Abstract
Objective
To present our treatment experiences and the follow-up data of patients with paradoxical embolism (PDE).
Methods
The clinical characteristics, management, and follow-up data of all included patients who were diagnosed with PDE at Fuwai Hospital from January 1994 to October 2015 were recorded.
Results
Twelve patients were included; all had a pulmonary embolism, and 8 had deep venous thrombosis. The artery embolisms involved the cerebral artery (7 patients), renal artery (2 patients), mesentery artery (2 patients), popliteal artery (1 patient), descending aorta thrombus (1 patient), and thrombus-straddled patent foramen ovale (PFO) (1 patient). PFO was found in 3 cases. One patient underwent thrombectomy and PFO closure; Six patients received thrombolysis; and 3 patients were implanted with a vena cava filter. Long-term anticoagulation with warfarin was recommended for each patient. One patient died from ventricular fibrillation despite cardiopulmonary resuscitation. Eleven patients were discharged with improvements. No late mortality occurred in 8 patients with a complete follow-up of 10.6–17.7 years. One had a recurrent deep venous thrombosis. No patient had a recurrent pulmonary or arterial embolism. Two patients changed their treatment from warfarin to aspirin; others remained on warfarin. Only 1 case had an occasional gum bleeding.
Conclusions
PDE treatment including thrombolysis, anticoagulation, and embolectomy should be individualized. We recommend long-term anticoagulation therapy to prevent the recurrence of PDE, especially to those with an intracardiac communication or persistent risk factors for re-thrombosis.
Keywords: Paradoxical embolism, Pulmonary embolism, Deep venous thrombosis, Patent foramen ovale
Introduction
Paradoxical embolism (PDE) refers to the blockage of an artery due to a passage of a clot from a systemic vein to a systemic artery without passing through the lungs, which ordinarily acts as a filter to remove blood clots from entering the arterial circulation. PDE occurs when there is a defect that allows a clot to cross directly from the right to the left side of the heart, as in patients with atrial septal defect or patent foramen ovale (PFO). Once in the arterial circulation, a clot can travel to any site where it can block an artery. PDE is frequently associated with cryptogenic stroke, peripheral embolism, and brain abscess.1 Cardiac sources account for 80% of all peripheral arterial thromboembolic events.2 PDE is comparatively rare and is recognized for less than 2% of systemic arterial emboli.3 However, the importance of PDE might be underestimated. Clinically, we often encounter patients with acute arterial embolisms with no identifiable significant atherosclerotic risk factors and can not find the sources of thrombus despite a thorough examination. Meacham et al4 reported that PDE could account for as many as 47,000 unexplained ischemic strokes each year in the United States. Our study aims to present the treatment experiences and follow-up data of our 12 cases with PDE, review and compare with prior reports, and enhance the recognition for PDE as a potential cause of arterial embolism.
Materials and methods
All patients who were diagnosed with PDE at Fuwai Hospital from January 1994 to October 2015 were included. All data related to the patients with PDE were collected, such as (1) demographic data; (2) case history and risk factors for thromboembolism; (3) clinical, laboratory, and imaging findings of deep venous thrombosis (DVT), pulmonary embolism (PE), and systemic arterial embolic events; (4) imaging findings of an abnormal communication that allowed a right-to-left shunt; (5) treatment and outcome; and (6) follow-up data. Follow-up data were obtained from hospital records or by telephone interviews with the patients or their family members. The follow-up period ended on November 1, 2015.
Results
Twelve patients were diagnosed with PDE during the past 21.8 years. The patients included 7 men and 5 women, with a median age of 53 years (range, 27–75 years) and a median body mass index (BMI) of 23.6 kg/m2 (range, 18.9–27.1 kg/m2). Eleven patients had 1 or more risk factors for thrombosis. Two patients had invasive tests through their femoral blood vessels, which may have directly led to thrombosis. No recognized risk factors were found in 1 patient. None of the patients had an identifiable coagulation defect (see Table 1 for more information).
Table 1.
Clinical data and follow-up results of the patients with paradoxical embolism.
| Case | Gender | Age, years | BMI, kg/m2 | Risk factors for thrombosis | Inducing or exacerbating factors | Symptoms | DVT | PE | Systemic arterial embolism | Treatment | Outcome | Follow-up, years |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 59 | – | CAG through femoral artery, hypertension, smoking | Defecation | Syncope | Right | Yes | Right CVA; mesentery artery | CPR | Died | – |
| 2 | M | 56 | 23.6 | radiofrequency ablation through femoral artery and vein, smoking | Defecation | Syncope | Bilateral | Yes | Right renal artery | CPR, warfarin | NA | NA |
| 3 | M | 34 | 23.7 | Smoking | – | Dyspnea, hemoptysis | Left | Yes | Thrombus-straddled PFO | Thrombectomy, IVCF, warfarin | Alive | 17.7 |
| 4 | M | 55 | 22.9 | History of L lower limb bruising, hypertension | Sitting still for 2 h | Dyspnea, abdominal and lower back pain | Left | Yes | Right renal artery mesentery artery | Urokinase, warfarin, IVCF | NA | NA |
| 5 | F | 75 | 23.8 | Venous varices in Bilateral lower limbs, hypertension, smoking | Defecation | Dyspnea, palpitation, CVA | Left | Yes | Left CVA | Urokinase, warfarin | NA | NA |
| 6 | M | 51 | 18.9 | Long-term standing work, smoking | Defecation | Dyspnea, convulsion | – | Yes | Bilateral CVA | Urokinase, warfarin | Alive | 13.7 |
| 7 | F | 27 | 21.0 | Peripartum | Delivery | Dyspnea, palpitation, pain in B lower limb, CVA | Bilateral | Yes | Right CVA | Urokinase, warfarin, IVCF | Alive | 13.5 |
| 8 | M | 39 | 24.8 | – | – | Dyspnea, pain in Left lower limb | Right | Yes | Lilateral popliteal artery | rt-PA, warfarin | Alive | 12.6 |
| 9 | M | 58 | 22.6 | Sedentary lifestyle, hypercholesterolemia | Driving for 4 h | Dyspnea | Left | Yes | Descending aorta thrombus | rt-PA, warfarin | Alive | 11.9 |
| 10 | F | 41 | 26.7 | Overweight | – | Dyspnea, CVA | – | Yes | Left CVA | Warfarin | Alive | 10.9 |
| 11 | F | 58 | 21.8 | Venous varices in the Left lower limb | Defecation | Dyspnea, cough, CVA | – | Yes | Left CVA | Warfarin | Alive | 10.8 |
| 12 | F | 42 | 27.1 | Overweight | – | Dyspnea, CVA | – | Yes | Right CVA | Warfarin, pulmonary endarterectomy | Alive | 10.6 |
BMI: body mass index; CAG: coronary angiogram; CPR: cardiopulmonary resuscitation; CVA: cerebrovascular accident; DVT: deep venous thrombosis; F: female; IVCF: inferior vena cava filter; M: male; NA: not available; PE: pulmonary embolism; rt-PA: recombinant tissue plasminogen activator.
The BMI of case 1 was unavailable.
All patients had a PE. Compressed ultrasonography of the lower limbs showed that 8 patients had a DVT, 3 had abnormally slow blood flow (cases 6, 11, and 12), and 1 had no abnormal findings (case 10). The arterial embolisms included 7 cerebrovascular events, 2 renal artery embolisms, 2 mesentery artery embolisms, and 1 each of popliteal artery embolism, descending aorta thrombus (Fig. 1), and thrombus-straddled PFO. Echocardiography showed no thrombus arising from the left heart. The disease onset was progressive in 4 patients and suddenly initiated or exacerbated in the other 8 patients: 5 related to defecation, 2 to immobility for several hours, and 1 to delivery of an infant. All symptoms were mainly related to PE/DVT and associated with arterial embolisms.
Fig. 1.

Computed tomography of the chest in case 9 before thrombolysis. The major pulmonary artery is enlarged. The distal right pulmonary artery is partly occluded; the left upper lobular artery is nearly totally occluded; and there is a thrombus floating in the lumen of the descending aorta. The arrows point to the thrombus.
Six patients received thrombolytic therapy, 4 with urokinase and 2 with recombinant tissue plasminogen activator (rt-PA); all received anticoagulants, heparin or low-molecular-weight heparin, followed by warfarin with a targeted international normalized ratio (INR) 2.0–3.0. Three received an implanted inferior vena cava filter (IVCF); 2 underwent cardiopulmonary resuscitation, and 1 died of ventricular fibrillation (case 1). PFO was found in 3 patients (cases 3, 9, and 11). Case 3 had an emergency thrombectomy for a thrombus-straddled PFO with a concurrent PFO repair. The other 2 cases with PFOs were not repaired. Case 12 underwent a pulmonary endarterectomy after anticoagulation for 6 months. Eleven patients were discharged with improvements. The descending aorta thrombus in case 9 disappeared after anticoagulation (Fig. 2). All the patients were recommended long-term anticoagulation with warfarin and monthly monitoring of INR.
Fig. 2.
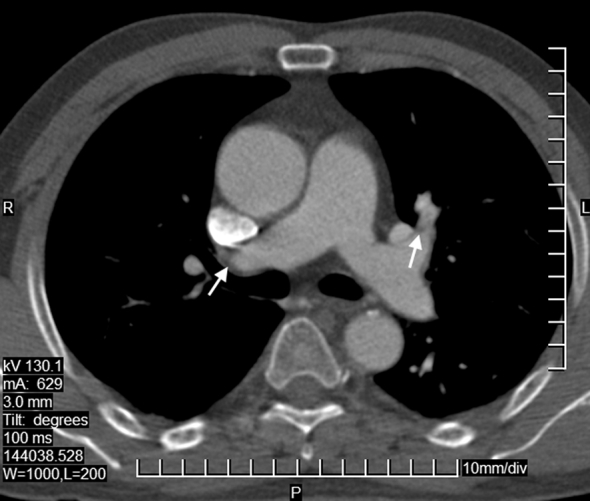
Computed tomography of the chest in case 9 after thrombolysis before discharge. The major pulmonary artery is enlarged. The walls of the right pulmonary artery and left upper lobular artery are irregular and thick. The thrombus in the distal right pulmonary artery is partly resolved, and the left upper lobular artery is partly recanalized. The thrombus floating in the lumen of the descending aorta disappeared. The arrows point to the thrombus.
Contact was lost after discharge in 4 patients; the other 8 patients had a complete follow-up (median period, 12.3 years; range, 10.6–17.7 years). They are all still on medication and perform their daily activities well. Six patients are on warfarin with an INR of 2.0–3.0. Cases 9 and 11 changed their treatment from warfarin to aspirin with a daily dose of 100 mg at 6 and 35 months after discharge for fear of bleeding and being tired of INR monitoring. Thrombi were residual in the lower limb in cases 3 and 8 and in the pulmonary artery in cases 9 and 10. None demonstrated recurrent symptoms or signs of either pulmonary or arterial emboli; however, case 7 had a recurrent DVT, and case 10 had occasional episodes of dizziness without evidence of stroke. No patient had bleeding complications secondary to anticoagulation, except for case 11, which had an occasional gum bleeding. See Table 1 for more information.
Discussion
Pathophysiology
According to Johnson,5 the diagnosis of PDE should be considered in the presence of (1) a systemic arterial embolus that did not arise from the left side of the heart, (2) venous thrombosis and /or PE, or (3) an intracardiac communication that permitted a right-to-left shunt. The diagnosis of PDE was termed definitive when made at autopsy or when a thrombus is seen crossing an intracardiac defect during echocardiography of an arterial embolus4, 6 and considered presumptive if the 3 criteria were fulfilled.6 The clinical diagnosis of PDE requires at least 2 of the 3 criteria.7 Following these diagnostic standards, only case 3 had a definitive PDE; cases 9 and 11 had a presumptive PDE; and others had a clinical PDE. However, based on the patients' histories and courses of the disease, the diagnosis of PDE was strongly suggested in all the 12 patients.
Incidence
PDE can occur only when there is an abnormal vascular or intracardiac defect. The most common defect associated with PDE was PFO. The closure of the foramen ovale is normally complete before the age of 2 years. If the foramen ovale was incompletely closed, it could be normally kept sealed by a pressure gradient between the left and right atrium but might be opened when the right atrial pressure increases and exceeds the left atrial pressure.8 Thus, the foramen ovale serves as a potential route for emboli arising from the right side of the heart. The incidence of PFO was 20.8% in the general population.9 In a study of 180 patients with PDE, 125 (69.4%) patients had a PFO, 63 (35%) had an atrial septal aneurysm, 24 (13.3%) had a PFO-like septal defect, and 31 (17.2%) had an atrial septal defect.10 In our case series, PFO was found in 3 (25%) patients via transthoracic echocardiography; no other abnormal vascular or intracardiac defects were found. However, the rate of PFO in our cases might be underestimated for the following reasons: (1) A Valsalva maneuver was not performed when the patients were receiving transthoracic echocardiography;11 and (2) none of our patients received a transesophageal echocardiogram, which could easily have revealed a right-to-left shunt at the atrial level, with a sensitivity and specificity level as high as 95%.12 In 6 patients, the onset of arterial embolism was definitely related to defecation or delivery, which could transiently open the foramen ovale. We believed that they all had a PFO.
Sandler and Martin13 reported that 79% of patients with PE had an evidence of DVT; if DVT was not detected, the entire thrombus may have already detached and embolized. In our patients with DVT, the source of thrombus was definitely identified from the lower limb in 8 (67%) patients. In cases 6, 11, and 12, the thrombus might have also arisen from the lower limb because the ultrasonography showed that the deep veins of their lower limbs were abnormal. The source of thrombus in case 10 was unknown because he had no identified risk factors for thrombosis and normal compressed ultrasonography results in the lower limb deep veins.
Distribution of arterial targets
The distribution of arterial emboli in PDE has differed among studies. According to D'Audiffret et al,14 the extremity arteries (55%) and cerebral arteries (37–50%) represented the most frequent targets; the visceral and coronary arteries were rarer targets (6–9% and 7–9%, respectively). Dubiel et al10 found the cerebrum as the most targeted (89.4%) area, with 57.8% cases of cerebral embolism and 31.7% cases of transient ischemic attacks; others were coronary (8.9%) and peripheral (1.7%) embolisms. In our case series, the cerebrum (58.3%) was the most commonly affected site, followed by the renal and mesentery arteries (16.7% respectively).
PDE with descending aorta thrombus and thrombus-straddled PFO was exceedingly rare with only several case reports.15, 16, 17, 18 We had 1 case of each. In a study by Loscalzo,6 23% of their cases had 2 separate embolic sites, and 10% had 3. Dubiel et al10 found that 12.8% of their patients with PDE experienced multifocal arterial embolisms. In our cases, 3 of the patients (cases 1, 4, and 6) had 3 embolized sites, and the others had 2.
Treatment
The therapeutic options for PDE included surgical embolectomy, thrombolysis, and anticoagulation. The overall survival appeared equivalent among these 3 therapeutic options, although more complications occurred with anticoagulation and thrombolysis.18 Ward et al19 recommended that hemodynamically significant PDE should be treated with thrombolytic therapy. We believe that therapeutic options should depend on the risk stratification of PE and systemic arterial embolism. In case 3 with thrombus-straddled PFO, surgical embolectomy was emergently performed because of a high risk for a serious arterial embolism. In the 6 patients who received thrombolytic therapy, cases 4, 8, and 9 received such therapy due to severe systemic arterial embolisms and cases 5, 6, and 7 due to PE with significant hemodynamics or right ventricular dysfunction. Case 2 received anticoagulation therapy rather than thrombolysis because of a cardiopulmonary resuscitation. The other 3 also received anticoagulation therapy because they had lower risks of PE or cerebrovascular events.
After a first PDE, the risk of recurrence was 3.4–3.8% per year.10 Nendaz et al20 recommended observation, antiplatelet and anticoagulation therapies, and closure of the PFO for the prevention of recurrent arterial emboli after PDE. Ward et al19 suggested that warfarin should be continued for 3–6 months or indefinitely if the patient had a PFO or pulmonary hypertension. In a systematic review, Khairy et al21 demonstrated that an implantation of a PFO closure device could prevent recurrent thromboembolic events better than a medical therapy alone (warfarin or salicylate). However, Travis et al22 recommended closure of a PFO for patients with PDE only in whom a systemic anticoagulant was contraindicated. Decousus et al23 reported a reduction in the occurrence of symptomatic or asymptomatic PE after IVCF implantation in patients with proximal DVT. In 8 patients with a complete follow-up in this study, 1 out of the 3 PFOs was repaired; 2 patients were implanted with an IVCF; they are all still on medication because they had an unsealed PFO, recurrence of DVT, persistent risk factors for thrombosis, or residual thrombus in the lower limb or pulmonary artery. There was no sign of pulmonary or arterial emboli nora clinically significant bleeding. The Cryptogenic Stroke Study has demonstrated that in patients with PFO, there was no significant difference in the time to the primary end points between those treated with warfarin and those treated with aspirin.24 Two of our patients who independently changed their treatment from warfarin to aspirin continued to perform well in their daily living.
Conclusions
PDE may not be rare as we have previously thought and may account for a significant minority of acute arterial occlusions in the absence of a clear cardiac or proximal arterial source. When a patient with systemic venous thrombosis also develops signs of systemic arterial embolism, the diagnosis of PDE should be considered, suggesting further studies. Recommendations for treatment should be individualized, including thrombolysis, anticoagulation, and /or embolectomy. We recommend a long-term anticoagulation therapy to prevent the recurrence of PDE for those with an abnormal intracardiac communication, persistent risk factors for thrombosis, or residual thrombus in the lower limb or pulmonary artery.
Conflicts of interest
All authors declare no conflicts of interest.
Acknowledgements
We honorably thank Professor Xing-Guo Sun, MD, National Center for Cardiovascular Disease, Fuwai Hospital, Chinese Academy of Medical Sciences, Peking Union Medical College, State Key Laboratory of Cardiovascular Disease, National Center for Cardiovascular Disease Clinical Medicine Research, Beijing 100037, China and Professor James E. Hansen, MD, Department of Medicine, Los Angeles Biomedical Research Center at Harbor-UCLA Medical Center, Torrance, CA 90503, USA for their assistance in English editing.
Edited by Wei-Zhu Liu
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Inoue T., Tadehara F., Hinoi T. Paradoxical peripheral embolism coincident with acute pulmonary thromboembolism. Intern Med. 2005;44:243–245. doi: 10.2169/internalmedicine.44.243. [DOI] [PubMed] [Google Scholar]
- 2.AbuRahma A.F., Richmond B.K., Robinson P.A. Etiology of peripheral arterial thromboembolism in young patients. Am J Surg. 1998;1762:158–161. doi: 10.1016/s0002-9610(98)00160-3. [DOI] [PubMed] [Google Scholar]
- 3.d'Audiffret A., Shenoy S.S., Ricotta J.J., Dryjski M. The role of thrombolytic therapy in the management of paradoxical embolism. Cardiovasc Surg. 1998;6:302–306. doi: 10.1016/s0967-2109(97)00154-3. [DOI] [PubMed] [Google Scholar]
- 4.Meacham R.R., 3rd, Headley A.S., Bronze M.S., Lewis J.B., Rester M.M. Impending paradoxical embolism. Arch Intern Med. 1998;158:438–448. doi: 10.1001/archinte.158.5.438. [DOI] [PubMed] [Google Scholar]
- 5.Johnson B.I. Paradoxical embolism. J Clin Pathol. 1951;4:316–332. doi: 10.1136/jcp.4.3.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loscalzo J. Paradoxical embolis: clinical presentation, diagnostic strategies, and therapeutic options. Am Heart J. 1986;112:141–145. doi: 10.1016/0002-8703(86)90692-7. [DOI] [PubMed] [Google Scholar]
- 7.Ucar Ozgul, Golbasi Zehra, Gulel Okan, Yildirim Nesligul. Paradoxical and pulmonary embolism due to a thrombus entrapped in a patent foramen ovale. Tex Heart Inst J. 2006;33:78–80. [PMC free article] [PubMed] [Google Scholar]
- 8.Wilmshurst P.T., de Belder M.A. Patent foramen ovale in adult life. Br Heart J. 1994;71:209–212. doi: 10.1136/hrt.71.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petty G.W., Khandheria B.K., Meissner I. Population-based study of the relationship between patent foramen ovale and cerebrovascular ischemic events. Mayo Clin Proc. 2006;81:602–608. doi: 10.4065/81.5.602. [DOI] [PubMed] [Google Scholar]
- 10.Dubiel M., Bruch L., Liebner M. Exclusion of patients with arteriosclerosis reduces long-term recurrence rate of presumed arterial embolism after PFO closure. J Interv Cardiol. 2007;20:275–281. doi: 10.1111/j.1540-8183.2007.00269.x. [DOI] [PubMed] [Google Scholar]
- 11.Cheng T.O. The proper conduct of Valsalva maneuver in the detection of patent foramen ovale. J Am Coll Cardiol. 2005;45:1145–1146. doi: 10.1016/j.jacc.2004.12.054. [DOI] [PubMed] [Google Scholar]
- 12.Chen W.J., Kuan P., Lien W.P., Lin F.Y. Detection of patent foramen ovale by contrast transesophageal echocardiography. Chest. 1992;101:1515–1520. doi: 10.1378/chest.101.6.1515. [DOI] [PubMed] [Google Scholar]
- 13.Sandler D.A., Martin J.F. Autopsy proven pulmonary embolism in hospital patients: are we detecting enough deep vein thrombosis? J R Soc Med. 1989;82:203–205. doi: 10.1177/014107688908200407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.d’Audiffret A., Pillai L., Dryjski M. Paradoxical emboli: the relationship between patent foramen ovale, deep vein thrombosis and ischaemic stroke. Eur J Vasc Endovasc Surg. 1999;17:468–471. doi: 10.1053/ejvs.1999.0776. [DOI] [PubMed] [Google Scholar]
- 15.Heckmann J.G., Wasmeier G., Dütsch F., Dütsch M., Dedow E. Patent foramen ovale as lifesaving purging valve. Eur J Emerg Med. 2006;13:230–232. doi: 10.1097/01.mej.0000206189.54577.21. [DOI] [PubMed] [Google Scholar]
- 16.Bracey T.S., Langrish C., Darby M. Cerebral infarction following thrombolysis for massive pulmonary embolism. Resuscitation. 2006;68:135–137. doi: 10.1016/j.resuscitation.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 17.Hsu C.H., Chao T.H., Tsai W.C. Intracardiac thrombosis in multiple chambers and descending aorta manifested as systemic and pulmonary thromboembolism. Echocardiography. 2005;22:671–674. doi: 10.1111/j.1540-8175.2005.40039.x. [DOI] [PubMed] [Google Scholar]
- 18.Willis S.L., Welch T.S., Scally J.P. Impending paradoxical embolism presenting as a pulmonary embolism, transient ischemic attack, and myocardial infarction. Chest. 2007;132:1358–1360. doi: 10.1378/chest.07-0100. [DOI] [PubMed] [Google Scholar]
- 19.Ward R., Jones D., Haponik E.F. Paradoxical embolism: an underrecognized problem. Chest. 1995;108:549–558. doi: 10.1378/chest.108.2.549. [DOI] [PubMed] [Google Scholar]
- 20.Nendaz M., Sarasin F.P., Bogousslavsky J. How to prevent stroke recurrence in patients with patent foramen ovale: anticoagulants, antiaggregants, foramen closure, or nothing? Eur Neurol. 1997;37:199–204. doi: 10.1159/000117442. [DOI] [PubMed] [Google Scholar]
- 21.Khairy P., O'Donnell C.P., Landzberg M.J. Transcatheter closure versus medical therapy of patent foramen ovale and presumed paradoxical thromboemboli: a systematic review. Ann Intern Med. 2003;139:753–760. doi: 10.7326/0003-4819-139-9-200311040-00010. [DOI] [PubMed] [Google Scholar]
- 22.Travis J.A., Fuller S.B., Ligush J., Jr., Plonk G.W., Jr., Geary R.L., Hansen K.J. Diagnosis and treatment of paradoxical embolus. J Vasc Surg. 2001;34:860–865. doi: 10.1067/mva.2001.118815. [DOI] [PubMed] [Google Scholar]
- 23.Decousus H., Leizorovicz A., Parent F. A clinical trial of vena filters in the prevention of pulmonary embolism in patients with proximal deep-vein thrombosis. Prevention du Risque d’Embolie Pulmonaire par Interruption Cave Study Group. N Engl J Med. 1998;338:409–415. doi: 10.1056/NEJM199802123380701. [DOI] [PubMed] [Google Scholar]
- 24.Homma S., Sacco R.L., Di Tullio M.R. PFO in Cryptogenic Stroke Study (PICSS) Investigators. Effect of medical treatment in stroke patients with patent foramen ovale: patent foramen ovale in Cryptogenic Stroke Study. Circulation. 2002;105:2625–2631. doi: 10.1161/01.cir.0000017498.88393.44. [DOI] [PubMed] [Google Scholar]


