Abstract
Objective
Wharton jelly-derived mesenchymal stem cells (WJMSCs) exhibit multilineage differentiation potential and can be used to treat multiple organs. However, diabetes affects the repair capability of MSCs. The aim of this study was to evaluate the effect of diabetic patient-derived serum on WJMSC behavior.
Methods
WJMSCs at passage 3 were treated with serum derived from type 2 diabetic patients. WJMSCs were characterized for surface markers expression by using immunocytochemistry technique. The effects on cell viability, proliferation, cell death rate, and vascular endothelial growth factor level were assessed by crystal violet staining, 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay, lactate dehydrogenase assay, and enzyme-linked immuno-sorbent assay, respectively. Oxidative stress was assessed by the estimation of free radical species malondialdehyde (MDA) and enzymes glutathione (GSH), catalase, and superoxide dismutase (SOD).
Results
WJMSCs isolated in this study were positive for CD29, CD49, CD73, CD90, CD105, and SSEA4 and negative for CD45 and CD34. Under diabetic stress conditions, WJMSCs showed low viability and high lactate dehydrogenase release. A low level of vascular endothelial growth factor was also observed after diabetic serum treatment. Antioxidant level was also lower in diabetic serum-treated WJMSCs compared to in normal serum-treated WJMSCs.
Conclusion
The results of the present study suggest that pre-treatment of WJMSCs with type 2 diabetic serum decreases the survival of WJMSCs. The findings of this study provide insight into diabetes-induced harmful effects on WJMSCs.
Keywords: Wharton jelly-derived mesenchymal stem cells, Type 2 diabetes mellitus, Oxidative stress, Vascular endothelial growth factor
Introduction
Wharton jelly-derived mesenchymal stem cells (WJMSCs) are a promising cell source for organ repair.1 The unique properties of WJMSCs are ease of isolation, immunological tolerance, and multilineage potential, which have led to the use of these cells for the therapeutic repair of many organs.2, 3
Diabetes mellitus is associated with enhanced reactive oxygen species (ROS) production.4 Previous studies have shown that ROS reacts with lipids, protein, and DNA to promote oxidative stress-induced cellular damage.5 These conditions inhibit cell proliferation and initiate cell apoptosis and cell death, limiting the survival of transplanted stem cells in diabetic conditions.6
The aim of this study was to evaluate the effect of diabetic patient-derived serum on WJMSC behavior. The effect of diabetic serum on WJMSCs has not been previously examined. We found that serum isolated from multiple diabetic patients constrained the proliferation and survival capacity of WJMSCs.
Material and methods
Blood collection
The study was performed with the approval of the ethics committee of The University of Lahore and informed consent of patients and controls. Blood samples were collected from newly diagnosed cases of type 2 diabetic patients with glycosylated hemoglobin level (Hb1Ac) >6.5%7 attending the department of diabetology. Blood samples were also taken from healthy control subjects with Hb1Ac in the range of <6.0%.
Isolation of human serum
Blood was drawn in the preprandial state by venipuncture into properly labeled vacutainers containing no additives (BD Biosciences, Franklin Lakes, NJ, USA). Blood samples were allowed to clot and then centrifuged for 10 min at 2000 ×g at 4 °C. The serum supernatant was isolated, sterile-filtered, aliquoted, and stored at −20 °C until use.
Isolation and culture of WJMSCs
The present study was approved by the Biosafety Board at The University of Lahore, Lahore, Pakistan. Samples were collected after obtaining written informed consent from mothers and all samples were screened for the absence of hepatitis B virus, hepatitis C virus, and human immunodeficiency virus. Samples were collected in sterile tubes and transported to the tissue culture laboratory immediately after collection. WJMSCs were isolated from umbilical cords by an explant culturing method as we previously described.8 Briefly, cord pieces were incubated in 3 mg/ml collagenase solution (Invitrogen, Carlsbad, CA, USA). After 3 h, Dulbecco's modified eagle medium-low glucose (DMEM LG) (Sigma Aldrich, St. Louis, MO, USA) containing 10% human serum, 100 U/ml penicillin, and 100 μg/ml streptomycin (Sigma–Aldrich) was added to the same flasks (Corning Inc., Corning, NY, USA) containing collagenase solution and placed at 37 °C in an incubator at 95% humidity and 5% CO2. The medium was renewed after three days. Cells were used at passage 3 for all further experiments.
