Abstract
Introduction: Inflammatory bowel diseases (IBDs) include Crohn's disease, and ulcerative colitis. Cannabis sativa preparations have beneficial effects for IBD patients. However, C. sativa extracts contain hundreds of compounds. Although there is much knowledge of the activity of different cannabinoids and their receptor agonists or antagonists, the cytotoxic and anti-inflammatory activity of whole C. sativa extracts has never been characterized in detail with in vitro and ex vivo colon models.
Material and Methods: The anti-inflammatory activity of C. sativa extracts was studied on three lines of epithelial cells and on colon tissue. C. sativa flowers were extracted with ethanol, enzyme-linked immunosorbent assay was used to determine the level of interleukin-8 in colon cells and tissue biopsies, chemical analysis was performed using high-performance liquid chromatography, mass spectrometry and nuclear magnetic resonance and gene expression was determined by quantitative real-time PCR.
Results: The anti-inflammatory activity of Cannabis extracts derives from D9-tetrahydrocannabinolic acid (THCA) present in fraction 7 (F7) of the extract. However, all fractions of C. sativa at a certain combination of concentrations have a significant increased cytotoxic activity. GPR55 receptor antagonist significantly reduces the anti-inflammatory activity of F7, whereas cannabinoid type 2 receptor antagonist significantly increases HCT116 cell proliferation. Also, cannabidiol (CBD) shows dose dependent cytotoxic activity, whereas anti-inflammatory activity was found only for the low concentration of CBD, and in a bell-shaped rather than dose-dependent manner. Activity of the extract and active fraction was verified on colon tissues taken from IBD patients, and was shown to suppress cyclooxygenase-2 (COX2) and metalloproteinase-9 (MMP9) gene expression in both cell culture and colon tissue.
Conclusions: It is suggested that the anti-inflammatory activity of Cannabis extracts on colon epithelial cells derives from a fraction of the extract that contains THCA, and is mediated, at least partially, via GPR55 receptor. The cytotoxic activity of the C. sativa extract was increased by combining all fractions at a certain combination of concentrations and was partially affected by CB2 receptor antagonist that increased cell proliferation. It is suggested that in a nonpsychoactive treatment for IBD, THCA should be used rather than CBD.
Keywords: : cannabinoid receptors, Cannabis, COX2, inflammatory bowel disease, MMP9, THCA
Introduction
Inflammatory bowel diseases (IBDs), Crohn's disease (CD), and ulcerative colitis (UC) are characterized by chronic intestinal inflammation. Both diseases are chronic, relapsing, and associated with genetic predisposing backgrounds. Their onset and reactivation are triggered by environmental factors that transiently break the mucosal barrier. This may alter the balance between beneficial and pathogenic enteric bacteria and consequently stimulate immune responses. Both CD and UC patients have activated innate (macrophage, neutrophil) and acquired (T and B cell) immune responses (e.g., Sartor1).
Epithelial cells in the gastrointestinal (GI) tract act as barriers against the intrusion of potentially deleterious luminal substances and microorganisms from the intestinal lumen, and play an important role in inflammatory responses. They express a variety of proinflammatory cytokines, which are upregulated in IBD patients.2 Therapies aimed at downregulating intestinal inflammation utilize both mediator-specific and nonspecific immune suppression, but with potentially considerable side effects.3
Different preparations of marijuana (Cannabis sativa) have been used for the treatment of GI problems, such as GI pain, gastroenteritis, and diarrhea.4,5 C. sativa contains more than 60 terpenophenolic compounds termed phytocannabinoids (reviewed by Aizpurua-Olaizola et al.6). Of these, Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD), which were discovered about 50 years ago, have been defined as the most active.7–9 Also, it was suggested that combination that exists in the whole extract is more active than a single compound solely (e.g., Russo and Taming10). Cannabinoids have been previously shown to be immune modulators. They shift the balance of pro- and anti-inflammatory cytokines and act to suppress cell-mediated immunity in different physiological systems.11 For example, Δ9-tetrahydrocannabivarin (THCV) was demonstrated to inhibit nitrite production in macrophages and thereby to play an immunomodulatory role.12
Phytocannabinoids have been shown to exert their anti-inflammatory functions on the GI tract by activating receptors that are part of the endocannabinoid system, mainly the G-protein-coupled cannabinoid receptor type 2 (CB2).4,13 In the human colonic epithelial cell line HT29, a number of cannabinoid receptor agonists and antagonists, including the plant-derived THC, have been shown to inhibit tumor necrosis factor alpha (TNFα)-induced interleukin-8 (IL-8) release. This inhibition was antagonized by a CB2 receptor antagonist.13 Later, a third cannabinoid receptor, GPR55, was identified and found to affect GI inflammation.14,15 Cannabinoids have been shown to be effective in a mouse model of colitis.16 Cannabinoids have also been shown to promote wound healing in the GI tract via activation of cannabinoid type 1 (CB1) receptor.17 In addition, we have recently reported clinical data from IBD patients. In a retrospective study, we interviewed 30 CD patients who were licensed to use medical Cannabis,18 while in a prospective trial we randomized 20 CD patients to receive either Cannabis or placebo for their IBD.19 Both revealed beneficial effects.
Cannabis sativa extracts contain hundreds of different compounds. The activity of many synthetic or isolated cannabinoids and their receptor agonists or antagonists has been investigated and verified. However, there seems to be an advantage of the unrefined content of the flower extract versus an isolated compound in IBD. For example, standardized C. sativa extract with high content of CBD given after the inflammatory insult was shown in an animal model of GI inflammation to attenuate injury and motility, further supporting the rationale of combining CBD with other Cannabis compounds.20 Also, in three models of seizure, Cannabis-derived botanical drug substances exerted significant anticonvulsant effects and were of similar efficacy with purified cannabidivarin.21
Since we could not find any detailed characterization of the anti-inflammatory activity of the whole C. sativa extract on both colonic epithelial cells and tissue derived from IBD patient colon, we decided to study the anti-inflammatory activity of C. sativa extracts on these models.
IL-8 was previously shown to be an indicator for the level of IBD-related inflammation in both cell models and in IBD patients (e.g., Refs.13,22–25). Therefore, it was chosen in the present study as the main marker for IBD-related inflammation in cell and colon tissues.
Here we show that the anti-inflammatory activity of Cannabis extracts on colon cells derived from Δ9-tetrahydrocannabinolic acid (THCA) present in fraction 7 (F7), while a combination of all fractions of Cannabis extract exerts an increased cytotoxic activity. Activity of the extract and the most active fraction was verified on colon tissue taken from IBD patients, and these were shown to suppress cyclooxygenase-2 (COX2) and metalloproteinase-9 (MMP9) gene expression in both cell culture and colon tissue.
Materials and Methods
Extraction of Cannabis
Fresh flowers of C. sativa strain AD were harvested from plants. They were either taken immediately for extraction and frozen at −80°C, or baked for 3 h at 150°C before extraction. Fresh and baked Cannabis flowers (2 g) were pulverized with liquid nitrogen and ground into fine powder. Absolute ethanol was added to each tube containing the powder at a sample-to-absolute ethanol ratio of 1:4 (w/v). The tubes were mixed thoroughly on a shaker for 30 min and then the extract was filtered through a filter paper. The filtrate was transferred to new tubes. The solvent was evaporated with a vacuum evaporator. The dried extract was resuspended in 1 mL of absolute methanol and filtered through a 0.45-μm syringe filter. The resuspended extract was diluted as to concentrations indicated in the Results section for each of the experiments and used for the treatment of cell cultures and biopsies in enzyme-linked immunosorbent assay (ELISA) experiments. The crude weight per milliliter for each extract was determined by drying 1 mL of the resuspended and filtered extract.
Chemical characterization
Standard preparation
The cannabinoid standards cannabigerol (CBG), CBD, cannabidiolic acid (CBDA), cannabinol (CBN), cannabigerolic acid (CBGA), THC, cannabichromene (CBC), and THCA were diluted to 10 ppm concentration with methanol and then subjected to high-performance liquid chromatography (HPLC) separation. For quantification of THC and THCA, the standards were dissolved in methanol at different concentrations from 5 to 40 ppm.
Sample preparation
For HPLC, the dry extract was resuspended in 1 mL methanol and filtered through a 0.45-μm syringe filter (Merck, Darmstadt, Germany). The filtered extract was diluted 10 times with methanol and then separated by HPLC.
