Abstract
Atherosclerosis is a chronic, progressive disease which eventually leads to coronary heart disease (CHD), ischemic stroke and other atherosclerotic cardiovascular disease (ASCVD). Numerous studies have demonstrated an atherogenic role of oxidized low-density lipoprotein (ox-LDL) in the progression of ASCVD. This article briefly reviews the atherogenic mechanism of ox-LDL, the methods of measuring ox-LDL in the circulation, effect of medical therapy and life-style modification on ox-LDL level, and the association between circulating ox-LDL and atherosclerosis, including clinical ASCVD events and subclinical atherosclerosis, in observational studies.
Keywords: Oxidized low density lipoprotein, Atherosclerosis, Cardiovascular disease, Subclinical atherosclerosis, Review
Introduction
Atherosclerosis is a chronic, progressive pathological process that involves vascular endothelial injury, lipid infiltration, macrophage activation, vascular smooth muscle cell proliferation, thrombosis, and inflammatory immune response, eventually leading to coronary heart disease (CHD), ischemic stroke, and other atherosclerotic cardiovascular diseases (ASCVDs). Among the pathogenesis of atherosclerosis, lipid deposition has been most extensively studied. It is well known that low-density lipoprotein (LDL), the major carrier of cholesterol, accumulates in the intima and stimulates the expression of adhesion molecules and chemoattractants on the surface of endothelial cells, activating the adhesion of circulating monocytes to the endothelium. After adhesion, the monocytes migrate into the intima, differentiate into macrophages, internalize and accumulate the cholesterol in cells, and eventually become foam cells that are characteristic of atherosclerosis.1 A study by Goldstein and Brown demonstrated that LDL receptors (LDL-Rs), which identify and internalize native LDL, played a vital role in the cellular metabolism of cholesterol.2 However, it was found that cholesterol accumulation also occurred in patients with familial hypercholesterolemia whose LDL-Rs were genetically impaired.2 Moreover, an in vitro experiment revealed that when incubated in high concentrations of native low-density lipoprotein cholesterol (LDL-C), macrophages did not convert to foam cells, which suggested that LDL-Rs expressed on macrophages may be down regulated in an environment of high LDL-C concentration.3 These observations implied that foam cells can be formed through the uptake of cholesterol by receptors other than LDL-Rs.
In 1984, Steinbrecher et al4 found that incubation of LDL with endothelium cells or smooth muscle cells could convert LDL into a modified form that is recognized by a novel receptor, increasing the rate of cholesterol uptake by macrophages. Oxidized LDL (ox-LDL) is the major modified form of native LDL because LDL particles are extremely sensitive to oxidative damage: each native LDL particle contains approximately 700 molecules of phospholipids, 600 molecules of free cholesterol, 1600 molecules of cholesterol ester, 185 molecules of triglycerides, and an apolipoprotein B-100 (apoB-100) protein with 4536 amino acids.1 Both the lipids and the proteins can be oxidized. The oxidation of native LDL is a complex process that can be divided into three stages. During the initial stage, known as the lag phase, endogenous antioxidants such as vitamin E are consumed. During the proliferation phase, polyunsaturated fatty acids (PUFAs) in the lipids of the LDL particles can be rapidly oxidized to fatty acid fragments, oxidized phospholipids (ox-PL), and oxygen free radicals. During the decomposition stage, fatty acid fragments are converted to aldehyde, which can interact with the lysine residue of apoB-100 to form new epitopes. Importantly, these new epitopes inhibit the ability of LDL to bind to the LDL-Rs expressed on macrophages.5
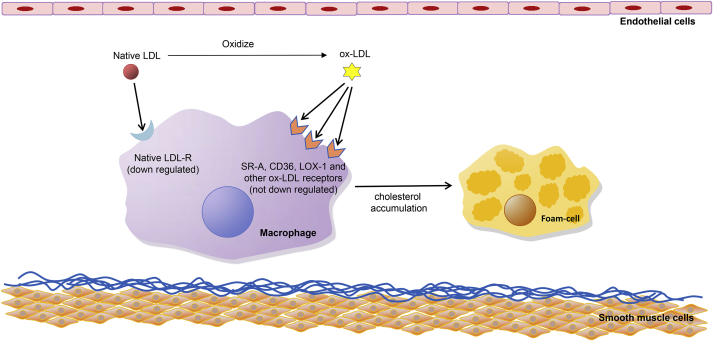
The most important atherogenic effect of ox-LDL is that this modification of native LDL shifts the recognition and internalization of the lipoprotein from the LDL-Rs to novel receptors termed as scavenger receptors (SRs).6 SRs are the cell surface receptors expressed on macrophages and other vascular cells that recognize and internalize ox-LDL rather than native LDL; these include SR-A, cluster differentiating 36 (CD36), SR-BI, cluster differentiating 68 (CD68), scavenger receptor for phosphatidylserine and oxidized lipoprotein (SR-PSOX), and lectin-like oxidized LDL receptor-1 (LOX-1), among others.7 Among these, SR-A binding to oxidized lysine residue of apoB-100 and CD36 binding to ox-PL are responsible for most of the ox-LDL uptake by macrophages in vitro.8, 9 The most important point is that contrary to LDL-R, SRs are not down regulated by elevated levels of intracellular cholesterol (Fig. 1).4 Uptake of ox-LDL by macrophages through SRs leads to remarkable cholesterol accumulation, converting macrophages to foam cells and promoting the development of atherosclerotic lesions.10
Fig. 1.