Characterization of WJMSCs
WJMSCs were characterized by immunocytochemistry analysis. Immunocytochemistry was performed by first fixing the cells with 4% paraformaldehyde (Sigma Aldrich). Non-specific binding was blocked with 10% donkey serum (Jackson ImmunoResearch, West Grove, PA, USA) for 30 min followed by incubation with the respective primary antibodies, i.e., CD29 conjugated to phycoerythrin (CD29-PE; BD Biosciences, USA), CD49-PE (BD Biosciences), CD73-PE (BD Biosciences), CD90 conjugated to fluorescein isothiocyanate (CD90-FITC; BD Biosciences), CD105 (BD Biosciences), SSEA4-FITC (Santa Cruz Biotechnology, Santa Cruz, CA, USA), CD34-FITC, and CD45-FITC (BD Biosciences) at a dilution of 1:100 for 1 hat 25 °C. For CD105, the cells were subsequently labeled with FITC-donkey anti-mouse (Jackson ImmunoResearch) for 1:200 for 1 h at 4 °C. Nuclei were stained with 4′,6-diamidino-2-phenylindole. Fluorescent images were captured by using an Olympus BX-61 microscope equipped with a DP-70 digital camera (Olympus, Tokyo, Japan).
Experimental design
WJMSCs at passage 3 were plated in a 6-well plate (Corning). WJMSCs were randomly assigned to one of two experimental groups as follows: (1) incubation with normal human serum (DMEM LG + 10% human serum from normal persons) (2) incubation with diabetic human serum (DMEM LG + 10% human serum from type 2 diabetic person), respectively.
Cell viability assay
Cell viability was evaluated by crystal violet staining (Invitrogen) after treatment with serum. Cells were incubated with 0.5% crystal violet for 20 min. After washing three times in phosphate-buffered saline (PBS) (Invitrogen), 1% sodium dodecyl sulfate (SDS) (Invitrogen) was added to solubilize the dye and absorbance was measured at 595 nm using a microplate reader (Molecular Devices, Sunnyvale, CA, USA).
Cell proliferation assay
To compare the proliferative potential of both treatment groups, the 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay was performed to measure cell proliferation. Before adding the MTT reagent, the cell monolayer was washed with PBS (Invitrogen), and then 500 μl 10% complete medium and 60 μl MTT solution was added and incubated for 2 h at 37 °C. To solubilize the crystals, 1.5 ml dimethyl sulfoxide (DMSO) (Invitrogen) was added and absorbance was measured at 570 nm.
Lactate dehydrogenase (LDH) assay
The cell supernatant was analyzed for cytotoxicity in an LDH assay according to the manufacturer's instructions (AMP Diagnostics, Graz, Austria). Briefly, 5 μl cell supernatant from both groups was mixed with 95 μl working reagent and incubated for 5 min. Absorbance was measured at a wavelength of 340 nm.
Enzyme-linked immunosorbent assay (ELISA) for vascular endothelial growth factor (VEGF)
The concentration of VEGF in the treatment groups were measured using a solid-phase sandwich ELISA. Briefly, a microplate was pre-coated with rabbit anti-VEGF monoclonal antibody (1× tris buffered saline (TBS), 5% milk, 0.10% Tween-20, 1:1000 antibody) overnight at 4 °C (SantaCruz Biotechnology). After washing three times, 100 μl medium was added and incubated for 18 h. After washing three times, blocking was conducted for 1 h by using 10% bovine serum albumin and then incubated with anti-rabbit secondary horseradish peroxidase-conjugated antibody (1:200) for 1 h at 4 °C. After washing, an equal volume of chromogenic solution, 3,3′,5,5′-tetramethylbenzidine (Invitrogen) and 0.1 mmol/L HCl was added, to stop the reaction. The optical density (OD) was measured at 450 nm using a microplate reader (Molecular Devices).
Estimation of oxidative stress
The cell supernatant from all treatment groups was removed and centrifuged at 12,000 ×g for 5 min to remove cell debris. This medium was used to analyze oxidative stress parameters e.g., lipid peroxidation (Malondialdehyde), superoxide dismutase (SOD), glutathione (GSH), and catalase (CAT) levels. Enzyme activity levels were expressed as OD. All assays were performed in triplicate according to our previously published protocol.8, 9, 10
Statistical analysis
Data were expressed as the mean ± standard error of mean (SEM). Statistical significance was assessed by Student t test and 2-way analysis of variance (ANOVA) with Bonferroni post hoc test. A P-value less than or equal to 0.05 was considered statistically significant. Statistical analysis was performed using GraphPad prism version 5.0 software (GraphPad, Inc., La Jolla, CA, USA).
Results
Demographic characteristics of subjects
Study subjects were divided based on health conditions into a normal group (12 subjects) or type 2 diabetic group (12 subjects). Normal subjects had a mean age of 52.0 ± 17.2 years, whereas the diabetic group had a mean age of 55.2 ± 10.1 years. Fasting blood glucose and hemoglobin A1c (HbA1c) levels were significantly higher in diabetic subjects (P < 0.001) compared to in normal subjects. Demographic characteristics are listed in Table 1.
Table 1.
General demographic characteristics of subjects.
| Group | n | Gender, Male |
Age | FBG, mmol/L | HbA1c, % | TC, mg/dl | TG, mg/dl | HDL, mg/dl | LDL, mg/dl |
|---|---|---|---|---|---|---|---|---|---|
| Normal subjects | 12 | 12 | 52 ± 17 | 4.0 ± 9.0 | 3.8 ± 2.4 | 175 ± 10 | 117 ± 23 | 40 ± 5 | 101 ± 24 |
| Diabetic subjects | 12 | 12 | 55 ± 10 | 6.9 ± 6.8 | 8.7 ± 7.5 | 265 ± 12 | 306 ± 18 | 26 ± 7 | 292 ± 22 |
| P | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
FBG: fasting blood glucose; HbA1c: hemoglobin A1c; TC: total cholesterol; TG: triglycerides; LDL: low-density lipoproteins; HDL: high-density lipoproteins.
All values are expressed as the mean ± standard error of mean (SEM).
Characterization of WJMSCs
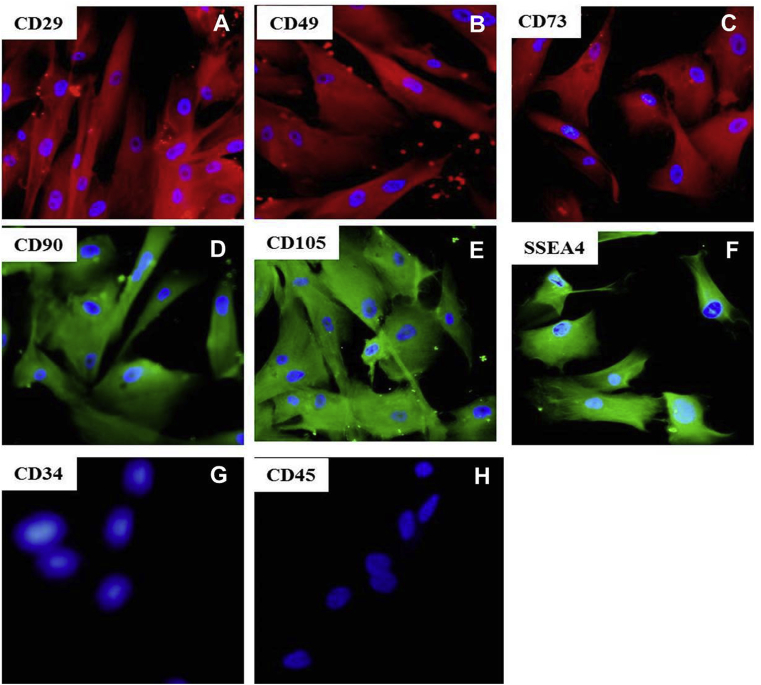
The WJMSCs were positive for MSCs markers (CD29, CD49, CD73, CD90, CD105, and SSEA4) and negative for hematopoietic markers (CD45 and CD34) as demonstrated by immunocytochemistry (Fig. 1).