HPLC separation
Sample separation was carried out in an UltiMate 3000 HPLC system coupled with WPS-3000(T) autosampler, HPG-3400 pump, and DAD-300 detector. The separation was performed on a Purospher RP-18 end capped column (250 mm×4.6 mm I.D.; Merck KGaA, Darmstadt, Germany) with a guard column (4 mm×4 mm I.D.). Solvent gradients were formed by isocratic proportion with 15% solvent A (0.1% acetic acid in water) and 85% solvent B (methanol) at a flow rate of 1.5 mL/min for 35 min. The compound peaks were detected at 220, 240, and 280 nm. The 220-nm peaks were taken for further processing. The extracts were fractionated into nine fractions according to the obtained chromatogram.
Mass spectrometry analysis
Analysis of the fractions was carried out using electrospray ionization (ESI) (quadrupole time-of-flight) 6545 (high resolution; Agilent). The mass spectrometry (MS) conditions were as follows: ESI-positive mode, m/z 50–1500, gas temperature 350°C, injection volume 5 μL, solvent composition 0.1% formic acid in water (46%), acetonitrile (50%), and water (4%; v/v).
Nuclear magnetic resonance analysis
1H and 13C spectra were recorded in a Bruker Avance-400 instrument (400.1 and 100.6 MHz, respectively) in CDCl3 as the solvent, containing tetramethylsilane as an internal reference, at 300 K. In addition, three 2D experiments were performed: COSY (1H–1H correlation), HMQC (one-bond 1H–13C correlation), and HMBC (long-range 1H–13C correlation).
Determination of anti-inflammation and cytotoxic activities in HCT116, HT29 and CaCO2 cells
HCT116 (ATCC CCL-247), HT29 (ATCC HTB-38), and CaCO2 (ATCC HTB-37) colon cells were grown at 37°C in a humidified 5% CO2–95% air atmosphere. Cells were maintained in McCoy's 5a modified medium (HCT116 and HT29) and Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (CaCO2). Cells were seeded, in triplicate, into a 24-well plate at a concentration of 50,000 cells per well in 500 μL of growing media and then incubated for 24 h at 37°C in a humidified 5% CO2–95% air atmosphere. When cell excitation was performed with TNFα (300 ng/mL recombinant human TNFα (PeproTech, Rocky Hill, NJ), cultures in each well were treated along with 50 μL plant extract, as described above. The supernatant was taken and the levels of IL-813 were measured 4 h after treatment using the commercial Human CXCL8/IL-8 DuoSet ELISA kit (R&D Systems, Minneapolis, MN). As a positive control, dexamethasone (Sigma-Aldrich, St. Louis, MO) at 200 and 400 μM final concentrations was used. The maximum involvement of the receptors (CB1, CB2, and GPR55) was examined by treating the cells with 20 μM of the CB1 receptor antagonist/inverse agonist rimonabant (Abcam, Cambridge, MA), CB2 receptor antagonist/inverse agonist SR144528 (Abcam), and GPR55 antagonist/inverse agonist CID16020046 (Sigma-Aldrich, Buchs, Switzerland). The whole extract from fresh flowers (C2F) or the active fraction (F7) was applied to cells along with TNFα 1 h after the antagonist treatment. Anti-inflammatory and cytotoxic activity of CBD (Restek, PA) was performed at different concentrations (16–252 μg/mL) on all three cell lines. Resazurin (R&D Systems) was used to check the cytotoxic effect of extracts. For this, 10% resazurin was added to each well of the treatments with different dilutions. Then, the plate was incubated for 2 h at 37°C in a humidified 5% CO2–95% air atmosphere. Supernatant (100 μL from each well) was transferred to a 96-well plate and the relative fluorescence at the excitation/emission of 544/590 nm was measured. Values were calculated as percentage of live cells relative to the nontreated (cells without TNFα and treatments) control after reducing autofluorescence of Alamar Blue without cells. For dose–response assays, data points were connected by nonlinear regression lines of the sigmoidal dose–response relationship. GraphPad Prism (version 6 for windows; GraphPad Software, Inc. San Diego) was used to produce dose–response curves and IC50 doses were calculated using nonlinear regression analysis.
Culture of biopsies
Three biopsies from both healthy and inflamed intestine of IBD patients were obtained from 29 patients with either CD or UC scheduled for colonoscopy as deemed necessary by their physician, Helsinki approval no. 0094-16. After obtaining informed consent, biopsies from inflamed and normal tissue were taken and placed in tissue culture media. On receiving the biopsies, phosphate-buffered saline was replaced with 75 μL dispase (StemCell Technologies, Cambridge, United Kingdom) and 150 μL collagenase 1A (StemCell Technologies) solution. Tubes were then incubated at 37°C for 1 h. After incubation, the tubes containing the biopsies were centrifuged at 8000 rpm (11,885 g) for 1 min. Then, the supernatant was removed and tissues were washed three times with Hank's balanced salt solution. After each wash, tubes were centrifuged as described above. Then, the tissues were placed on a small Petri dish and cut into 2–3 pieces with a clean scalpel. The pieces were then placed on Millicell hydrophilic polytetrafluoroethylene tissue culture inserts (Millipore, 30 mm, 0.4 μm). The inserts were placed in six-well plastic tissue culture dishes (Costar 3506) along with 1.5 mL of tissue culture medium (DMEM supplemented with 10% v/v heat-inactivated fetal calf serum, 100 U/mL penicillin, 100 μg/mL streptomycin, 50 μg/mL leupeptin, 1 mM phenylmethanesulfonylfluoride, and 50 μg/mL soybean trypsin inhibitor). When dexamethasone was included in the media, its concentration was 200 μg/mL. This was followed by treating the tissues with extracts as mentioned above, or leaving them untreated (control). The supernatants were taken after overnight incubation and used for determination of IL-8 and IL-6 cytokine profile by measuring the levels with a commercial ELISA kit. Levels of cytokines from inflamed, Cannabis-treated, and nontreated tissue were compared.
Quantitative real-time PCR
Cells were seeded into a six-well plate at a concentration of 1,500,000 cell/mL per well. After 24 h of incubation at 37°C in a humidified 5% CO2–95% air atmosphere, cells were treated with TNFα (final concentration of 1 ng/mL) and incubated overnight under the same conditions. Nontreated cells or cells treated only with TNFα served as negative and positive controls, respectively. Cells were then reincubated for 5 h with C2F (200 μg crude dry extract/mL) or F7 (from 200 μg crude dry extract/mL of C2F) at 37°C in a humidified 5% CO2–95% air atmosphere. The next day, cells were harvested and total RNA was extracted using TRI reagent (Sigma-Aldrich) according to the manufacturer's protocol. For biopsies, all treatments were added overnight, after which the biopsies were stored at −20°C in RNA Save solution (Biological Industries, Beit Haemek, Israel). RNA was extracted from frozen biopsies. Tissue samples were homogenized with an appropriate homogenizer in TRI reagent, as done for the cells. RNA (50 ng for biopsies: four UC patients and one CD patient) or 2.5 μg (for cells) was reverse-transcribed in a total volume of 20 μL using Maxima reverse transcriptase (Thermo Scientific, Boston, MA) according to the manufacturer's protocol. All primers were designed using Primer3Plus software. PCR was performed in triplicate using a Rotor-Gene 6000 instrument (Qiagen, Zurich, Switzerland) and Maxima SyGreen Mix (Thermo Scientific) according to the manufacturer's protocol. The expression of each target gene was normalized to the expression of GAPDH mRNA in the 2ΔΔCt and is presented as the ratio of the target gene to GAPDH mRNA, expressed as 2ΔCt, where Ct is the threshold cycle and ΔCt=(Ct Target gene − Ct GAPDH). Experiments were repeated three times. The primers were as follows: for COX2 (forward) 5′-ATTGACCAGAGCAGGCAGAT-3′ and (reverse) 5′-CAGGATACAGCTCCACAGCA-3′, and for MMP9 (forward) 5′-TTGACAGCGACAAGAAGTGG-3′ and (reverse) 5′-TCACGTCGTCCTTATGCAAG-3′.
Statistical analyses
Results are presented as mean±standard error of replicate analyses and are either representative of or include at least two independent experiments. Means of replicates were subjected to statistical analyses by the Tukey–Kramer test (p≤0.05) using the JMP statistical package and considered significant when p≤0.05.
