Abstract
The tumor microenvironment is profoundly immunosuppressive. This creates a major barrier for attempts to combine immunotherapy with conventional chemotherapy or radiation, because the tumor antigens released by these cytotoxic agents are not cross-presented in an immunogenic fashion. In this Focused Research Review, we focus on mouse preclinical studies exploring the role of immunosuppressive Tregs expressing the PTEN lipid phosphatase, and the links between PTEN+ Tregs and the immunoregulatory enzyme indoleamine 2,3-dioxygenase (IDO). IDO has received attention because it can be expressed by a variety of human tumor types in vivo, but IDO can also be induced in host immune cells of both humans and mice in response to inflammation, infection or dying (apoptotic) cells. Mechanistically, IDO and PTEN+ Tregs are closely connected, with IDO causing activation of the PTEN pathway in Tregs. Genetic ablation or pharmacologic inhibition of PTEN in mouse Tregs destabilizes their suppressive phenotype, and this prevents transplantable and autochthonous tumors from creating their normal immunosuppressive microenvironment. Genetic ablation of either IDO or PTEN+ Tregs in mice results in a fundamental defect in the ability to maintain tolerance to antigens associated with apoptotic cells, including dying tumor cells. Consistent with this, pharmacologic inhibitors of either pathway show synergy when combined with cytotoxic agents such as chemotherapy or radiation. Thus, we propose that IDO and PTEN+ Tregs represent closely linked checkpoints that can influence the choice between immune activation versus tolerance to dying tumor cells.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-017-2010-2) contains supplementary material, which is available to authorized users.
Keywords: Tolerance; Regulatory T cells; Chemotherapy; Indoleamine 2,3-dioxygenase; PTEN; Regulatory myeloid suppressor cells
Cross-presentation of tumor antigens is actively suppressed in tumors
In this Focused Research Review, we will address recent findings related to two linked hypotheses: The first is that immunogenic cross-presentation of tumor-derived antigens is actively inhibited in the tumor microenvironment. Although dying tumor cells release many antigens, and cell death generates many potentially inflammatory signals, the usual outcome is not immune activation but merely further immunosuppression and tolerance. We hypothesize that one of the main reasons for this failure is a dominant tolerizing network that exists within the tumor, driven by active inhibitory mechanisms such as regulatory T cells (Tregs) activated via the phosphatase and tensin homolog (PTEN) lipid-phosphatase pathway (PTEN+ Tregs), and the effects of the immunoregulatory enzyme indoleamine 2,3-dioxygenase (IDO). Normally, this suppressive network is dominant. If, however, these inhibitory signals can be blocked, then our second hypothesis proposes that the tumor milieu can now be rapidly reprogrammed into a highly activating and immunogenic microenvironment. In this reprogrammed milieu, the same tumor antigens now become spontaneously immunogenic.
Blocking the inhibitory mechanisms that underlie tumor-induced immunosuppression has become a major focus in tumor immunology. To date, however, most of the attention has been focused on effector T cells: either by blocking T cell-associated checkpoints such as CTLA-4 and PD-1, or by adoptive-transfer of pre-activated effector cells such as CAR-T cells. Less attention has been paid to enhancing the upstream process of antigen-presentation by host APCs. However, this upstream cross-presentation step is critical. Without effective antigen-presentation, it is not possible to use the full array of endogenous tumor neoantigens to activate the host’s own T cells.
It is now clear that a robust endogenous host T cell response constitutes a major determinant of success in conventional checkpoint-blockade therapy [1–7]. In the case of adoptive cellular therapy, the collateral recruitment of new specificities of endogenous host T cells (“epitope spreading”) may likewise be crucial to maintain long-term therapeutic response [8]. As proposed by Chen and Mellman, in order to create a long-term, self-sustaining immune response that cures the tumor, it is critical that the host immune response be enlisted to form a spontaneous, self-amplifying “cancer-immunity cycle” [9]. However, generating this endogenous T cell response requires activated, immunogenic host APCs, plus a receptive, pro-inflammatory tumor milieu. Unfortunately, this is not the usual case in tumors, and eliciting such a milieu requires more than simply blocking PD-1 or CTLA-4. We propose that it will require manipulation of a different set of immune checkpoints, focused on the upstream process of immunogenic antigen-presentation—factors which are influenced by immunosuppressive pathways such as IDO and activated Tregs.
Immune response to dying tumor cells is an active choice
A synchronous release of tumor antigens occurs during the wave of tumor cell death following chemotherapy or radiation. This review will focus on how the immune system chooses between activation versus tolerance to this wave of dying tumor cells. The key conceptual point proposed is that the immune response to antigens from dying tumor cells is not determined primarily by the pathway by which the tumor cells die, or by the type of chemotherapy delivered; but rather is dictated by active, external signals delivered by the local microenvironment. We propose that even during apoptosis (a classically “silent” form of cell death) the immune system still has the potential to cross-present tumor antigens in a robustly immunogenic way—unless this is actively suppressed. Conversely, even during classically “immunogenic” cell death (ICD) [10], the dominant suppressive signals within the tumor microenvironment may force the immune system to remain unresponsive, and prevent activation.
Thus, the response to antigens released by dying tumor cells is not fixed and inherent, but rather is a choice dictated by active signals—and these signals can be modified. This point becomes critical when immunologic therapy is combined with chemotherapy or radiation. Such combinations are a subject of high current interest [10–13], and it would have a major clinical impact if the antigens released by conventional treatments could be used to stimulate an aggressive, synergistic immune response. Although in principle this ought to be possible [10], in practice it has been very difficult to achieve [12]. Here we discuss recent data addressing the hypothesis that one fundamental reason for this failure is that the antigen-presenting milieu is normally suppressed by inhibitory mechanisms such as IDO and PTEN+ Tregs.
Activation of Tregs via the PTEN pathway
Tregs are a major mechanism of immune suppression in tumors [14]. While they may suppress effector T cells directly, Tregs can also have potent inhibitory effects on local APCs as well [15, 16]. At rest, Tregs are not spontaneously suppressive [17], so they must receive activation signals in order to become functional. Treg activation requires TCR engagement [18], but it is also highly influenced by additional modulating signals from the local milieu, which can profoundly affect the functional properties of the activated Tregs [19]. In the following discussion, we will describe data drawn primarily from mouse models of melanoma and lymphoma. However, we will also describe data from models of constitutive self-tolerance in mice without tumors, suggesting that these pathways represent fundamental regulatory mechanisms in the normal immune system. We hypothesize that the impact of these findings is not restricted to one particular type of tumor, or only to tumor cells that happen to express IDO. Rather, we speculate that IDO induction and PTEN+ Tregs may represent a more fundamental response by the host immune system, elicited by any dying cells. These tolerogenic host mechanisms become pathologically exaggerated and overexpressed in the tumor milieu, but the pathways themselves are basic mechanisms of self-tolerance.