Fig. 1.
Characterization of WJMSCs: A) CD29; B) CD49; C) CD73; D) CD90; E) CD105; F) SSEA4; G) CD34; H) CD45. 100×, WJMSCs: Wharton jelly-derived mesenchymal stem cells.
Effect of diabetic patient-derived serum on cell viability, LDH, and proliferation of WJMSCs
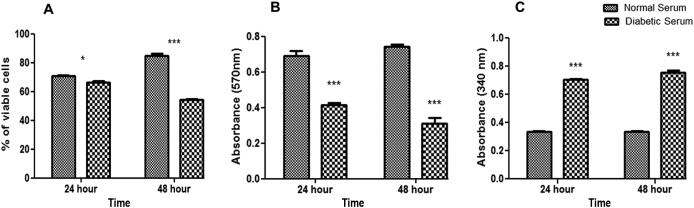
Decreased cell viability was observed in the 48-h diabetic serum-pretreated group (54.0 ± 0.8%) compared to in the normal serum-pretreated group (85.0 ± 1.3%) based on the crystal violet assay results (Fig. 2A). Furthermore, cell proliferation was evaluated after treatment with normal and diabetic serum. Diabetic serum pretreatment at 48 h resulted in significantly low proliferations (0.32 ± 0.03 nm) compared to in the normal serum group (0.75 ± 0.01 nm) (Fig. 2B). Cell damage demonstrated by the LDH assay was significantly high after 48-h diabetic serum treatment (0.76 ± 0.01 nm) compared to in the normal serum group (0.33 ± 0.00 nm) (Fig. 2C).
Fig. 2.
Effect of diabetic patient-derived serum on cell viability, LDH, and proliferation of WJMSCs. A) Crystal violet assay: decreased cell viability in diabetic serum group. B) Cell proliferation: reduced cell proliferation in diabetic serum group. NS: non-significant, *P < 0.05, **P < 0.01, ***P < 0.001. C) LDH assay: increased LDH release in diabetic serum group compared to in normal serum group. LDH: lactate dehydrogenase. WJMSCs: Wharton jelly-derived mesenchymal stem cells.
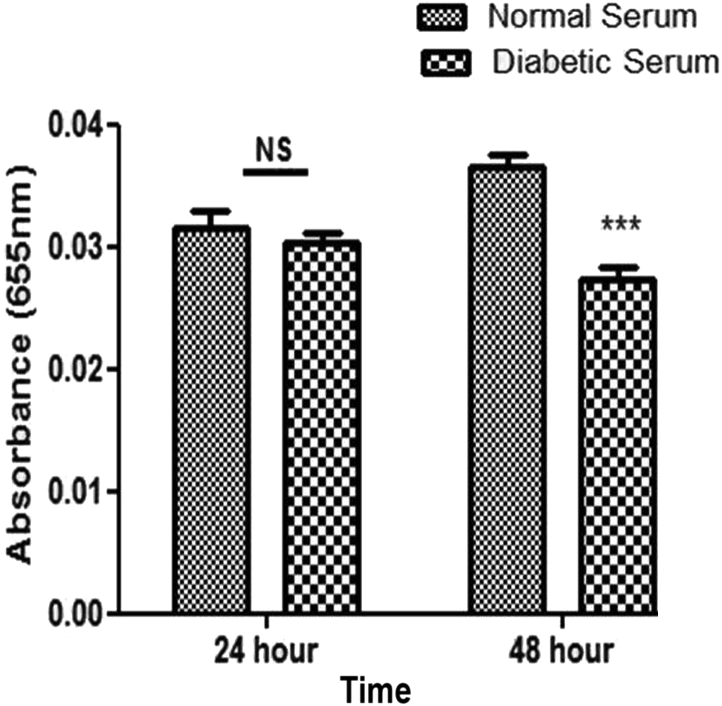
Effect of diabetic patient-derived serum on VEGF level of WJMSCs
The level of VEGF protein was determined by ELISA (Fig. 3). Compared to the normal serum-treated group (0.03 ± 0.00 nm), VEGF protein levels were significantly lower in the diabetic serum-treated group (0.02 ± 0.00 nm) after 48 h.
Fig. 3.
VEGF expression by ELISA: decreased VEGF level in diabetic serum group compared to in normal serum group. NS; non-significant, *P < 0.05, **P < 0.01, ***P < 0.001. VEGF: vascular endothelial growth factor; ELISA: enzyme-linked immunosorbent assay.
Evaluation of oxidative stress
Activities of antioxidant enzymes were evaluated; SOD (0.48 ± 0.02 nm), GSH (0.21 ± 0.00 nm), and CAT (0.72 ± 0.03 nm) were significantly lower in the diabetic serum-treated group than in the normal serum group for SOD (0.85 ± 0.03 nm), GSH (0.26 ± 0.00 nm), and CAT (0.49 ± 0.02 nm) (Fig. 4 A–C). Moreover, malondialdehyde was higher in the diabetic serum-treated group (0.61 ± 0.02 nm) after 48 h compared to in the normal serum-treated group (0.22 ± 0.00 nm) (Fig. 4 D).
Fig. 4.
Activity of antioxidant enzymes. A) Superoxide dismutase (SOD), B) glutathione reductase (GSH), C) catalase (CAT), and D) malondialdehyde (MDA). NS; non-significant, *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
The regenerative capacity of diabetic patients-derived stem cells is affected by age and duration of diabetes,11 and thus affects autologous transplantation. Non-autologous stem cells are preferred for transplantation to regenerate damaged tissues in diabetic patients. WJMSCs represent a better alternative source for non-autologous stem cells, as they are naive and low-immunogenic and show high proliferation.11, 12, 13 However, it is unknown whether the performance of serum isolated from diabetic patients affects WJMSC efficiency. An important aspect of the study was to evaluate whether WJMSCs survive in the harsh diabetic environment. Thus, we designed an in vitro model of type 2 diabetic conditions by culturing WJMSCs in serum isolated from type 2 diabetic patients. We investigated the behavior of WJMSCs in response to serum from type 2 diabetic patients. WJMSCs treated with type 2 diabetic patient-derived serum exhibited decreased cell viability and proliferation, but increased LDH activity. These results suggest that diabetes enhances the apoptosis and necrosis of stem cells via high glucose-induced overproduction of ROS.
It is well-documented that angiogenic processes are impaired in diabetic conditions.14, 6 Hence, it was hypothesized that serum from type 2 diabetic patients affects the process of angiogenesis. Our results showed that VEGF level is impaired in type 2 diabetic patient sera.
In diabetic patients, oxidative stress increases because of diminished antioxidant activity, which further augments oxidative stress-induced complications.15, 16, 17 The consequences of high glucose-induced oxidative stress on WJMSCs have not been examined. Low levels of antioxidant activity in WJMSCs after long exposure to diabetic patient-derived serum suggest that increased ROS is released in the blood circulation of diabetic patients, limiting the survival of WJMSCs.
In conclusion, we evaluated the survival and proliferative ability of WJMSCs in type 2 diabetic patient serum. Type 2 diabetic patient sera reduced the proliferation and survival of WJMSCs. To overcome the limitations of poor survival and proliferation of WJMSCs in the diabetic environment, preconditioning strategies are required to augment the survival of WJMSCs.
Conflicts of interest
None declared.