Mechanisms of ox-LDL uptake by macrophages. Native LDL can hardly induce foam-cell formation because of the down regulation of LDL-R. Ox-LDL induces cholesterol accumulation in macrophages through rapid uptake by the SRs, which are not down regulated in response to an increase intracellular cholesterol. LDL: low-density lipoprotein; ox-LDL: oxidized low-density lipoprotein; LDL-R: low-density lipoprotein receptor; SR-A: scavenger receptor A; CD36: cluster differentiating 36; LOX-1: lectin-like oxidized low density lipoprotein receptor 1.
To date, three monoclonal antibodies have been developed for determination of circulating ox-LDL by enzyme linked immunosorbent assay (ELISA): ox-LDL-4E6,11 ox-LDL-E06,12 and ox-LDL-DLH3 antibody.13 Ox-LDL-4E6 was the first to be established and widely used. It can directly recognize the epitope that is generated after modification of lysine residues in apoB-100 by aldehydes, while DLH3 and E06 antibody recognize oxidized phosphatidylcholine (ox-PC) generated after modification of phospholipids containing PUFAs.14 However, ox-LDL with fewer than 60 lysine modifications cannot be detected using the ox-LDL-4E6 antibody.14
Association between circulating ox-LDL and atherosclerosis
We searched PubMed and the Cochrane Library for observational epidemiological studies on associations between circulating ox-LDL and atherosclerosis. The key words “oxidized low density lipoprotein”, “cardiovascular disease,” and “atherosclerosis” were used to build our search strategy. Thus far, 21 case–control or prospective cohort studies15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35 have reported the association between circulating ox-LDL and atherosclerosis in PubMed and Cochrane Library, including 19 studies15, 16, 17, 18, 19, 21, 22, 23, 24, 25, 26, 28, 29, 30, 31, 32, 33, 34, 35 on clinical ASCVD events and three studies16, 20, 27 on subclinical atherosclerosis (one study reported the data for both clinical and subclinical atherosclerosis16).
Association between circulating ox-LDL and clinical ASCVD events
Among six hospital-based cohort studies,15, 18, 23, 28, 32, 33 two studies found that after adjusting for LDL-C, increased level of circulating ox-LDL was significantly associated with the risk of clinical ASCVD events; one study reported that among 1371 patients with acute myocardial infarction (AMI), the hazard ratio (HR) of death due to cardiovascular diseases (CVD), or the occurrence of unstable angina pectoris (UAP) or myocardial infarction (MI) at the 6-month follow-up was 1.66 (95% confidence interval (CI): 1.07–2.57) in patients with the highest tertile of circulating ox-LDL, compared with those with the lowest tertile.23 Another study showed that in 246 patients with more than 50% stenosis, the risk of major adverse coronary events (MACE) increased by 215% (95% CI: 47%–576%) for patients with the highest quartile, compared with those with the lowest quartile.33 One hospital-based cohort study without adjustment of LDL-C also reported a significant association between circulating ox-LDL and clinical ASCVD events: among 425 patients with acute coronary syndrome (ACS), the HR of AMI recurrence or ACS-related death at the 5-year follow-up was 2.88 (95% CI: 1.93–4.32) for 1 mmol/L increase in circulating ox-LDL.15 However, the remaining three studies did not find any significant association between circulating ox-LDL and clinical ASCVD events, regardless of adjustment of LDL-C in multivariate models. A study by Finckh reported that the HR of MACE among 118 patients with rheumatoid arthritis (RA) was 1.90 (95% CI: 0.69–5.29) for every 1 U/ml increase in circulating ox-LDL.18 A study by Braun et al32 demonstrated that after adjusting for LDL-C in a model, the HR of MACE was 0.85 (95% CI: 0.63–1.15) among 687 patients with coronary artery disease (CAD) whose circulating ox-LDL level was higher than the median, while a study by Johnston et al28 reported similar result without adjustment of LDL-C in their model (odds ratio [OR] = 1.71, 95% CI: 0.84–3.46).