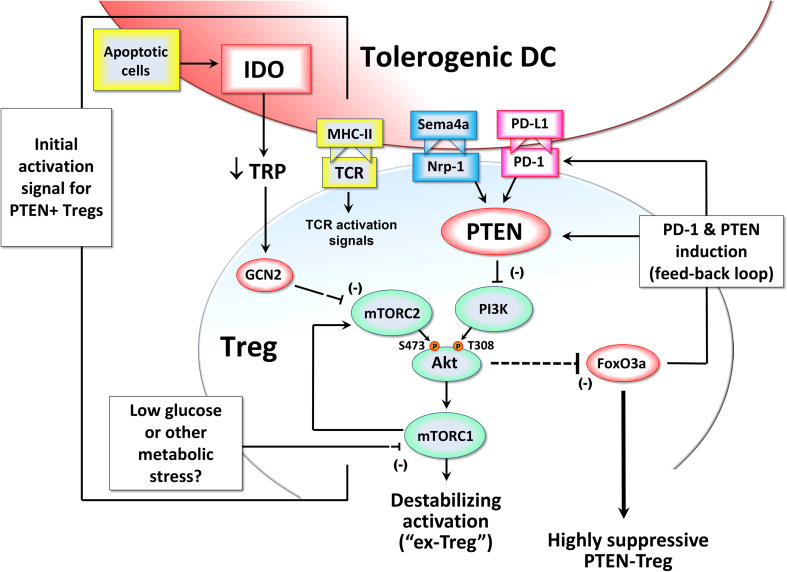
We have recently described a Treg activation pathway that is triggered when Tregs are stimulated under conditions that block signaling via Akt kinase and the mechanistic target of rapamycin (mTOR) (Fig. 1). Within the tumor microenvironment, there are multiple signals that can inhibit the Akt → mTOR pathway, including metabolic stress [20], the neuropilin-1 receptor [21], and—as shown in the diagram—exposure to DCs expressing the IDO enzyme [22]. IDO depletes the essential amino acid tryptophan. In vitro, depriving T cells of tryptophan is able to activate the amino acid-sensitive general control nonderepressible-2 kinase (GCN2) [23, 24]. In addition, the mTOR pathway itself is also a major cellular sensor of amino acid sufficiency [25]; and in some settings the GCN2 and mTOR pathways appear linked [26]. In vivo, the biology of GCN2 is complex. It can be expressed by multiple cell types in addition to T cells, and it may be responsive to more than just amino acid deprivation [27, 28]. Mice lacking GCN2 are unable to respond to induction of IDO by challenge with apoptotic cells [29]; and GCN2-knockout (KO) mice show an autoimmune-prone phenotype similar to IDO-KO mice [29, 30]. How much of this is due to sensing alterations in tryptophan is currently unknown. However, even simple dietary restriction of amino acids in mice can be sufficient to activate the GCN2 pathway in vivo [27, 31], so total depletion of tryptophan is not required to activate GCN2. Thus it may be that local reduction of tryptophan in the tumor microenvironment by IDO could play a role in activating the GCN2 pathway in Tregs.
Fig. 1.
Hypothetical model in which Treg activation is controlled by signals affecting the Akt/mTOR pathway, FoxO3 and a feedback loop between FoxO3, PD–1 and PTEN. The figure is reproduced from Ref. [71] with copyright permission of the publisher
By whatever mechanism, in our in vitro cell-culture models we found that GCN2 was important in allowing IDO to inhibit activity of mTOR kinase complex-2 (mTORC2) in Tregs, thus preventing the phosphorylation of Akt on its activating Ser473 site [22]. (Although this effect was lost in the absence of GCN2, amino acid insufficiency can also directly inhibit mTORC1, as noted above; and mTORC1, Akt and mTORC2 all participate in a feedback loop; so the interaction is complex.) Nevertheless, the effect of tryptophan and other metabolic stress on Akt activity is an important finding, because Akt is emerging as a key control-point for Treg activation in tumors [22, 32]. Unlike effector T cells, which require Akt signaling for normal activation, excessive Akt activity in Tregs inhibits their function [33]. More specifically, Akt destabilizes the Tregs so that they lose their suppressive activity [22, 34, 35]. As we have shown, these destabilized Tregs may then become “helper-like” cells with pro-inflammatory activity [36–38]. Such cells have been termed “ex-Tregs” [34, 39, 40], and we have shown that they can play an important role as helper cells in anti-tumor immune responses [37]. Thus, taken together, these data suggest that one key consequence of IDO exposure during Treg activation is to inhibit Akt, and thus maintain the suppressive Treg phenotype (prevent destabilization).
Akt is known to trigger inactivation and degradation of the transcription factors Forkhead box O3 (FoxO3) and FoxO1 [41], both of which are important for Treg function [42]. We find that IDO, by inhibiting the mTOR/Akt axis, allows Tregs to successfully up-regulate FoxO3 during activation, and with it a FoxO3-dependent suppressive program. Part of this program includes up-regulation of PD-1 on the activated Tregs [22]. When this PD-1 is engaged by its ligands, it activates the lipid phosphatase PTEN [43]. PTEN then acts to inhibit PI3K activity and thus block phosphorylation of Akt on its other activating site, at Thr308. Together, the result is a positive-feedback loop, as shown in Fig. 1, that maintains the sustained inhibition of Akt and expression of FoxO3. Thus, we propose that once Tregs undergo initial activation in the presence of IDO (or any other upstream signal that inhibits the Akt → mTOR pathway), the activated Tregs put in place the PTEN-driven feedback loop, which then stably maintains the highly suppressive Treg phenotype, as long as PD-1 is engaged by its ligands. And, since we have previously shown that IDO-activated Tregs potently induce the up-regulation of PD-ligand expression on DCs [44], the PD-1 → PTEN feedback loop is likely to remain permanently active in the tumor.
Thus, the PD-1 → PTEN feedback loop offers a molecular explanation for our long-standing observation that IDO-induced Treg activity becomes strictly dependent on the PD-1/PD-ligand pathway in order to maintain suppression [44–47]. Taken together, these data suggest a model in which IDO—or, potentially, a variety of other Treg-activating signals in the tumor microenvironment—all converge to trigger a suppressive program in the Tregs, driven by PTEN. This implicates the PTEN enzyme in Tregs as a previously unsuspected molecular target for tumor immunotherapy.
One final point to emphasize from this model is that the mTOR/Akt pathway in Tregs, with the downstream PD-1 → PTEN feedback loop that it controls, might also function as a response pathway for a variety of local environmental signals. Using in vitro models, we found that simply inhibiting the mTOR pathway with compounds such as rapamycin or PP242 at the time of Treg activation was sufficient to trigger the whole PD-1 → PTEN feedback loop, and thus confer potent, self-sustaining suppressor activity on the Tregs [22]. In this case, the antigen-presenting cells themselves did not need to express IDO, or even create a low-tryptophan signal, as long as the in vitro milieu caused mTOR to be inhibited (e.g., by rapamycin). The tumor microenvironment is highly stressful and creates multiple conditions that might inhibit mTOR in Tregs (e.g., low glucose, low energy stores, deprivation of a variety of amino acids). Even in the specific case of IDO, the IDO enzyme in the tumor may not need to be expressed by a professional APC, but simply by the tumor cells themselves as an “environmental” factor, conditioning the tumor milieu. Although this hypothesis is still speculative, it might be that any signal in the tumor microenvironment that acts to prevent mTOR signaling during Treg activation will have the effect of triggering the PD-1 → PTEN pathway, and thus driving Treg suppressor activity.
Biologic effects of the PTEN pathway in Tregs
PTEN is an important but incompletely understood regulator of T cell activation. Mice with a targeted ablation of PTEN in all T cells develop spontaneous lymphomas [48], as well as lymphoproliferative disorders and defects in self-tolerance and autoimmunity [48, 49]. This appears related to dysregulated TCR signaling and excessively prolonged immune activation [50]. In the Treg lineage, several groups including our own have recently studied the functional consequences of targeted deletion of PTEN in Tregs [22, 34, 51]. Ablation of PTEN rendered Tregs chronically unstable, with gradual conversion into pro-inflammatory “ex-Tregs” as the mice aged [34]. This was consistent with previous reports from our group and others, indicating that Tregs are susceptible to loss of suppressor activity and functional re-programming under conditions of inflammation [36–39, 46]. Consistent with the model in Fig. 1, the PTEN pathway thus appeared to be an important mechanism by which the normal suppressive Treg phenotype was stabilized and maintained in vivo.
Functionally, the Treg instability resulting from loss of PTEN caused mice to progressively develop spontaneous lupus-like autoimmunity as they aged [22, 34, 51]. The age at which autoimmunity manifested differs between the different cre/lox systems used in these studies, but in our particular strain the mice do not become symptomatic until late in life. When young, these mice were healthy and fertile. Strikingly, however, we found that even young, healthy PTENTreg-KO mice (which had not yet developed autoimmunity) immediately lost tolerance to self-antigens if they were challenged with large numbers of apoptotic cells [22]. Control, wild-type mice were unaffected by exposure to the same apoptotic cells; but the mice lacking PTEN+ Tregs rapidly developed lethal lupus autoimmunity. This inability to maintain self-tolerance when challenged with apoptotic cells is similar to the defect seen with IDO1-KO mice under the same conditions [30]. However, unlike the PTENTreg-KO mice, the IDO1-KO hosts only developed autoimmunity when directly challenged with apoptotic cells, not spontaneously over time. This may reflect the wider range of upstream factors such as PD-1 and neuropilin-1 that converge on the PTEN pathway in Tregs, in addition to IDO. Taken together, these findings suggested that the PTEN pathway in Tregs appeared critical in defining how the immune system responded to apoptotic cells in vivo: immunosuppression and tolerance if PTEN+ Tregs were intact; but immune activation and autoimmunity if PTEN+ Tregs were absent.
The tumor microenvironment becomes spontaneously immunogenic in the absence of PTEN+ Tregs
These findings were from mice without tumors, challenged with syngeneic apoptotic cells, but we hypothesized that these findings might also have important implications for the response to dying tumor cells. As mentioned above, we had noted that many of the Tregs in tumors constitutively expressed PTEN, and the same cells also co-expressed FoxO3 and PD-1. When tumors were implanted in PTENTreg-KO hosts, the impact on tumor growth was profound [22]. In the absence of the PTEN+ Treg population, even aggressive tumors such as B16F10 were unable to create their usual immunosuppressive tumor microenvironment; and instead became spontaneously immunogenic, chronically inflamed, and could barely grow. The immune response elicited by tumors in mice lacking PTEN+ Tregs included spontaneous activation of host T cells; chronic production of inflammatory cytokines such as IL-6 and IL-12; and (importantly for purposes of this discussion) constitutive maturation of activated CD103+ dendritic cells within the tumor. Thus, the PTEN+ Treg population appeared to coordinately control multiple features of the suppressive tumor microenvironment; and, in the absence of these Tregs, the tumor milieu was transformed into the type of robustly immunogenic microenvironment that would be desirable for immunotherapy.
Manipulating the PTEN pathway for therapy
Based on this, we asked whether a similar immunogenic milieu could be therapeutically created in wild-type hosts simply by destabilizing the PTEN+ Treg population. The idea of destabilizing the Tregs in tumors—rather than trying to physically deplete or eliminate them—is potentially an attractive concept, because this may represent a point of vulnerability for activated Tregs. During activation, Tregs must actively work to maintain their suppressive phenotype, via pathways such as Ezh2 [52] and the Helios transcription factor [53]. Without this active stabilization, exposure to inflammatory signals such as IL-6 causes Tregs to spontaneously down-regulate key transcription factors such as Eos/Ikzf4, resulting in transformation of the Tregs into a pro-inflammatory “helper-like” phenotype [36, 54].
To test whether Tregs in tumors could be destabilized by pharmacologically blocking PTEN, we inhibited the PTEN phosphatase enzyme using the orthovanadate drug VO-OHpic [55]. Many cells can express PTEN, so VO-OHpic treatment in vivo was not selective only for Tregs. However, effector T cells and inflammatory APCs expressed little PTEN during activation, and we were unable to detect any direct effect of the PTEN-inhibitor attributable to these populations (i.e., when PTEN was selectively ablated in the Tregs, then there was no further detectable effect of treating the PTENTreg-KO mice with VO-OHpic) [22]. In contrast, Tregs appeared to be heavily dependent on the PTEN pathway, and became sensitive to destabilization when mice were treated with the PTEN-inhibitor. The result was that when VO-OHpic was combined with even modest doses of chemotherapy, we found that the drugs displayed a potent, synergistic anti-tumor effect, with rapid activation of the immune system and shrinkage of the tumors. This was not restricted to transplantable tumors models, since autochthonous melanoma showed the same pattern of immune activation and regression when treated with VO-OHpic plus chemotherapy.
Thus, taken together, the PTEN pathway in Tregs appeared to represent a novel and potentially tractable target for immunotherapy. In wild-type mice, where tumors were already established and suppressive at the time of treatment, the PTEN-inhibitor drug by itself did not trigger spontaneous tumor regression. However, inhibiting PTEN rendered the Tregs susceptible to rapid destabilization whenever inflammation was created by chemotherapy or other immunotherapy—even if that inflammation by itself would not have had any anti-tumor effect. Treg destabilization was driven by inflammatory cytokines, specifically including the local production of IL-6, as we have previously described [36]. If Tregs lacked the high-affinity IL-6 receptor (IL-6RaTreg-KO mice) then they showed no destabilization when treated with VO-OHpic+ chemotherapy, and the treatment lost all effect [22]. All anti-tumor effect of PTEN-inhibitor was also lost in Rag-deficient hosts, or if CD8+ T cells were depleted, so the synergy between chemotherapy and PTEN-inhibitor was strictly immune-mediated [22].
It remains to be determined whether the PTEN pathway plays the same role in human Tregs as in mouse. Patients with germline mutations in PTEN have been reported to have an increased risk of autoimmunity [56], but interpretation of this finding is complicated by the fact that PTEN is expressed in B cells and effector T cells, in addition to Tregs. In mouse tumors, we found that PTEN+ Tregs co-expressed PD-1 and the C-C chemokine receptor type 4 (CCR4). A population of activated Tregs with a similar PD-1+ CCR4+ phenotype has been demonstrated in human tumors [14], but whether these are dependent on PTEN, and whether they can be destabilized by PTEN-inhibitor drugs, have not yet been tested. With respect to the use of PTEN-inhibitor drugs such as VO-OHpic for clinical therapy, none of the existing compounds show strict specificity for PTEN (i.e., they may also affect one or more other phosphatase enzymes) [57]. However, since the desired biologic effect is simply to inhibit PTEN sufficiently to destabilize the highly PTEN-dependent Tregs, it may be that exquisite specificity is not required, as long as this objective can be met within an acceptable toxicity profile.
Characteristics of DCs and T cells in the activated tumor microenvironment
Examining the downstream mechanism, the key effect of destabilizing PTEN+ Tregs was to transform the tumor microenvironment into an activating, pro-inflammatory milieu. Prior to treatment, the majority of DCs in tumors expressed inhibitory PD-L1, and there was little expression of costimulatory CD86 or inflammatory cytokines. However, within 1–2 days of treatment with PTEN-inhibitor + chemotherapy the number of CD103+ DCs in tumors had markedly increased, and these DCs all expressed CD80, CD86 and inflammatory cytokines (IL-6 and IL-12); while the expression of PD-L1 and PD-L2 was markedly reduced.
Consistent with a more immunogenic APC population, the effector T cells in the tumor became activated [22]. Prior to treatment, tumors contained CD8+ T cells, but these were functionally unresponsive: they were mostly PD-1+; did not express effector molecules such as granzyme B; and were unable to proliferate (anergic/exhausted). However, within 1–2 days of treatment with PTEN-inhibitor + chemotherapy, CD8+ T cells in the tumor up-regulated CD69 and granzyme B, became able to proliferate, and expressed IFNγ and the cell-surface integrin CD103. It is not yet known whether this T cell activation represents turnover (i.e., replacement of the anergic T cells by a new population of activated cells), or in situ re-activation of the formerly anergic T cells. However, the rapidity with which this occurred (within 24–48 h) suggested that the responding T cells were not naïve, but must have been a pre-existing memory population. In either case, destabilizing PTEN+ Tregs triggered a change in the antigen-presenting milieu within the tumor, which led to robust T cell activation.
IDO, PTEN+ Tregs and the response to apoptotic tumor cells
Physiologic tolerance to apoptotic self-cells
As mentioned above, IDO is one of the upstream signals that can activate PTEN+ Tregs. IDO contributes to multiple forms of acquired peripheral tolerance (reviewed in Ref. [58]), but we hypothesize that the direct link between IDO and PTEN+ Tregs might become especially important when the immune system encountered dying tumor cells. In mice without tumors, previous work had shown that IDO plays an important and non-redundant role in enforcing tolerance to apoptotic cells [29, 30, 59]. Exposure to apoptotic cells rapidly up-regulates IDO expression [30]; and this IDO was a required signal for downstream induction of tolerogenic IL-10 and TGFβ by the apoptotic cells, and for recruiting suppressive Tregs [29, 59]. When mice lacking the IDO1 gene were challenged with a wave of apoptotic thymocytes, they were unable to maintain the normal tolerance to self-antigens, and rapidly developed lupus-like autoimmunity to antigens associated with apoptotic cells [29, 30]. Thus, IDO-deficient mice had a fundamental defect in tolerance to apoptotic cells, which was similar to mice lacking PTEN in Tregs, as described above [22]. This is consistent with the hypothesis that these two mechanisms form a linked system for inhibiting immune responses to dying cells in vivo.
Response to apoptotic tumor cells
In the case of tumor cells, injection of apoptotic tumor cells into normal mice caused direct activation of local IDO in the draining lymph nodes [22]. This occurred even in mice with no prior exposure to tumors, and so appeared to be a direct effect of the dying cells themselves. This IDO signal rapidly activated PTEN+ Tregs in draining lymph nodes, and these PTEN+ Tregs then suppressed all T cell response to neoantigens present on the apoptotic tumor cells. However, if either IDO or PTEN pathways were pharmacologically blocked or genetically ablated, then T cells now were able to respond robustly to the same tumor cell-associated antigens [22]. Thus, the apoptotic tumor cells themselves were intrinsically immunogenic, but the T cell response was actively suppressed by induction of the IDO → PTEN+ Treg pathway.
This has potentially important implications for the immune response to chemotherapy and radiation of tumors. These treatments generate large numbers of dying tumor cells, but the antigens are released into an unreceptive microenvironment dominated by IDO and PTEN+ Tregs. When tested in murine tumor models, combination of IDO-inhibitor drugs with chemotherapy or radiation showed synergistic anti-tumor effect [60–63]. Quantitatively, the effect of inhibiting IDO was not as dramatic as the effect of PTEN-inhibition (perhaps consistent with the fact that PTEN is also downstream of multiple other Treg-activating signals in the tumor, in addition to IDO). However, IDO-inhibitor drugs are well-tolerated in the clinic, even with prolonged administration [64, 65]. Phase II clinical trials combining IDO-inhibitors with chemotherapy and/or radiation in pancreatic cancer and brain tumors are currently in progress [66–68].
Inhibitors of PTEN are still at the preclinical-development stage. Based on the progressive autoimmunity seen in PTENTreg-KO mice, it may be that PTEN-inhibitor drugs will show more risk of autoimmune toxicity if used for prolonged periods. However, if the goal is only to enhance the immune response to the transient wave of antigens released by chemotherapy or radiation, then prolonged administration may not be required. We speculate that the critical window of time may be only the few days during which cells are dying after the insult. With intermittent pulsed therapy, it may be possible to destabilize PTEN+ Tregs in the tumor long enough to break tolerance to dying tumors cells, without blocking PTEN long enough to trigger spontaneous loss of self-tolerance elsewhere. In this regard, it is relevant to note that administration of VO-OHpic to mice during chemotherapy caused extensive destabilization of Tregs inside the tumor and associated tumor-draining LNs (both of which became inflamed), whereas elsewhere in the same animal the Tregs were not destabilized and there was no inflammation in other LNs [22].
One final consideration for PTEN-inhibitor drugs is that PTEN is a tumor-suppressor gene, and its loss can contribute to malignant transformation. However, the oncogenic impact of disrupting a tumor-suppressor gene occurs over a prolonged period of time. Short-term intermittent use, such as for pulsed immunotherapy, presents much less of a concern [57, 69].
Future implications: combining immunotherapy with chemotherapy and radiation
In principle, there is an important translational opportunity for synergies between standard-of-care cytotoxic therapy (chemotherapy and radiation) and active immunotherapy [12]. The caveat, however, is that the immunotherapy needs to be of a type that targets and successfully re-defines the antigen-presenting milieu in the tumor. If this can be accomplished—as, for example, with IDO and PTEN inhibitors—then there is potential for synergy in both directions: the dying tumor cells are now allowed to act as an endogenous “vaccine” for the immune system [11]; while, conversely, the activated immune system becomes a potent additional effector mechanism for the chemotherapy and radiation. Hints of the inherent power of such an activated immune system are already being seen in the form of anecdotal “late-responders” to conventional checkpoint blockade [70]. If this immunologic effector arm can be reliably recruited in response to conventional chemotherapy and radiation, then the potential for clinical impact would be large.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Supported in part by Grants CA096651 and CA103320 from the National Institutes of Health, and the Beloco foundation.
Abbreviations
- CCR4
C-C chemokine receptor type 4
- FoxO3
Forkhead box O3
- GCN2
General control nonderepressible-2
- KO
Knockout
- mTOR
Mechanistic target of rapamycin
- mTORC
mTOR kinase complex
- PTEN
Phosphatase and tensin homolog
- Tregs
Regulatory T cells
Compliance with ethical standards
Conflict of interest
David Munn is a consultant to NewLink Genetics Corporation and holds intellectual property in IDO-inhibitors and PTEN-inhibitors. Theodore Johnson receives funding for clinical trials of IDO-inhibitors from NewLink Genetics, Inc. The authors declare that there are no other conflicts of interest.
Footnotes
This paper is a Focussed Research Review based on a presentation given at the conference Regulatory Myeloid Suppressor Cells: From Basic Discovery to Therapeutic Application which was hosted by the Wistar Institute in Philadelphia, PA, USA, 16th–19th June, 2016. It is part of a Cancer Immunology, Immunotherapy series of Focussed Research Reviews.
References
- 1.McGranahan N, Furness AJ, Rosenthal R, Ramskov S, Lyngaa R, Saini SK, Jamal-Hanjani M, Wilson GA, Birkbak NJ, Hiley CT, Watkins TB, Shafi S, Murugaesu N, Mitter R, Akarca AU, Linares J, Marafioti T, Henry JY, Van Allen EM, Miao D, Schilling B, Schadendorf D, Garraway LA, Makarov V, Rizvi NA, Snyder A, Hellmann MD, Merghoub T, Wolchok JD, Shukla SA, Wu CJ, Peggs KS, Chan TA, Hadrup SR, Quezada SA, Swanton C. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351:1463–1469. doi: 10.1126/science.aaf1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kvistborg P, Philips D, Kelderman S, Hageman L, Ottensmeier C, Joseph-Pietras D, Welters MJ, van der Burg S, Kapiteijn E, Michielin O, Romano E, Linnemann C, Speiser D, Blank C, Haanen JB, Schumacher TN. Anti-CTLA-4 therapy broadens the melanoma-reactive CD8+ T cell response. Sci Transl Med. 2014;6:254ra128. doi: 10.1126/scitranslmed.3008918. [DOI] [PubMed] [Google Scholar]
- 3.Cha E, Klinger M, Hou Y, Cummings C, Ribas A, Faham M, Fong L. Improved survival with T cell clonotype stability after anti-CTLA-4 treatment in cancer patients. Sci Transl Med. 2014;6:238ra70. doi: 10.1126/scitranslmed.3008211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reissfelder C, Stamova S, Gossmann C, Braun M, Bonertz A, Walliczek U, Grimm M, Rahbari NN, Koch M, Saadati M, Benner A, Buchler MW, Jager D, Halama N, Khazaie K, Weitz J, Beckhove P. Tumor-specific cytotoxic T lymphocyte activity determines colorectal cancer patient prognosis. J Clin Invest. 2015;125:739–751. doi: 10.1172/JCI74894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, West AN, Carmona M, Kivork C, Seja E, Cherry G, Gutierrez AJ, Grogan TR, Mateus C, Tomasic G, Glaspy JA, Emerson RO, Robins H, Pierce RH, Elashoff DA, Robert C, Ribas A. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, Kohrt HE, Horn L, Lawrence DP, Rost S, Leabman M, Xiao Y, Mokatrin A, Koeppen H, Hegde PS, Mellman I, Chen DS, Hodi FS. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daud AI, Loo K, Pauli ML, Sanchez-Rodriguez R, Sandoval PM, Taravati K, Tsai K, Nosrati A, Nardo L, Alvarado MD, Algazi AP, Pampaloni MH, Lobach IV, Hwang J, Pierce RH, Gratz IK, Krummel MF, Rosenblum MD. Tumor immune profiling predicts response to anti-PD-1 therapy in human melanoma. J Clin Invest. 2016;126:3447–3452. doi: 10.1172/JCI87324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verdegaal EM, de Miranda NF, Visser M, Harryvan T, van Buuren MM, Andersen RS, Hadrup SR, van der Minne CE, Schotte R, Spits H, Haanen JB, Kapiteijn EH, Schumacher TN, van der Burg SH. Neoantigen landscape dynamics during human melanoma-T cell interactions. Nature. 2016;536:91–95. doi: 10.1038/nature18945. [DOI] [PubMed] [Google Scholar]
- 9.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 10.Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell. 2015;28:690–714. doi: 10.1016/j.ccell.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 11.Belvin M, Mellman I. Is all cancer therapy immunotherapy? Sci Transl Med. 2015;7(315):315fs48. doi: 10.1126/scitranslmed.aad7661. [DOI] [PubMed] [Google Scholar]
- 12.Medler TR, Cotechini T, Coussens LM. Immune response to cancer therapy: mounting an effective antitumor response and mechanisms of resistance. Trends Cancer. 2015;1:66–75. doi: 10.1016/j.trecan.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bezu L, Gomes-de-Silva LC, Dewitte H, Breckpot K, Fucikova J, Spisek R, Galluzzi L, Kepp O, Kroemer G. Combinatorial strategies for the induction of immunogenic cell death. Front Immunol. 2015;6:187. doi: 10.3389/fimmu.2015.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishikawa H, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Curr Opin Immunol. 2014;27:1–7. doi: 10.1016/j.coi.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Bauer CA, Kim EY, Marangoni F, Carrizosa E, Claudio NM, Mempel TR. Dynamic Treg interactions with intratumoral APCs promote local CTL dysfunction. J Clin Invest. 2014;124:2425–2440. doi: 10.1172/JCI66375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joshi NS, Akama-Garren EH, Lu Y, Lee DY, Chang GP, Li A, DuPage M, Tammela T, Kerper NR, Farago AF, Robbins R, Crowley DM, Bronson RT, Jacks T. Regulatory T cells in tumor-associated tertiary lymphoid structures suppress anti-tumor T cell responses. Immunity. 2015;43:579–590. doi: 10.1016/j.immuni.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thornton AM, Piccirillo CA, Shevach EM. Activation requirements for the induction of CD4+ CD25+ T cell suppressor function. Eur J Immunol. 2004;34:366–376. doi: 10.1002/eji.200324455. [DOI] [PubMed] [Google Scholar]
- 18.Levine AG, Arvey A, Jin W, Rudensky AY. Continuous requirement for the TCR in regulatory T cell function. Nat Immunol. 2014;15:1070–1078. doi: 10.1038/ni.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaudhry A, Rudensky AY. Control of inflammation by integration of environmental cues by regulatory T cells. J Clin Invest. 2013;123:939–944. doi: 10.1172/JCI57175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blagih J, Coulombe F, Vincent EE, Dupuy F, Galicia-Vazquez G, Yurchenko E, Raissi TC, van der Windt GJ, Viollet B, Pearce EL, Pelletier J, Piccirillo CA, Krawczyk CM, Divangahi M, Jones RG. The energy sensor AMPK regulates T cell metabolic adaptation and effector responses in vivo. Immunity. 2015;42:41–54. doi: 10.1016/j.immuni.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 21.Delgoffe GM, Woo SR, Turnis ME, Gravano DM, Guy C, Overacre AE, Bettini ML, Vogel P, Finkelstein D, Bonnevier J, Workman CJ, Vignali DA. Stability and function of regulatory T cells is maintained by a neuropilin-1-semaphorin-4a axis. Nature. 2013;501:252–256. doi: 10.1038/nature12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma MD, Shinde R, McGaha T, Huang L, Holmgaard RB, Wolchok JD, Mautino MR, Celis E, Sharpe A, Francisco LM, Powell DJ, Jr, Yagita H, Mellor AL, Blazar BR, Munn DH. The PTEN pathway in Tregs is a critical driver of the suppressive tumor microenvironment. Science Advances. 2015;1:e1500845. doi: 10.1126/sciadv.1500845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Munn DH, Sharma MD, Baban B, Harding HP, Zhang Y, Ron D, Mellor AL. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity. 2005;22:633–642. doi: 10.1016/j.immuni.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 24.Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, Vacca C, Orabona C, Bianchi R, Belladonna ML, Volpi C, Santamaria P, Fioretti MC, Puccetti P. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. J Immunol. 2006;176:6752–6761. doi: 10.4049/jimmunol.176.11.6752. [DOI] [PubMed] [Google Scholar]
- 25.Jewell JL, Russell RC, Guan KL. Amino acid signalling upstream of mTOR. Nat Rev Molec Cell Biol. 2013;14:133–139. doi: 10.1038/nrm3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ye J, Palm W, Peng M, King B, Lindsten T, Li MO, Koumenis C, Thompson CB. GCN2 sustains mTORC1 suppression upon amino acid deprivation by inducing Sestrin2. Genes Dev. 2015;29:2331–2336. doi: 10.1101/gad.269324.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ravindran R, Loebbermann J, Nakaya HI, Khan N, Ma H, Gama L, Machiah DK, Lawson B, Hakimpour P, Wang YC, Li S, Sharma P, Kaufman RJ, Martinez J, Pulendran B. The amino acid sensor GCN2 controls gut inflammation by inhibiting inflammasome activation. Nature. 2016;531:523–527. doi: 10.1038/nature17186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van de Velde LA, Guo XJ, Barbaric L, Smith AM, Oguin TH, 3rd, Thomas PG, Murray PJ. Stress kinase GCN2 controls the proliferative fitness and trafficking of cytotoxic T cells independent of environmental amino acid sensing. Cell reports. 2016;17:2247–2258. doi: 10.1016/j.celrep.2016.10.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ravishankar B, Liu H, Shinde R, Chaudhary K, Xiao W, Bradley J, Koritzinsky M, Madaio MP, McGaha TL. The amino acid sensor GCN2 inhibits inflammatory responses to apoptotic cells promoting tolerance and suppressing systemic autoimmunity. Proc Natl Acad Sci USA. 2015;112:10774–10779. doi: 10.1073/pnas.1504276112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ravishankar B, Liu H, Shinde R, Chandler P, Baban B, Tanaka M, Munn DH, Mellor AL, Karlsson MC, McGaha TL. Tolerance to apoptotic cells is regulated by indoleamine 2,3-dioxygenase. Proc Natl Acad Sci USA. 2012;109:3909–3914. doi: 10.1073/pnas.1117736109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peng W, Robertson L, Gallinetti J, Mejia P, Vose S, Charlip A, Chu T, Mitchell JR. Surgical stress resistance induced by single amino acid deprivation requires Gcn2 in mice. Sci Transl Med. 2012;4:118ra11. doi: 10.1126/scitranslmed.3002629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ali K, Soond DR, Pineiro R, Hagemann T, Pearce W, Lim EL, Bouabe H, Scudamore CL, Hancox T, Maecker H, Friedman L, Turner M, Okkenhaug K, Vanhaesebroeck B. Inactivation of PI(3)K p110delta breaks regulatory T-cell-mediated immune tolerance to cancer. Nature. 2014;510:407–411. doi: 10.1038/nature13444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crellin NK, Garcia RV, Levings MK. Altered activation of AKT is required for the suppressive function of human CD4+ CD25+ T regulatory cells. Blood. 2007;109:2014–2022. doi: 10.1182/blood-2006-07-035279. [DOI] [PubMed] [Google Scholar]