Acknowledgments
This work was supported by research grants from The University of Lahore, Lahore, Pakistan. The authors thank Jinnah Hospital and Sheikh Zyed Hospital for providing support in sample collection.
Edited by Wei-Zhu Liu
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Kim D.W., Staples M., Shinozuka K. Wharton's jelly-derived mesenchymal stem cells: phenotypic characterization and optimizing their therapeutic potential for clinical applications. Int J Mol Sci. 2013;14:11692–11712. doi: 10.3390/ijms140611692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar S., Singh N.P. Stem cells: a new paradigm. Indian J Hum Genet. 2006;12:4–10. [Google Scholar]
- 3.Cutler A.J., Limbani V., Girdlestone J., Navarrete C.V. Umbilical cord-derived mesenchymal stromal cells modulate monocyte function to suppress T cell proliferation. J Immunol. 2010;185:6617–6623. doi: 10.4049/jimmunol.1002239. [DOI] [PubMed] [Google Scholar]
- 4.Giacco F., Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fiorentino T.V., Prioletta A., Zuo P. Hyperglycemia-induced oxidative stress and its role in diabetes mellitus related cardiovascular diseases. Curr Pharm Des. 2013;19:5695–5703. doi: 10.2174/1381612811319320005. [DOI] [PubMed] [Google Scholar]
- 6.Khan M., Ali F., Mohsin S. Preconditioning diabetic mesenchymal stem cells with myogenic medium increases their ability to repair diabetic heart. Stem Cell Res Ther. 2013;4:58. doi: 10.1186/scrt207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Diabetes Association Standards of medical care in diabetes-2010. Diabetes Care. 2010;33:S11–S61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wajid N., Naseem R., Anwar S.S. The effect of gestational diabetes on proliferation capacity and viability of human umbilical cord-derived stromal cells. Cell Tissue Bank. 2015;16:389–397. doi: 10.1007/s10561-014-9483-4. [DOI] [PubMed] [Google Scholar]
- 9.Ali F., Naqvi S.A., Bismillah M., Wajid N. Comparative analysis of biochemical parameters in diabetic and non-diabetic acute myocardial infarction patients. Indian Heart J. 2016;68:325–331. doi: 10.1016/j.ihj.2015.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ali F., Rafique H., Wajid N. N-acetylcysteine prevents cord derived stem cells from H2O2 induced injury in vitro. EJPMR. 2015;2:589–598. [Google Scholar]
- 11.Kalaszczynska I., Ferdyn K. Wharton's jelly derived mesenchymal stem cells: future of regenerative medicine? Recent findings and clinical significance. Biomed Res Int. 2015;2015 doi: 10.1155/2015/430847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bárcia R.N., Santos J.M., Filipe M. What makes umbilical cord tissue-derived mesenchymal stromal cells superior immunomodulators when compared to bone marrow derived mesenchymal stromal cells? Stem Cells Int. 2015;2015 doi: 10.1155/2015/583984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanafi M.M., Ramesh A., Gupta P.K., Bhonde R.R. Influence of hypoxia, high glucose, and low serum on the growth kinetics of mesenchymal stem cells from deciduous and permanent teeth. Cells Tissues Organs. 2013;198:198–208. doi: 10.1159/000354901. [DOI] [PubMed] [Google Scholar]
- 14.Dunn L.L., Simpson P.J., Prosser H.C. A critical role for thioredoxin-interacting protein in diabetes-related impairment of angiogenesis. Diabetes. 2014;63:675–687. doi: 10.2337/db13-0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maritim A.C., Sanders R.A., Watkins J.B., 3rd Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 16.Naudi A., Jove M., Ayala V. Cellular dysfunction in diabetes as maladaptive response to mitochondrial oxidative stress. Exp Diabetes Res. 2012;2012 doi: 10.1155/2012/696215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hwang I., Lee J., Huh J.Y. Catalase deficiency accelerates diabetic renal injury through peroxisomal dysfunction. Diabetes. 2012;61:728–738. doi: 10.2337/db11-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]