Two of the three community-based cohort studies revealed a significant association between increased level of circulating ox-LDL at baseline and high risk of occurrence of clinical ASCVD events in the general population.16, 25 One study that enrolled 2793 subjects without ASCVD at baseline found that after adjusting for LDL-C in the model, the HR of occurrence of ACS at the 10-year follow-up was 1.70 (95% CI: 1.02–2.84) for every 1 unit increase in log-transformed circulating ox-LDL level.16 Another study found similar results in 765 participants free of ASCVD at baseline. The HR for occurrence of CVD was 2.4 (95% CI: 1.3–4.3) in patients with the highest tertile of circulating ox-LDL compared with those with the lowest tertile, after adjusting for LDL-C.25 However, one study that enrolled 1025 older community-dwelling individuals did not find a significant association between baseline ox-LDL levels and CVD-related deaths at a 9-year follow-up after LDL-C adjustment in a multivariate model (HR = 1.17 for the highest tertile versus the lowest tertile; 95% CI: 0.79–1.93).17
Four case–control studies21, 24, 31, 35 including one nested case–control study21 revealed that after adjustment of LDL-C, higher level of circulating ox-LDL was significantly associated with clinical ASCVD events: the OR ranged from 1.67 to 5.03 in patients with the highest quantile of circulating ox-LDL compared with those with the lowest quantile21, 24, 35; the OR was 2.93 (95% CI: 2.89–2.98) for every 1 U/L increase in circulating ox-LDL level.31 The four case–control studies that did not adjust LDL-C in multivariate models also reported that circulating ox-LDL was associated with clinical ASCVD events19, 29, 30, 34: the OR ranged from 2.15 to 20.60 in patients with the highest quantile of circulating ox-LDL compared with those with the lowest quantile19, 30, 34 and the OR was 1.05 (95% CI: 1.02–1.09) for every 1 U/L increase in circulating ox-LDL.29 The remaining two studies did not find a significant association between circulating ox-LDL and clinical ASCVD events: the study by Wu et al26 reported that after LDL-C adjustment, the OR for the highest quintile as compared with the lowest quintile was 1.64 (95% CI: 0.83–3.25) and 1.53 (95% CI: 0.65–3.57), respectively, in men and women; the study by Drogan et al,22 which did not adjust LDL-C in a multivariate model, demonstrated an OR of 1.31 (95% CI: 0.56–3.05) in patients with the highest quartile of circulating ox-LDL, compared with those with the lowest quartile.
Association between circulating ox-LDL and subclinical atherosclerosis
The relationship between circulating ox-LDL and subclinical atherosclerosis was observed in one case–control study20 and two community-based cohort studies.16, 27 The study by Tsimikas et al27 was a community-based cohort study consisting of 765 participants without ASCVD at baseline. After adjusting for LDL-C, the OR of new-onset carotid plaques at the 5-year follow-up was 1.44 (95% CI: 1.06–1.96) for every 1 unit increase in log-transformed circulating ox-LDL levels. Significant association between ox-LDL and carotid plaques was also found in one case–control study (OR = 1.02 for every 1 U/L increase in circulating ox-LDL, 95% CI: 1.01–1.03).20 However, one community-based cohort study that enrolled 1427 subjects found a different result. After LDL-C adjustment, the OR of subclinical atherosclerosis, which was compositely defined as top-quintile carotid intima media thickness (IMT) or ankle-brachial index (ABI) < 0.9 at the 10-year follow-up, was 0.96 (95% CI: 0.67–1.37) for per unit increase in log-transformed circulating ox-LDL level.16
The different association of circulating ox-LDL with clinical ASCVD events and subclinical atherosclerosis may be explained by two main reasons. Firstly, the inconsistent and composite definition of subclinical atherosclerosis in different studies may lead to different conclusions. Secondly, fewer studies were available on subclinical atherosclerosis than on clinical ASCVD events, owing to the difficulty in measuring subclinical atherosclerosis in a large sample size. Although some cross-sectional studies investigated the association between circulating ox-LDL and carotid IMT, they are less powerful in etiological investigations, given the nature of the study design.36, 37, 38, 39
Apart from ASCVD, circulating ox-LDL was also reported to be associated with diabetes,40, 41 metabolic syndrome,42 obesity,41 and fatty liver,43 which may increase the risk of ASCVD.
Effect of medical therapy and lifestyle modifications on ox-LDL level
Several studies have focused on the strategies for reducing circulating ox-LDL levels. A 12-week healthy-life exercise program was reported to reduce circulating ox-LDL levels and carotid IMT in obese elderly women.44 Tavridou et al45 reported that treatment with simvastatin 40 mg/d for 12 weeks significantly reduced circulating ox-LDL levels by 6.5% ± 5.2% and 31.1% ± 5.0% in hypercholesterolemia subjects with and without CHD, respectively. Likewise, a study by Aydin et al46 showed that both rosuvastatin 20 mg/d and atorvastatin 80 mg/d had comparable effects on circulating ox-LDL levels. A study by Ndrepepa et al47 demonstrated that compared to patients with CAD who did not receive statins, patients who received statins had lower levels of circulating ox-LDL and less severe CAD. Similar results were found in studies that enrolled patients with acute ischemic stroke (AIS). A study by Tsai et al48 revealed that statins reduced plasma ox-LDL levels and improved the prognosis of patients with AIS. Another study by Kei et al49 showed that various types of statins differed in their abilities to reduce circulating ox-LDL levels. It was demonstrated that compared with patients who received add-on fenofibrate treatment, the reduction of circulating ox-LDL levels was significant in the groups of patients who received add-on extended release nicotinic acid/laropiprant (ER-NA/LRPT) and rosuvastatin monotherapy. It is important to note that none of the above studies were randomized controlled trials that focused on ox-LDL. Meanwhile, with the discovery of specific receptors such as the LOX-1 for ox-LDL, novel therapeutic targets are promising for regulating ox-LDL levels.50
Conclusion
Since 1984, when ox-LDL was first described, many studies on the relationship between ox-LDL and atherosclerosis have been published. Over the past decade, extensive basic and epidemiologic research evidence has been gathered on the role of ox-LDL in the progression of atherosclerosis. However, it is unclear whether the effects of ox-LDL vary at different stages of atherosclerosis. The optimal treatment strategies for reducing circulating ox-LDL levels and the eventual risk of developing a cardiovascular disease also remain elusive. Therefore, further research addressing these aspects is warranted.
Conflict of interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81541096) and the National Science & Technology Pillar Program during the 13th Five-Year Plan Period of China (2016YFC0900902).