- 34.Huynh A, DuPage M, Priyadharshini B, Sage PT, Quiros J, Borges CM, Townamchai N, Gerriets VA, Rathmell JC, Sharpe AH, Bluestone JA, Turka LA. Control of PI(3) kinase in Treg cells maintains homeostasis and lineage stability. Nat Immunol. 2015;16:188–196. doi: 10.1038/ni.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kitz A, de Marcken M, Gautron AS, Mitrovic M, Hafler DA, Dominguez-Villar M. AKT isoforms modulate Th1-like Treg generation and function in human autoimmune disease. EMBO Rep. 2016;17:1169–1183. doi: 10.15252/embr.201541905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharma MD, Huang L, Choi JH, Lee EJ, Wilson JM, Lemos H, Pan F, Blazar BR, Pardoll DM, Mellor AL, Shi H, Munn DH. An inherently bifunctional subset of Foxp3 T helper cells is controlled by the transcription factor Eos. Immunity. 2013;38:998–1012. doi: 10.1016/j.immuni.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharma MD, Hou DY, Baban B, Koni PA, He Y, Chandler PR, Blazar BR, Mellor AL, Munn DH. Reprogrammed Foxp3(+) regulatory T cells provide essential help to support cross-presentation and CD8(+) T cell priming in naive mice. Immunity. 2010;33:942–954. doi: 10.1016/j.immuni.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharma MD, Hou DY, Liu Y, Koni PA, Metz R, Chandler P, Mellor AL, He Y, Munn DH. Indoleamine 2,3-dioxygenase controls conversion of Foxp3 + Tregs to TH17-like cells in tumor-draining lymph nodes. Blood. 2009;113:6102–6111. doi: 10.1182/blood-2008-12-195354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martinez-Llordella M, Ashby M, Nakayama M, Rosenthal W, Bluestone JA. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009;10:1000–1007. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bailey-Bucktrout SL, Martinez-Llordella M, Zhou X, Anthony B, Rosenthal W, Luche H, Fehling HJ, Bluestone JA. Self-antigen-driven activation induces instability of regulatory T cells during an inflammatory autoimmune response. Immunity. 2013;39:949–962. doi: 10.1016/j.immuni.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hedrick SM, Hess Michelini R, Doedens AL, Goldrath AW, Stone EL. FOXO transcription factors throughout T cell biology. Nat Rev Immunol. 2012;12:649–661. doi: 10.1038/nri3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kerdiles YM, Stone EL, Beisner DL, McGargill MA, Ch’en IL, Stockmann C, Katayama CD, Hedrick SM. Foxo transcription factors control regulatory T cell development and function. Immunity. 2010;33:890–904. doi: 10.1016/j.immuni.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharma MD, Baban B, Chandler P, Hou DY, Singh N, Yagita H, Azuma M, Blazar BR, Mellor AL, Munn DH. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J Clin Invest. 2007;117:2570–2582. doi: 10.1172/JCI31911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baban B, Chandler PR, Johnson BA, 3rd, Huang L, Li M, Sharpe ML, Francisco LM, Sharpe AH, Blazar BR, Munn DH, Mellor AL. Physiologic control of IDO competence in splenic dendritic cells. J Immunol. 2011;187:2329–2335. doi: 10.4049/jimmunol.1100276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baban B, Chandler PR, Sharma MD, Pihkala J, Koni PA, Munn DH, Mellor AL. IDO activates regulatory T cells and blocks their conversion into Th17-like T cells. J Immunol. 2009;183:2475–2483. doi: 10.4049/jimmunol.0900986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang L, Lemos HP, Li L, Li M, Chandler PR, Baban B, McGaha TL, Ravishankar B, Lee JR, Munn DH, Mellor AL. Engineering DNA nanoparticles as immunomodulatory reagents that activate regulatory T cells. J Immunol. 2012;188:4913–4920. doi: 10.4049/jimmunol.1103668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suzuki A, Yamaguchi MT, Ohteki T, Sasaki T, Kaisho T, Kimura Y, Yoshida R, Wakeham A, Higuchi T, Fukumoto M, Tsubata T, Ohashi PS, Koyasu S, Penninger JM, Nakano T, Mak TW. T cell-specific loss of Pten leads to defects in central and peripheral tolerance. Immunity. 2001;14:523–534. doi: 10.1016/S1074-7613(01)00134-0. [DOI] [PubMed] [Google Scholar]
- 49.Di Cristofano A, Kotsi P, Peng YF, Cordon-Cardo C, Elkon KB, Pandolfi PP. Impaired Fas response and autoimmunity in Pten ± mice. Science. 1999;285:2122–2125. doi: 10.1126/science.285.5436.2122. [DOI] [PubMed] [Google Scholar]
- 50.Liu X, Karnell JL, Yin B, Zhang R, Zhang J, Li P, Choi Y, Maltzman JS, Pear WS, Bassing CH, Turka LA. Distinct roles for PTEN in prevention of T cell lymphoma and autoimmunity in mice. J Clin Invest. 2010;120:2497–2507. doi: 10.1172/JCI42382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shrestha S, Yang K, Guy C, Vogel P, Neale G, Chi H. Treg cells require the phosphatase PTEN to restrain Th1 and Tfh cell responses. Nat Immunol. 2015;16:178–187. doi: 10.1038/ni.3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.DuPage M, Chopra G, Quiros J, Rosenthal WL, Morar MM, Holohan D, Zhang R, Turka L, Marson A, Bluestone JA. The chromatin-modifying enzyme Ezh2 is critical for the maintenance of regulatory T cell identity after activation. Immunity. 2015;42:227–238. doi: 10.1016/j.immuni.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim HJ, Barnitz RA, Kreslavsky T, Brown FD, Moffett H, Lemieux ME, Kaygusuz Y, Meissner T, Holderried TA, Chan S, Kastner P, Haining WN, Cantor H. Stable inhibitory activity of regulatory T cells requires the transcription factor Helios. Science. 2015;350:334–339. doi: 10.1126/science.aad0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang HY, Barbi J, Wu CY, Zheng Y, Vignali PD, Wu X, Tao JH, Park BV, Bandara S, Novack L, Ni X, Yang X, Chang KY, Wu RC, Zhang J, Yang CW, Pardoll DM, Li H, Pan F. MicroRNA-17 modulates regulatory T cell function by targeting Co-regulators of the Foxp3 transcription factor. Immunity. 2016;45:83–93. doi: 10.1016/j.immuni.2016.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mak LH, Vilar R, Woscholski R. Characterisation of the PTEN inhibitor VO-OHpic. J Chem Biol. 2010;3:157–163. doi: 10.1007/s12154-010-0041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heindl M, Händel N, Ngeow J, Kionke J, Wittekind C, Kamprad M, Rensing-Ehl A, Ehl S, Reifenberger J, Loddenkemper C, Maul J, Hoffmeister A, Aretz S, Kiess W, Eng C, Uhlig HH. Autoimmunity, intestinal lymphoid hyperplasia, and defects in mucosal B-cell homeostasis in patients with PTEN hamartoma tumor syndrome. Gastroenterology. 2012;142(1093–6):e6. doi: 10.1053/j.gastro.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 57.Spinelli L, Lindsay YE, Leslie NR. PTEN inhibitors: an evaluation of current compounds. Adv Biol Regul. 2015;57:102–111. doi: 10.1016/j.jbior.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 58.Munn DH, Mellor AL. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 2013;34:137–143. doi: 10.1016/j.it.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ravishankar B, Shinde R, Liu H, Chaudhary K, Bradley J, Lemos HP, Chandler P, Tanaka M, Munn DH, Mellor AL, McGaha TL. Marginal zone CD169+ macrophages coordinate apoptotic cell-driven cellular recruitment and tolerance. Proc Natl Acad Sci USA. 2014;111:4215–4220. doi: 10.1073/pnas.1320924111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Muller AJ, Duhadaway JB, Donover PS, Sutanto-Ward E, Prendergast GC. Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat Med. 2005;11:312–319. doi: 10.1038/nm1196. [DOI] [PubMed] [Google Scholar]
- 61.Hou DY, Muller AJ, Sharma MD, Duhadaway JB, Banerjee T, Johnson M, Mellor AL, Prendergast GC, Munn DH. Inhibition of IDO in dendritic cells by stereoisomers of 1-methyl-tryptophan correlates with anti-tumor responses. Cancer Res. 2007;67:792–801. doi: 10.1158/0008-5472.CAN-06-2925. [DOI] [PubMed] [Google Scholar]
- 62.Li M, Bolduc AR, Hoda MN, Gamble DN, Dolisca SB, Bolduc AK, Hoang K, Ashley C, McCall D, Rojiani AM, Maria BL, Rixe O, MacDonald TJ, Heeger PS, Mellor AL, Munn DH, Johnson TS. The indoleamine 2,3-dioxygenase pathway controls complement-dependent enhancement of chemo-radiation therapy against murine glioblastoma. J Immunother Cancer (JITC) 2014;2:21. doi: 10.1186/2051-1426-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wainwright DA, Chang AL, Dey M, Balyasnikova IV, Kim CK, Tobias A, Cheng Y, Kim JW, Qiao J, Zhang L, Han Y, Lesniak MS. Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4, and PD-L1 in mice with brain tumors. Clin Cancer Res. 2014;20:5290–5301. doi: 10.1158/1078-0432.CCR-14-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Soliman HH, Minton SE, Han HS, Ismail-Khan R, Neuger A, Khambati F, Noyes D, Lush R, Chiappori AA, Roberts JD, Link C, Vahanian NN, Mautino M, Streicher H, Sullivan DM, Antonia SJ. A phase I study of indoximod in patients with advanced malignancies. Oncotarget. 2016;7:22928–22938. doi: 10.18632/oncotarget.8216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Beatty GL, O’Dwyer PJ, Clark J, Shi JG, Bowman KJ, Scherle PA, Newton RC, Schaub R, Maleski J, Leopold L, Gajewski TF. First-in-human phase 1 study of the oral inhibitor of indoleamine 2,3-dioxygenase-1 epacadostat (INCB024360) in patients with advanced solid malignancies. Clin Cancer Res. 2017 doi: 10.1158/1078-0432.CCR-16-2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bahary N, Garrido-Laguna I, Cinar P, O’Rourke Ma, Somer BG, Nyak-Kapoor A, Lee JS, Munn DH, Kennedy EP, Vahanian NN, Link CJ, Wang-Gillam A (2016). Phase 2 trial of the indoleamine 2,3-dioxygenase pathway (IDO) inhibitor indoximod plus gemcitabine/nab-paclitaxel for the treatment of metastatic pancreas cancer: interim analysis. ASCO Annual Meeting Proceedings: (Abstract 3020)
- 67.Johnson TS, Giller CA, Heger IM, Kennedy EP, Kolhe RB, Mourad WF, Rojiani AM, Sadek RF, Vahanian NN, Macdonald TJ, Munn DH (2016). Phase 1 trial of indoximod in combination with temozolomide-based therapy for children with progressive primary brain tumors (NCT02502708). Pediatr Blood Cancer 63(Suppl. 1):S72-S3. Abstract from the 29th Annual Meeting of the American Society of Pediatric Hematology/Oncology (ASPHO), Minneapolis MN, May 2016
- 68.Zakharia Y, Munn D, Link C, Vahanian N, Kennedy E (2016). Interim analysis of Phase 1b/2 combination of the IDO pathway inhibitor indoximod with temozolomide for adult patients with temozolomide-refractory primary malignant brain tumors. Neuro-Oncology 18(suppl. 6):vi13-4. Abstract from the 21st Annual Meeting of the Society for Neuro-Oncology (SNO), Scottsdale AZ, November 2016
- 69.Nardella C, Clohessy JG, Alimonti A, Pandolfi PP. Pro-senescence therapy for cancer treatment. Nat Rev Cancer. 2011;11:503–511. doi: 10.1038/nrc3057. [DOI] [PubMed] [Google Scholar]
- 70.Hodi FS, Hwu WJ, Kefford R, Weber JS, Daud A, Hamid O, Patnaik A, Ribas A, Robert C, Gangadhar TC, Joshua AM, Hersey P, Dronca R, Joseph R, Hille D, Xue D, Li XN, Kang SP, Ebbinghaus S, Perrone A, Wolchok JD. Evaluation of immune-related response criteria and RECIST v1.1 in patients with advanced melanoma treated with pembrolizumab. J Clin Oncol. 2016;34:1510–1517. doi: 10.1200/JCO.2015.64.0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Munn DH, Mellor AL. IDO in the tumor microenvironment: inflammation, counter-regulation, and tolerance. Trends Immunol. 2016;37:193–207. doi: 10.1016/j.it.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.