Results
C. sativa extracts from fresh flowers are highly active in reducing inflammation in colon cell lines
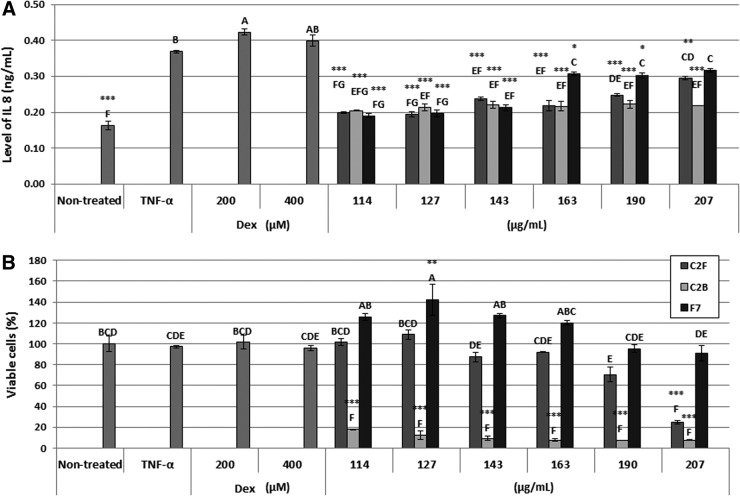
Anti-inflammation activity was determined for absolute ethanol extracts of fresh (C2F) and baked (C2B) flowers of C. sativa (Cs-AD var.). The activity was determined as the level of IL-8 in HCT116 colon cancer cell cultures pretreated with TNFα to induce IL-8 expression and then treated with C2F or C2B (Fig. 1A). Notably, IL-8 was used in several other studies, in HCT116 as well as other cell models and in IBD patients as an indicator for the level of IBD-related inflammation (e.g., Refs.13,22–25). At different concentrations of C2F and C2B (114–207 μg/mL), extracts significantly reduced IL8 levels when compared to TNFα. Under these conditions, dexamethasone (at concentrations of 200 and 400 μM) was inactive in reducing IL-8 level (Fig. 1A).
FIG. 1.
(A) Anti-inflammatory activity at different concentrations of Cannabis sativa ethanolic fresh flower extracts (C2F; 114–207 μg/mL), baked flower extracts (C2B; 114–207 μg/mL), F7 from fresh flower extracts (an HPLC fraction of C2F at concentrations of 114–207 μg/mL), and Dex at 200 and 400 μM on HCT 116 cells measured as level of IL-8 (ng/mL). HCT116 cells were seeded (50,000 per well) in triplicate in 500 μL growing media and incubated for 24 h at 37°C in a humidified 5% CO2–95% air atmosphere. Cells were treated with 300 ng/mL TNFα and 50 μL of C. sativa ethanol extract of C2F or fractions for 4 h. Nontreated are the cells without TNF-α and treatments. Levels of IL-8 were measured from the supernatant using a commercial kit. Values (ng/mL) were calculated relative to a TNFα-treated control. (B) Determination of HCT116 cell viability using Alamar Blue fluorescence (resazurin assay) as a function of live cell number. Cells were seeded and treated as described in (A). Next, the cells were incubated with Alamar Blue for 2 h. Relative fluorescence at the excitation/emission of 544/590 nm was measured. Values were calculated as percentage of live cells relative to the nontreated (cells without TNFα and treatments) control after reducing the autofluorescence of Alamar Blue without cells. Error bars indicate±SE (n=3). *ΔΔCt **ΔΔCt *** Indicate data statistically significantly different in comparison with the control (TNFα-treated cells) at p≤0.01, p≤0.001, p≤0.0001, respectively. Levels with different letters are significantly different from all combinations of pairs by Tukey's HSD. HPLC, high-performance liquid chromatography; HSD, honest significant difference; Dex, dexamethasone; IL, interleukin; TNFα, tumor necrosis factor alpha; SE, standard error; F7, fraction 7.
To determine that the reduction in IL-8 is due to anti-inflammatory rather than cytotoxic effect, cell viability was examined for the C2F and C2B treatments at different concentrations. At most examined concentrations C2B had significant cytotoxic activity, whereas C2F did not (Fig. 1B). These results suggest that although the reduction in IL-8 level following treatment with C2B may be derived from cell death, that reduction following treatment with C2F is solely based on anti-inflammatory activity. Similar results on the activity of C2F and C2B were obtained in HT29 and CaCO2 cells (Supplementary Figs. S1 and S2); slightly increased cytotoxicity at the higher concentration was determined for C2F in the CaCO2 cell line (Supplementary Fig. S2). Taken together, results demonstrated the strong, dose-dependent, anti-inflammation activity of C. sativa C2F, mostly absent in C2B.
Chemical composition of C. sativa extracts from fresh and baked flowers
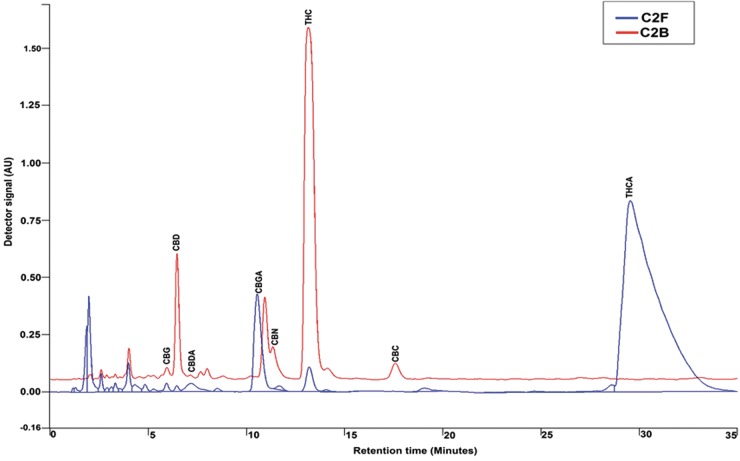
HPLC chromatogram and main active compounds were determined for the anti-inflammation active extract, C2F, and for the inactive extract, C2B (Fig. 2; Table 1). Eight major cannabinoids were identified in the fresh and baked crude extracts at 220 nm. These peaks were identified as CBG, CBD, CBDA, CBN, CBGA, THC, CBC, and THCA, with retention times of 5.9, 6.4, 7.9, 10.9, 11.3, 13.1, 17.5, and 29.3 min, respectively, relative to the HPLC profile of cannabinoid standards (not shown). The levels of CBD, CBN, and THC were 36, 14, and 32 times higher in the C2B versus C2F extract. CBC was not identified in C2F but appeared in C2B. The levels of THCA and CBDA in C2B were reduced 1200 and 1.5 times, respectively, compared to those in C2F (Fig. 2; Table 1), due to decarboxylation of CBDA and THCA during heating (e.g., Smith and Vaughan26).
FIG. 2.
HPLC chromatograms of C. sativa ethanolic extracts. Chromatogram of fresh Cannabis extract (C2F; 0.1 mg/mL) and baked (i.e., fresh flowers that were baked at 150°C for 3 h) Cannabis extract (C2B; 0.1 mg/mL) obtained from isocratic elution with a mixture of 15% water containing 0.1% acetic acid (solvent A) and 85% MeOH (solvent B) for a total run time of 35 min at 220 nm. The samples were injected at a concentration of 0.58 mg/mL in a volume of 20 μL for C2B and 0.33 mg/mL in a volume of 20 μL for C2F.
Table 1.
High-Performance Liquid Chromatography Peak Area and Percentage of Area for Ethanol Extract of Cannabis sativa Fresh (C2F) and Baked (C2B) Flowers
| C2F | C2B | ||||
|---|---|---|---|---|---|
| RT (Min) | Area of peak (mAU) | Area (%) | Area of peak (mAU) | Area (%) | Identified compounds |
| 2.604 | 7,279,491 | 0.61 | 4,834,101 | 0.63 | |
| 4.015 | 15,943,959 | 1.33 | 19,329,498 | 2.52 | |
| 5.947 | 5,640,210 | 0.47 | 9,625,348 | 1.26 | CBG |
| 6.466 | 3,614,080 | 0.30 | 84,359,176 | 11.00 | CBD |
| 7.996 | 14,896,186 | 1.24 | 6,041,859 | 0.79 | CBDA |
| 10.931 | 115,095,840 | 9.61 | 68,550,632 | 8.94 | CBN |
| 11.339 | 3,592,090 | 0.30 | 32,233,628 | 4.20 | CBGA |
| 13.146 | 25,512,280 | 2.13 | 518,303,552 | 67.59 | THC |
| 17.575 | 0 | 0.00 | 22,981,120 | 3.00 | CBC |
| 29.373 | 1,006,329,152 | 84.01 | 544,761 | 0.07 | THCA |
| 1,197,903,288 | 100.00 | 766,803,675 | 100.00 | ||
RT, retention time; CBG, cannabigerol; CBD, cannabidiol; CBDA, cannabidiolic acid; CBN, cannabinol, CBGA, cannabigerolic acid; THC, Δ9-tetrahydrocannabinol; CBC, cannabichromene; THCA, Δ9-tetrahydrocannabinolic acid.
Identification of an active fraction of the fresh flower extract of C. sativa and determination of activity of combinations of fractions
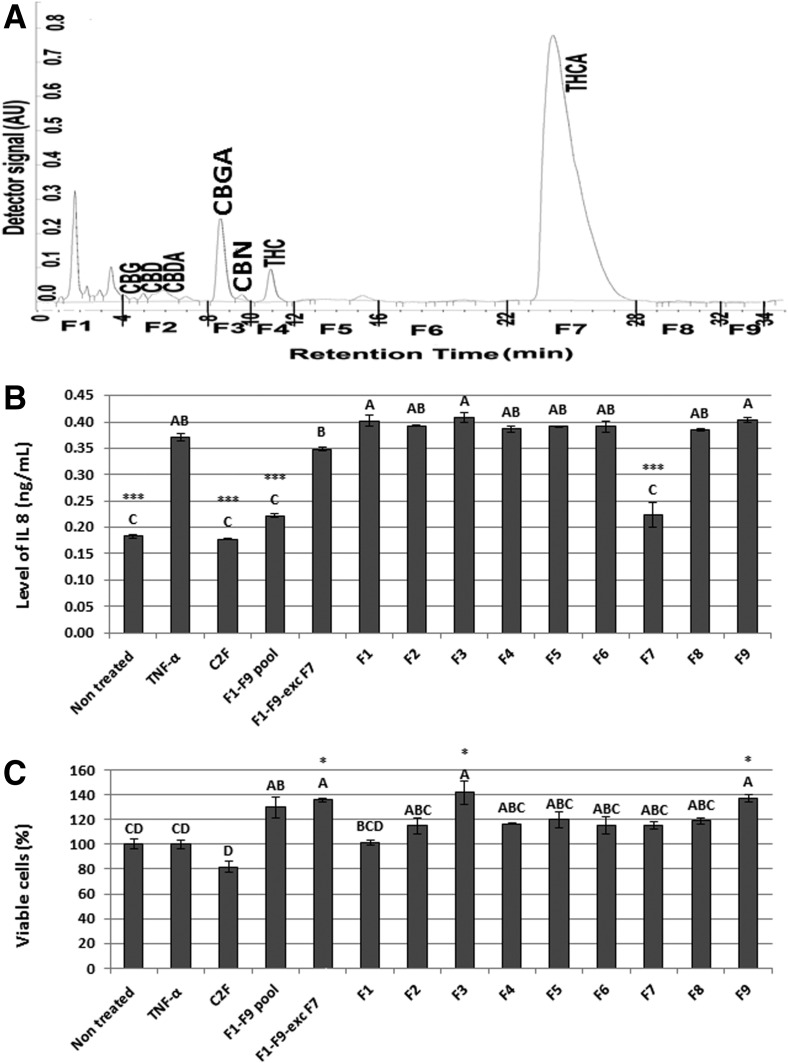
C2F (at a concentration of 163 μg/mL) was fractionated (Fig. 3A) by HPLC. Fractions were collected and were examined for anti-inflammatory activity, determined as the level of IL-8 in HCT116 cells. One fraction, F7, significantly reduced the level of IL-8 to that of the whole extract treatment (C2F and F1–F9 pooled; Fig. 3B). No significant reduction in IL-8 levels was observed for any of the other fractions (Fig. 3A, B). None of the fractions reduced cell viability, whereas treatment with some showed even increased cell proliferation (e.g., F3, F9; Fig. 3C). Similar results of anti-inflammatory activity were obtained for F7 in HT29 and CaCO2 cells (Supplementary Figs. S1 and S2).
FIG. 3.
(A) HPLC profile of fractions of C. sativa ethanolic extract (C2F). HPLC profile was obtained from isocratic elution with a mixture of 15% water containing 0.1% acetic acid (solvent A) and 85% MeOH (solvent B) for a total run time of 40 min at 220 nm. The sample was injected at a concentration of 0.1 mg crude dried extract/mL in a volume of 50 μL per cycle. Fractions were collected every 2 min. F1–F9 represent the nine fractions collected by HPLC. (B) Anti-inflammatory activity of C. sativa ethanolic extracts (C2F; 163 μg/mL), fractions F1–F9 pooled together (HPLC fractions of C2F at concentrations of 163 μg/mL), F1–F9-excluding F7 (HPLC fractions of C2F at concentrations of 163 μg/mL), F1–F9 (each an HPLC fraction of C2F at concentrations of 163 μg/mL) on HCT 116 cells measured as level of IL-8 (ng/mL). HCT116 cells were seeded (50,000 per well) in triplicate in 500 μL growing media and incubated for 24 h at 37°C in a humidified 5% CO2–95% air atmosphere. Cells were treated with 300 ng/mL TNFα and 50 μL of C. sativa ethanol extract of C2F or fractions for 4 h. Nontreated are the cells without TNFα and treatments. Levels of IL-8 were measured from the supernatant using a commercial kit. Values (ng/mL) were calculated relative to a TNFα-treated control. (C) Determination of HCT116 cell viability using Alamar Blue fluorescence (resazurin assay) as a function of live cell number. Cells were seeded and treated as described in (B). Next, the cells were incubated with Alamar Blue for 2 h. Relative fluorescence at the excitation/emission of 544/590 nm was measured. Values were calculated as percentage of live cells relative to the nontreated (cells without TNFα and treatments) control after reducing the autofluorescence of Alamar Blue without cells. Error bars indicate±SE (n=3). *ΔΔCt *** Indicate data statistically significantly different in comparison with the control (TNF-α treated cells) at p≤0.01 and p≤0.0001, respectively. Levels with different letters are significantly different from all combinations of pairs by Tukey's HSD.
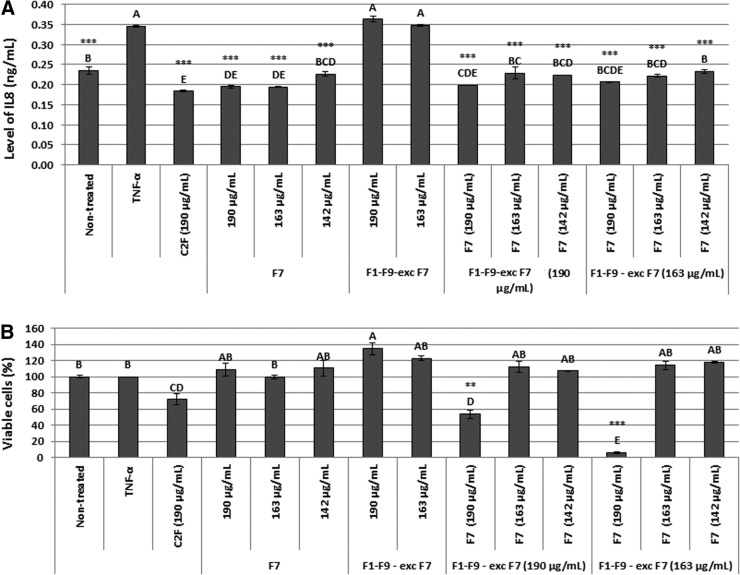
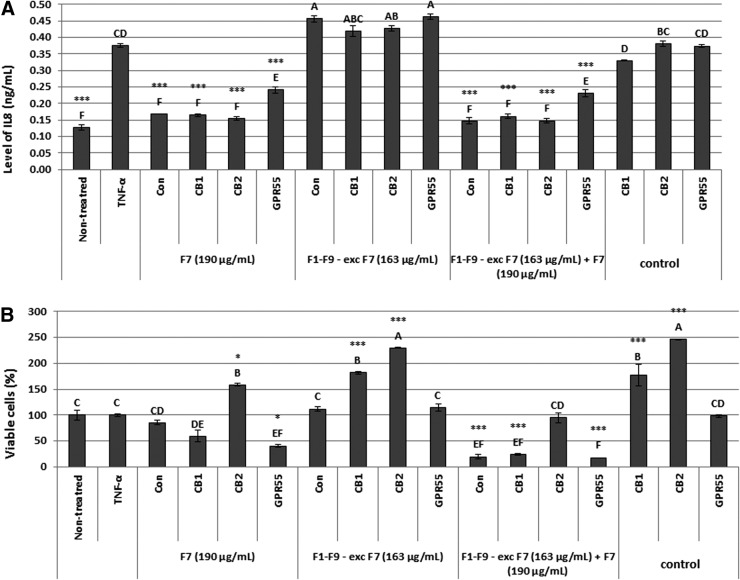
Next, F1–F9 pool-excluding F7 and combined treatment of all fractions, including F7, at different concentrations, were examined on HCT116 cells for anti-inflammatory and cytotoxic activities (Fig. 4). As expected, F7 and C2F had similar anti-inflammatory activity, whereas F1–F9-excluding F7 treatment were inactive. However, once F7 was added to F1–F9-excluding F7 treatment, anti-inflammatory activity was retained (Fig. 4A). As for the cytotoxic activity, a marked induction of cytotoxicity was found for combined treatment of F1–F9-excluding F7 and addition of F7 at concentrations of 190 and 190 μg/mL, respectively, and even more profoundly, at concentrations of 163 and 190 μg/mL, for F1–F9-excluding F7 and F7, respectively (Fig. 4B).
FIG. 4.
(A) Anti-inflammatory activity of C. sativa ethanolic extracts (C2F; 163 μg/mL), F7 at three different concentrations (an HPLC fraction of C2F at concentrations of 142, 163, and 190 μg/mL), fractions F1–F9-excluding F7 (F1–F9-exc F7) at two concentrations (HPLC fractions of C2F at concentrations of 163 and 190 μg/mL), combination of each concentration of F1–F9-excluding F7 along with each concentration of F7 on HCT 116 cells measured as level of IL-8 (ng/mL). HCT116 cells were seeded (50,000 per well) in triplicate in 500 μL growing media and incubated for 24 h at 37°C in a humidified 5% CO2–95% air atmosphere. Cells were treated with 300 ng/mL TNFα and 50 μL of C. sativa ethanol extract of C2F or fractions for 4 h. Nontreated are the cells without TNFα and treatments. Levels of IL-8 were measured from the supernatant using a commercial kit. Values (ng/mL) were calculated relative to a TNFα-treated control. (B) Determination of HCT116 cell viability using Alamar Blue fluorescence (resazurin assay) as a function of live cell number. Cells were seeded and treated as described in (A). Next, the cells were incubated with Alamar Blue for 2 h. Relative fluorescence at the excitation/emission of 544/590 nm was measured. Values were calculated as percentage of live cells relative to the nontreated (cells without TNFα and treatments) control after reducing the autofluorescence of Alamar Blue without cells. Error bars indicate±SE (n=3). **ΔΔCt *** Indicate data statistically significantly different in comparison with the control (TNFα-treated cells) at p≤0.001 and p≤0.0001, respectively. Levels with different letters are significantly different from all combinations of pairs by Tukey's HSD.
These results suggest that F7 denotes anti-inflammatory activity in colon cell lines, whereas certain combinations of treatment with all fractions of the extract lead to a significant increase in the cytotoxic activity.
The active fraction of C. sativa extract contains mainly THCA
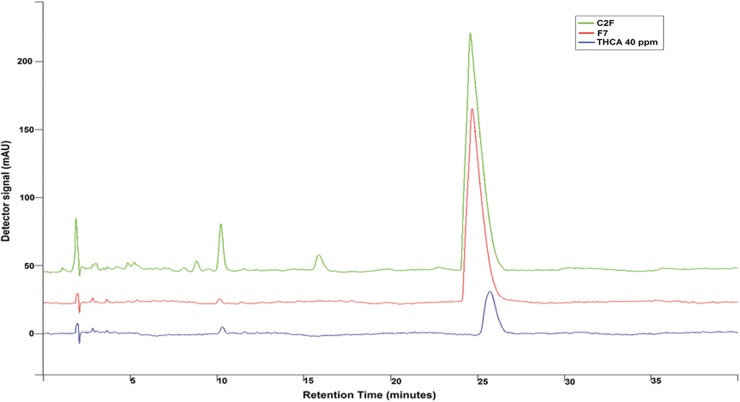
The chemical composition of the active fraction (F7) was analyzed by HPLC and ESI–MS. F7 was obtained as a broad peak in the HPLC chromatogram. To analyze its structure and verify its purity, it was analyzed at different dilutions, in comparison to a THCA standard. The results suggested that F7 is THCA (Fig. 5). ESI–MS results further confirmed that F7 contains THCA: C22H30O4 (358.214); m/z (MH+) 359.222, (MNa+) 381.203. 1H and 13C spectra were taken to verify the exact structure and determine the purity of F7. The nuclear magnetic resonance results showed that F7 is indeed THCA, at over 90% purity.
FIG. 5.
HPLC profile of C2F, F7, and THCA. Chromatograms of THCA standard at 40 ppm (marked in blue), whole C. sativa extract at 0.1 mg/mL (marked in green), and F7 at 0.04 mg/mL (marked in red). All samples were injected in a volume of 20 μL and were obtained from isocratic elution with a mixture of 15% water containing 0.1% acetic acid (solvent A) and 85% MeOH (solvent B) for a total run time of 40 min at 220 nm. THCA, Δ9-tetrahydrocannabinolic acid.
GPR55 receptor antagonist significantly reduces the anti-inflammatory activity of F7, whereas CB2 receptor antagonist significantly increases HCT116 cell proliferation
To determine whether activity of fractions of C2F in HCT116 cells is conferred via the CBs or GPR55 receptors, we determined the effect of CB1, CB2, and GPR55 receptor antagonists (rimonabant, SR144528 and CID16020046, respectively) on their anti-inflammatory or cytotoxic activities. CB1 and CB2 receptor antagonists did not change the anti-inflammatory activity of F7 or F1–F9 (Fig. 6A). However, addition of GPR55 antagonist led to a significant reduction in activity and to an increase in IL-8 levels in these treatments (Fig. 6A). Addition of GPR55 antagonist did not change IL-8 level in control (Fig. 6A). As for the cytotoxic activity, a significant reduction in F1–F9-excluding F7 with addition of F7 (163 and 190 μg/mL, respectively) activity was found for CB2 antagonist. However, CB2 antagonist increases cell number even in the control (Fig. 6B), suggesting that it counteracts the fractions' activity by inducing HCT116 cell proliferation (Fig. 6B).
FIG. 6.
(A) Anti-inflammatory activity of C. sativa F7 at three different concentrations (an HPLC fraction of C2F at concentrations of 190 μg/mL), fractions F1–F9-excluding F7 (F1–F9-exc F7; HPLC fractions of C2F at concentrations of 163 μg/mL), combination of F1–F9-excluding F7 along with F7 measured as level of IL-8 on HCT116 cells, with and without antagonists to CB1, CB2, and GPR55 receptors (antagonists at a concentration of 20 μM, CB1, CB1 receptor antagonist rimonabant; CB2, CB2 receptor antagonist SR144528; GPR55, GPR55 antagonist CID16020046). HCT116 cells were seeded (50,000 per well) in triplicate in 500 μL growing media and incubated for 24 h at 37°C in a humidified 5% CO2–95% air atmosphere. Cells were treated with 300 ng/mL TNFα and 50 μL of C. sativa ethanol extract of C2F fractions for 4 h. Treatments with F7, F1–F9, and combination of fractions without antagonists served as a positive control (Con). Nontreated are the cells without TNFα and treatments. Levels of IL-8 were measured from the supernatant using a commercial kit. Values (ng/mL) were calculated relative to control. (B) Determination of HCT116 cell viability using Alamar Blue fluorescence (resazurin assay) as a function of cytotoxic effect. HCT116 cells were seeded (50,000 per well) in triplicate in 500 μL growing media and incubated for 24 h at 37°C in a humidified 5% CO2–95% air atmosphere. Cells were seeded and treated as described in (A). Next, the cells were incubated with Alamar Blue for 2 h. Relative fluorescence at the excitation/emission of 544/590 nm was measured. Values were calculated as percentage of live cells relative to the nontreated (cells without TNFα and treatments) control after reducing the autofluorescence of Alamar Blue without cells. Error bars indicate±SE (n=3). *ΔΔCt *** Indicate data statistically significantly different in comparison with the control (TNFα-treated cells) at p≤0.01 and p≤0.0001, respectively. Levels with different letters are significantly different from all combinations of pairs by Tukey's HSD. CB1, cannabinoid receptor type 1; CB2, cannabinoid receptor type 2.
Transcripts for CB1, CB2, and GPR55 were detected by qPCR in HCT116 cells. Expression of CB2 and GPR55 was significantly increased on treatment with TNFα in these cells (values are the steady-state level of gene expression in TNFα-treated vs. nontreated cells; Table 2).
Table 2.
Relative Gene Expression in HCT116 Cells
| Gene | Mean relative expression | Std Err | Statistics |
|---|---|---|---|
| CB1 | 1.31 | 0.19 | AB |
| CB2 | 5.84 | 1.04 | A |
| GPR55 | 5.26 | 1.57 | B |
CB1, CB2, and GPR55 gene expression was measured after overnight treatment of the cells with TNFα. Values of gene transcripts were determined as a ratio between target genes (CB1, CB2, and GPR55) versus a reference gene (GAPDH), in TNFα-treated versus untreated cells using the 2ΔΔCt method.
CB1, cannabinoid receptor type 1; CB2, cannabinoid receptor type 2; TNFα, tumor necrosis factor alpha.
CBD reduces inflammation only at lower concentration yet its cytotoxic activity is dose dependent
The fraction of C2F containing CBG, CBD, and CBDA (F2) did not show any anti-inflammatory activity in HCT116 cells in terms of IL-8 reduction (Fig. 3A). This is in contrast to several publications that have suggested that CBD is the main anti-inflammatory compound for IBD (reviewed by Esposito et al.27). To further examine CBD activity, we determined the anti-inflammatory and cytotoxic activity of pure CBD (purity was verified by HPLC, not shown) in HCT116, HT29, and CaCO2 cells. Treatment with purified CBD at different concentrations (16–252 μg/mL) leads to a significant reduction in IL-8 levels at lower concentrations of CBD (Supplementary Fig. S3A). Yet, no anti-inflammatory activity for CBD was determined for the higher CBD concentrations in HCT116 cells (Supplementary Fig. S3A). CBD was active in reduction of IL-8 levels in CaCO2 and HT29 cells (Supplementary Fig. S3A). However, treatments with CBD lead to a dose-dependent cell death in HCT116 and in CaCO2 cells, and to a lesser extent in HT29 cells (Supplementary Fig. S3B).
Treatment with C. sativa extracts C2F and F7 leads to reduction in IL-8 levels in patient colon tissue
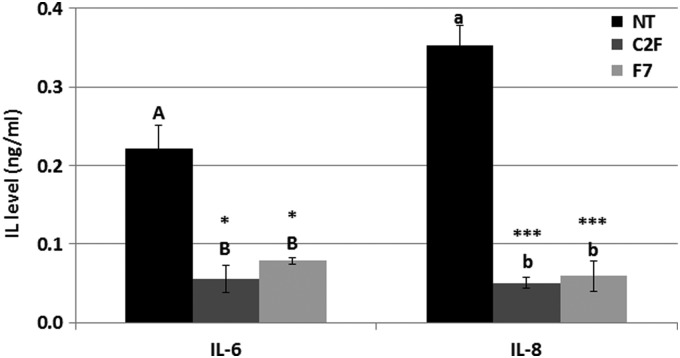
Since cell lines do not fully reflect the conditions in colon tissue, we further verified C2F and F7 inflammation-reducing activity in biopsies of colon tissue taken from IBD patients. Biopsies were maintained ex vivo and the levels of IL-8 and IL-6 were determined in nontreated versus C2F- and F7-treated tissue. Treatment with C2F reduced significantly both IL-8 and IL-6 levels compared to nontreated controls (n=29). These results confirmed the anti-inflammatory effect of C2F and F7 on colon tissues derived from IBD patients (Fig. 7).
FIG. 7.
Anti-inflammatory activity of C. sativa fresh flower ethanol extracts (C2F; 0.2 mg/mL) and F7 (0.08 mg/mL) measured as level of IL-8 and IL-6 from biopsies of intestine bowel disease patients. Biopsies from inflamed and normal tissue were taken and processed in tissue culture media and then treated with C2F (n=29), F7 (n=9), and NT controls (n=29) for 16 h. Levels of IL-8 and IL-6 (ng/mL) were measured from the supernatant using a commercial kit. Error bars indicate±SE. *ΔΔCt *** Indicate data statistically significantly different in comparison with the control (NT tissues) at p≤0.05, p≤0.0001, respectively. Levels with different letters are significantly different from all combinations of pairs by Tukey's HSD. NT, nontreated.
Treatment of HCT116 cells and biopsies with C. sativa extracts C2F and F7 leads to reduction in MMP9 and COX2 expression
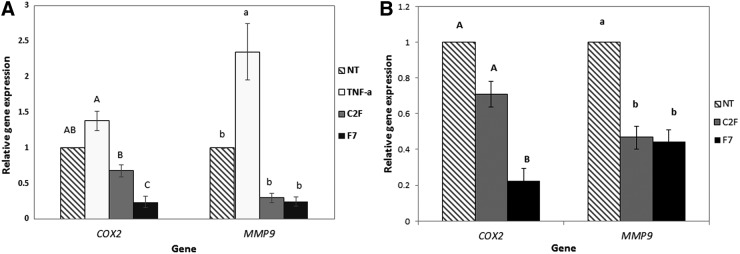
COX2 expression is induced in the large intestine of IBD patients (reviewed by Wang and DuBois28) and MMP9 is among the predominant proteinases expressed in the gut mucosa during active IBD, associated with disease severity.29 The steady-state levels of MMP9 and COX2 expression were examined as markers for inflammation level in HCT116 cells and colon biopsies of IBD patients (four UC and one CD). Expression of both COX2 and MMP9 was significantly induced in HCT116 cells treated with TNFα and significantly reduced by treatment with C2F and F7. F7 was more effective at reducing COX2 expression than C2F (Fig. 8A). In colon tissues, expression of both COX2 and MMP9 was downregulated by C2F and F7 treatments (Fig. 8B). As with the cell lines, F7 was more effective than C2F at reducing COX2 expression (Fig. 8B).
FIG. 8.
COX2 and MMP9 gene expression. (A) HCT116 cell line. Cells were seeded (1,500,000 per well) in triplicate in 500 μL growing media and incubated for 24 h at 37°C in a humidified 5% CO2–95% air atmosphere. Cells were treated with 50 ng/mL TNFα overnight and then treated with C. sativa C2F or F7 added 5 h before RNA extraction. (B) Colon biopsies. C2F and F7 were added overnight to four ulcerative colitis patients and one Crohn's disease patient biopsies at concentrations of 0.2 and 0.07 mg/mL, respectively. RNA was extracted and reverse transcribed, and values of the steady-state level of gene transcripts were determined as the ratio between the target gene (COX2 or MMP9) and a reference gene (GAPDH), and that of treatment versus no treatment (NT) cells, using the 2ΔΔCt method. The experiment was performed in three biological replicates, with three technical repeats for each (n=3). SE was calculated for three biological replicates for each examined treatment. Different letters above bars indicate statistically significant differences between means by one-way analysis of variance with Tukey's HSD (p<0.01). COX2, cyclooxygenase-2; MMP9, metalloproteinase-9.
Discussion
The present study suggests that the anti-inflammatory activity of Cannabis extracts on colon epithelial cells derives from a fraction of the extract that contains THCA. This conclusion is based on several lines of evidence. First, fresh flower ethanolic extracts of C. sativa led to reduction of IL-8 levels in HCT116, HT29, and CaCO2 colon cell lines, determined as reduction in IL-8 secretion. Second, fractionation of the fresh flower extract yielded only one fraction, F7, which retained activity. Third, chemical analysis showed that F7 contains mainly THCA. Also, a combination of F7 with the other F1–F9 fractions led to anti-inflammatory activity in HCT116 cells similar to that of the whole extract. Furthermore, under the examined conditions, F7 did not show cytotoxic activity, further suggesting that under the examined conditions, F7 activity is anti-inflammatory and does not derive simply from cell death.
As previously indicated, THC is considered to be one of the most active compounds in C. sativa. However, other phytocannabinoids, including CBG, CBN, and THCV, are now known to have therapeutic effects.10 As for THCA, other cell-based experiments have demonstrated its immunomodulatory, anti-inflammatory, neuroprotective, and antineoplastic activity (reviewed in Moreno-Sanz30). For example, in lipopolysaccharide-activated U937 macrophages and peripheral blood macrophages, THCA-A inhibits the release of TNFα and COX1 and COX2 expression in a dose-dependent manner and prostaglandin production.31,32
In agreement, C2F and F7 were found to inhibit the expression of COX2. COX1 and COX2 catalyze the production of prostaglandins from arachidonic acid, and prostaglandins are known to be important in mediating the inflammatory process. COX2 is an immediate early response gene induced mainly at sites of inflammation in response to inflammatory stimuli, whereas it is normally absent from most cells. It is induced in the large intestine of IBD patients and in inflamed tissues of an IL-10-deficient mouse model of IBD (reviewed by Wang and DuBois28). Reduction of COX2 has been suggested as a major target for the treatment of IBD (e.g., El Miedany et al.33). In agreement, in in vitro enzyme-based COX1/COX2 inhibition assay and in HT29 cell line, COX1 and COX2 enzyme activity was inhibited by THCA, however, only in the high millimolar concentration range.32
Our results showed that C2F and F7 inhibit the expression of MMP9 as well. MMP9 is among the predominant proteinases expressed in the gut mucosa during active IBD and it is associated with disease severity.29 In addition, MMP9 levels are significantly higher in active IBD and in UC compared to CD.34 Indeed, in UC patients, fecal MMP9 levels are significantly correlated with several measurement parameters, such as Mayo score, endoscopic score, and serum C-reactive protein levels, suggesting that it is a good selective marker for evaluation of disease activity in UC patients.35 The inhibition of MMP9 expression by C2F and F7 in both cell lines and IBD patients (three UC and one CD) provides another indication of their possible efficacy against colon inflammation.
Perhaps even more significant, inhibition of COX2 and reduction of prostaglandin production have been proposed to play an important role in the inhibition of colon cancer development.28 Importantly, COX2 expression is elevated in up to 90% of colorectal carcinomas and is correlated with poor prognosis, whereas it has been shown in mice that for cancer therapy, selective COX2 inhibitors may enhance the activity of antiangiogenic agents against pre-established metastases.36
In this light, our findings that a certain combination of F7 with the other fractions highly induces cell death suggest interaction between THCA and other C. sativa compounds for this activity. Interactions between C. sativa-derived compounds were notified before for different activities.10 Practically, using certain combinations of C. sativa compounds may further potentiate the use of C. sativa as an anticancer drug.
Phytocannabinoids as well as endocannabinoids bind to target receptors and activate various signaling pathways thereby affecting several biological processes. The main receptors for endocannabinoids are CB1 and CB2 (reviewed by Chiurchiù et al.37). CB1 and CB2 are expressed in different tissues and cells. CB1 is mainly expressed in the nervous system and is involved in the regulation of cognitive, memory, motor functions, and analgesia. CB2 is expressed by the cells of the immune system and is associated with the modulation of different immune functions (reviewed by Chiurchiù et al.37). CB2 was also found in additional tissues, including epithelial cells of the colon.4,37 Upregulation of CB2 is associated with chronic inflammation of the nervous system37 and modulation of intestinal inflammation.4
We found that CB2 receptor antagonist led to an increase in the percentage of live cells even in the absence of C. sativa treatment, suggesting that CB2 activity may be negatively involved with cell proliferation. However, CB2 was shown to be expressed with great intensity in epithelial cells of colorectal cancer tumor and correlated with tumor growth and disease progression. Moreover, its expression was suggested to be a marker for poor prognosis.38 Also, Romano et al. found that Cannabis extract with high content of CBD inhibits colorectal cancer cell proliferation and attenuates colon carcinogenesis. This activity involved CB1 and CB2 receptor activation.39
GPR55, activated by phytocannabinoids and endocannabinoids, is widely expressed in several tissues, including the GI and in different human innate and adaptive immune cells.40 Our results suggest that the anti-inflammatory activity of F7 is affected by GPR55 receptor antagonists, suggesting that the anti-inflammatory activity of F7 is mediated, at least partially, via GPR55. In contrast, the findings that GPR55 negatively affects the ability of monocytes to phagocytose, enhances IL-12 and TNFα production, and that pharmacological blockade of GPR55 reduces intestinal inflammation in mice by reducing leukocyte migration and activation, positioning GPR55 with a proinflammatory role.14,40
Our results also show a dose-dependent cytotoxic activity for CBD in all three examined cell lines, in agreement to other studies suggesting CBD to be cytotoxic (e.g., ChoiPark et al.41). However, we found anti-inflammatory activity for CBD only for the low concentrations, and in a bell-shaped rather than dose-dependent manner. At high CBD concentrations, even a proinflammatory effect was detected. Similar results for bell-shaped anti-inflammatory activity were found for CBD in an animal model of inflammation.42 Yet, CBD is still considered to be a strong anti-inflammatory agent.27 Our results suggest that in a nonpsychoactive treatment for IBD, THCA should be used rather than CBD.
An important advantage of using THCA (present in F7) is its lack of narcotic activity. This is due to two pharmacological traits: decreased central nervous system penetration due to the presence of a carboxylic group and a probable inability to convert to THC in vivo.30 Hence, the use of C. sativa extract that contains THCA could have major implications for the medical use of THCA, the relevant fraction in combination with other fractions or, alternatively, raw, unheated Cannabis preparations. Such preparations might promote more precise treatment with medical Cannabis for IBD patients, maximizing Cannabis's therapeutic gain while minimizing the undesirable psychoactive side effects. Our results further underscore the need to allocate the right treatment with the right compounds for each medical indication treated by Cannabis.
Supplementary Material
Abbreviations Used
- CB1
cannabinoid receptor type 1
- CB2
cannabinoid receptor type 2
- CBC
cannabichromene
- CBD
cannabidiol
- CBDA
cannabidiolic acid
- CBG
cannabigerol
- CBGA
cannabigerolic acid
- CBN
cannabinol
- CD
Crohn's disease
- COX2
cyclooxygenase-2
- Dex
dexamethasone
- DMEM
Dulbecco's modified Eagle's medium
- ELISA
enzyme-linked immunosorbent assay
- ESI
electrospray ionization
- F7
fraction 7
- GI
gastrointestinal
- HPLC
high-performance liquid chromatography
- IBD
inflammatory bowel disease
- IL
interleukin
- MMP9
metalloproteinase-9
- MS
mass spectrometry
- NT
nontreated
- RT
retention time
- SE
standard error
- THC
Δ9-tetrahydrocannabinol
- THCA
Δ9-tetrahydrocannabinolic acid
- THCV
Δ9-tetrahydrocannabivarin
- TNFα
tumor necrosis factor alpha
- UC
ulcerative colitis
Acknowledgment
Research was performed under the authorization of IMCA (Israel Medical Cannabis Agency) for research in medical cannabis.
Author Disclosure Statement
O.S. was paid as an employee of PLANTEXT, Israel. For other authors, no competing financial interests exist.
References
- 1.Sartor RB. Mechanisms of disease: pathogenesis of Crohn's disease and ulcerative colitis. Nat Rev Gastroenterol Hepatol. 2006;3:390–407 [DOI] [PubMed] [Google Scholar]
- 2.Sturm A, Dignass AU. Epithelial restitution and wound healing in inflammatory bowel disease. World J Gastroenterol. 2008;14:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D'Haens GR, Sartor RB, Silverberg MS, et al. . Future directions in inflammatory bowel disease management. J Crohns Colitis. 2014;8:726–734 [DOI] [PubMed] [Google Scholar]
- 4.Wright K, Duncan M, Sharkey K. Cannabinoid CB2 receptors in the gastrointestinal tract: a regulatory system in states of inflammation. Br J Pharmacol. 2008;153:263–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schicho R, Storr M. Cannabis finds its way into treatment of Crohn's disease. Pharmacology. 2013;93:1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aizpurua-Olaizola O, Soydaner U, Öztürk E, et al. . Evolution of the cannabinoid and terpene content during the growth of Cannabis sativa plants from different chemotypes. J Nat Prod. 2016;79:324–331 [DOI] [PubMed] [Google Scholar]
- 7.Mechoulam R, Shani A, Edery H, et al. . Chemical basis of hashish activity. Science. 1970;169:611–612 [DOI] [PubMed] [Google Scholar]
- 8.Mechoulam R, Gaoni Y. Hashish—iv: the isolation and structure of cannabinolic cannabidiolic and cannabigerolic acids. Tetrahedron. 1965;21:1223–1229 [DOI] [PubMed] [Google Scholar]
- 9.Mechoulam R, Parker LA, Gallily R. Cannabidiol: an overview of some pharmacological aspects. J Clin Pharmacol. 2002;42:11S–19S [DOI] [PubMed] [Google Scholar]
- 10.Russo EB. Taming THC: potential Cannabis synergy and phytocannabinoid‐terpenoid entourage effects. Br J Pharmacol. 2011;163:1344–1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greineisen WE, Turner H. Immunoactive effects of cannabinoids: considerations for the therapeutic use of cannabinoid receptor agonists and antagonists. Int Immunopharmacol. 2010;10:547–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romano B, Pagano E, Orlando P, et al. . Pure Δ9-tetrahydrocannabivarin and a Cannabis sativa extract with high content in Δ9-tetrahydrocannabivarin inhibit nitrite production in murine peritoneal macrophages. Pharmacol Res. 2016;113:199–208 [DOI] [PubMed] [Google Scholar]
- 13.Ihenetu K, Molleman A, Parsons ME, et al. . Inhibition of interleukin-8 release in the human colonic epithelial cell line HT-29 by cannabinoids. Eur J Pharmacol. 2003;458:207–215 [DOI] [PubMed] [Google Scholar]
- 14.Ryberg E, Larsson N, Sjögren S, et al. . The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol. 2007;152:1092–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stančić A, Jandl K, Hasenöhrl C, et al. . The GPR55 antagonist CID16020046 protects against intestinal inflammation. Neurogastroenterol Motil. 2015;27:1432–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Storr MA, Keenan CM, Zhang H, et al. . Activation of the cannabinoid 2 receptor (CB2) protects against experimental colitis. Inflamm Bowel Dis. 2009;15:1678–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Izzo AA, Camilleri M. Cannabinoids in intestinal inflammation and cancer. Pharmacol Res. 2009;60:117–125 [DOI] [PubMed] [Google Scholar]
- 18.Naftali T, Lev LB, Yablecovitch D, et al. . Treatment of Crohn's disease with Cannabis: an observational study. Isr Med Assoc J. 2011;13:455–458 [PubMed] [Google Scholar]
- 19.Naftali T, Schleider LB, Dotan I, et al. . Cannabis induces a clinical response in patients with Crohn's disease: a prospective placebo-controlled study. Clin Gastroenterol Hepatol. 2013;11:1276–1280 [DOI] [PubMed] [Google Scholar]
- 20.Pagano E, Capasso R, Piscitelli F, et al. . An orally active Cannabis extract with high content in cannabidiol attenuates chemically-induced intestinal inflammation and hypermotility in the mouse. Front Pharmacol. 2016;7:341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hill TD, Cascio MG, Romano B, et al. . Cannabidivarin‐rich Cannabis extracts are anticonvulsant in mouse and rat via a CB1 receptor‐independent mechanism. Br J Pharmacol. 2013;170:679–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chuang AY, Chuang JC, Zhai Z, et al. . NOD2 expression is regulated by microRNAs in colonic epithelial HCT116 cells. Inflamm Bowel Dis. 2014;20:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Subramanian S, Rhodes JM, Hart CA, et al. . Characterization of epithelial IL‐8 response to inflammatory bowel disease mucosal E. coli and its inhibition by mesalamine. Inflamm Bowel Dis. 2008;14:162–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Banks C, Bateman A, Payne R, et al. . Chemokine expression in IBD. Mucosal chemokine expression is unselectively increased in both ulcerative colitis and Crohn's disease. J Pathol. 2003;199:28–35 [DOI] [PubMed] [Google Scholar]
- 25.Mitsuyama K, Toyonaga A, Sasaki E, et al. . IL‐8 as an important chemoattractant for neutrophils in ulcerative colitis and Crohn's disease. Clin Exp Immunol. 1994;96:432–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith R, Vaughan C. The decomposition of acidic and neutral cannabinoids in organic solvents. J Pharm Pharmacol. 1977;29:286–290 [DOI] [PubMed] [Google Scholar]
- 27.Esposito G, Filippis DD, Cirillo C, et al. . Cannabidiol in inflammatory bowel diseases: a brief overview. Phytother Res. 2013;27:633–636 [DOI] [PubMed] [Google Scholar]
- 28.Wang D, DuBois RN. The role of COX-2 in intestinal inflammation and colorectal cancer. Oncogene. 2010;29:781–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castaneda FE, Walia B, Vijay-Kumar M, et al. . Targeted deletion of metalloproteinase 9 attenuates experimental colitis in mice: central role of epithelial-derived MMP. Gastroenterology. 2005;129:1991–2008 [DOI] [PubMed] [Google Scholar]
- 30.Moreno-Sanz G. Can you pass the acid test? Critical review and novel therapeutic perspectives of δ9-tetrahydrocannabinolic acid A. Cannabis Cannabinoid Res. 2016;1:124–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verhoeckx KC, Korthout HA, van Meeteren-Kreikamp A, et al. . Unheated Cannabis sativa extracts and its major compound THC-acid have potential immuno-modulating properties not mediated by CB 1 and CB 2 receptor coupled pathways. Int Immunopharmacol. 2006;6:656–665 [DOI] [PubMed] [Google Scholar]
- 32.Ruhaak LR, Felth J, Karlsson PC, et al. . Evaluation of the cyclooxygenase inhibiting effects of six major cannabinoids isolated from Cannabis sativa. Biol Pharm Bull. 2011;34:774–778 [DOI] [PubMed] [Google Scholar]
- 33.El Miedany Y, Youssef S, Ahmed I, et al. . The gastrointestinal safety and effect on disease activity of etoricoxib, a selective COX-2 inhibitor in inflammatory bowel diseases. Am J Gastroenterol. 2006;101:311–317 [DOI] [PubMed] [Google Scholar]
- 34.Kolho K-L, Sipponen T, Valtonen E, Savilahti E. Fecal calprotectin, MMP-9, and human beta-defensin-2 levels in pediatric inflammatory bowel disease. Int J Colorectal Dis. 2014;29:43–50 [DOI] [PubMed] [Google Scholar]
- 35.Annaházi A, Molnár T, Farkas K, et al. . Fecal MMP‐9: a new noninvasive differential diagnostic and activity marker in ulcerative colitis. Inflamm Bowel Dis. 2012;19:316–320 [DOI] [PubMed] [Google Scholar]
- 36.Xu L, Stevens J, Hilton MB, Seaman S, et al. . COX-2 inhibition potentiates antiangiogenic cancer therapy and prevents metastasis in preclinical models. Sci Transl Med. 2014;6:242ra84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chiurchiù V, Leuti A, Maccarrone M. Cannabinoid signaling and neuroinflammatory diseases: a melting pot for the regulation of brain immune responses. J Neuroimmune Pharmacol. 2015;10:268–280 [DOI] [PubMed] [Google Scholar]
- 38.Martínez-Martínez E, Gómez I, Martín P, et al. . Cannabinoids receptor type 2, CB2, expression correlates with human colon cancer progression and predicts patient survival. Oncoscience. 2015;2:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Romano B, Borrelli F, Pagano E, et al. . Inhibition of colon carcinogenesis by a standardized Cannabis sativa extract with high content of cannabidiol. Phytomedicine. 2014;21:631–639 [DOI] [PubMed] [Google Scholar]
- 40.Chiurchiù V, Lanuti M, De Bardi M, et al. . The differential characterization of GPR55 receptor in human peripheral blood reveals a distinctive expression in monocytes and NK cells and a proinflammatory role in these innate cells. Int Immunol. 2014:dxu097. [DOI] [PubMed] [Google Scholar]
- 41.ChoiPark WH, Baek SH, Chu JP, et al. . Cannabidiol induces cytotoxicity and cell death via apoptotic pathway in cancer cell lines. Biomol Ther. 2008;16:87–94 [Google Scholar]
- 42.Gallily R, Yekhtin Z, Hanuš LO. Overcoming the bell-shaped dose–response of cannabidiol by using Cannabis extract enriched in cannabidiol. Pharmacol Pharm. 2015;6:75 [Google Scholar]
References
Cite this article as: Nallathambi R, Mazuz M, Ion A, Selvaraj G, Weininger S, Fridlender M, Nasser A, Sagee O, Kumari P, Nemichenizer D, Mendelovitz M, Firstein N, Hanin O, Konikoff F, Kapulnik Y, Naftali T, Koltai H (2017) Anti-inflammatory activity in colon models is derived from Δ9-tetrahydrocannabinolic acid that interacts with additional compounds in Cannabis extracts, Cannabis and Cannabinoid Research 2:1, 167–182, DOI: 10.1089/can.2017.0027.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.