Edited by Wei-Zhu Liu
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Linton M.F., Yancey P.G., Davies S.S. The role of lipids and lipoproteins in atherosclerosis. In: De Groot L.J., Beck-Peccoz P., Chrousos G., editors. Endotext, South Dartmouth (MA) 2000. [Google Scholar]
- 2.Brown M.S., Goldstein J.L. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986;232:34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein J.L., Ho Y.K., Basu S.K., Brown M.S. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci U S A. 1979;76:333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steinbrecher U.P., Parthasarathy S., Leake D.S., Witztum J.L., Steinberg D. Modification of low density lipoprotein by endothelial cells involves lipid peroxidation and degradation of low density lipoprotein phospholipids. Proc Natl Acad Sci U S A. 1984;81:3883–3887. doi: 10.1073/pnas.81.12.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinbrecher U.P. Oxidation of human low density lipoprotein results in derivatization of lysine residues of apolipoprotein B by lipid peroxide decomposition products. J Biol Chem. 1987;262:3603–3608. [PubMed] [Google Scholar]
- 6.Jürgens G., Hoff H.F., Chisolm G.M., 3rd, Esterbauer H. Modification of human serum low density lipoprotein by oxidation–characterization and pathophysiological implications. Chem Phys Lipids. 1987;45:315–336. doi: 10.1016/0009-3084(87)90070-3. [DOI] [PubMed] [Google Scholar]
- 7.Trpkovic A., Resanovic I., Stanimirovic J. Oxidized low-density lipoprotein as a biomarker of cardiovascular diseases. Crit Rev Clin Lab Sci. 2015;52:70–85. doi: 10.3109/10408363.2014.992063. [DOI] [PubMed] [Google Scholar]
- 8.Di Pietro N., Formoso G., Pandolfi A. Physiology and pathophysiology of oxLDL uptake by vascular wall cells in atherosclerosis. Vasc Pharmacol. 2016;84:1–7. doi: 10.1016/j.vph.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 9.Kunjathoor V.V., Febbraio M., Podrez E.A. Scavenger receptors class A-I/II and CD36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J Biol Chem. 2002;277:49982–49988. doi: 10.1074/jbc.M209649200. [DOI] [PubMed] [Google Scholar]
- 10.Witztum J.L., Steinberg D. The oxidative modification hypothesis of atherosclerosis: does it hold for humans? Trends Cardiovasc Med. 2001;11:93–102. doi: 10.1016/s1050-1738(01)00111-6. [DOI] [PubMed] [Google Scholar]
- 11.Holvoet P., Donck J., Landeloos M. Correlation between oxidized low density lipoproteins and von Willebrand factor in chronic renal failure. Thromb Haemost. 1996;76:663–669. [PubMed] [Google Scholar]
- 12.Itabe H., Yamamoto H., Imanaka T. Sensitive detection of oxidatively modified low density lipoprotein using a monoclonal antibody. J Lipid Res. 1996;37:45–53. [PubMed] [Google Scholar]
- 13.Palinski W., Hörkkö S., Miller E. Cloning of monoclonal autoantibodies to epitopes of oxidized lipoproteins from apolipoprotein E-deficient mice. Demonstration of epitopes of oxidized low density lipoprotein in human plasma. J Clin Investig. 1996;98:800–814. doi: 10.1172/JCI118853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsimikas S. Measures of oxidative stress. Clin Lab Med. 2006;26:571–590. doi: 10.1016/j.cll.2006.06.004. v-vi. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y.C., Tang Y., Chen Y. Oxidized low-density lipoprotein and C-reactive protein have combined utility for better predicting prognosis after acute coronary syndrome. Cell Biochem Biophys. 2014;68:379–385. doi: 10.1007/s12013-013-9718-1. [DOI] [PubMed] [Google Scholar]
- 16.Gómez M., Vila J., Elosua R. Relationship of lipid oxidation with subclinical atherosclerosis and 10-year coronary events in general population. Atherosclerosis. 2014;232:134–140. doi: 10.1016/j.atherosclerosis.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 17.Zuliani G., Morieri M.L., Volpato S. Determinants and clinical significance of plasma oxidized LDLs in older individuals. A 9 years follow-up study. Atherosclerosis. 2013;226:201–207. doi: 10.1016/j.atherosclerosis.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Finckh A., Courvoisier D.S., Pagano S. Evaluation of cardiovascular risk in patients with rheumatoid arthritis: do cardiovascular biomarkers offer added predictive ability over established clinical risk scores? Arthr Care Res (Hoboken) 2012;64:817–825. doi: 10.1002/acr.21631. [DOI] [PubMed] [Google Scholar]
- 19.Huang Y., Hu Y., Mai W. Plasma oxidized low-density lipoprotein is an independent risk factor in young patients with coronary artery disease. Dis Mark. 2011;31:295–301. doi: 10.3233/DMA-2011-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fang R., Zhang N., Wang C. Relations between plasma ox-LDL and carotid plaque among Chinese Han ethnic group. Neurol Res. 2011;33:460–466. doi: 10.1179/016164111X13007856083927. [DOI] [PubMed] [Google Scholar]
- 21.Tsimikas S., Mallat Z., Talmud P.J. