multiple sclerosis: Levels of interleukin-10-secreting blood mononuclear cells are low in untreated patients but augmented during interferon-beta-1b treatment. Scand J Immunol. 1999;49:554–61. doi: 10.1046/j.1365-3083.1999.00546.x. [DOI] [PubMed] [Google Scholar]
- 24.Nakahara J, Maeda M, Aiso S, Suzuki N. Current concepts in multiple sclerosis: Autoimmunity versus oligodendrogliopathy. Clin Rev Allergy Immunol. 2012;42:26–34. doi: 10.1007/s12016-011-8287-6. [DOI] [PubMed] [Google Scholar]
- 25.De Vito P, Balducci V, Leone S, Percario Z, Mangino G, Davis PJ, et al. Nongenomic effects of thyroid hormones on the immune system cells: New targets, old players. Steroids. 2012;77:988–95. doi: 10.1016/j.steroids.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 26.Klecha AJ, Barreiro Arcos ML, Frick L, Genaro AM, Cremaschi G. Immune-endocrine interactions in autoimmune thyroid diseases. Neuroimmunomodulation. 2008;15:68–75. doi: 10.1159/000135626. [DOI] [PubMed] [Google Scholar]
- 27.De Vito P, Incerpi S, Pedersen JZ, Luly P, Davis FB, Davis PJ. Thyroid hormones as modulators of immune activities at the cellular level. Thyroid. 2011;21:879–90. doi: 10.1089/thy.2010.0429. [DOI] [PubMed] [Google Scholar]
- 28.Yao C, Zhang J, Wang L, Guo Y, Tian Z. Inhibitory effects of thyroxine on cytokine production by T cells in mice. Int Immunopharmacol. 2007;7:1747–54. doi: 10.1016/j.intimp.2007.09.015. [DOI] [PubMed] [Google Scholar]


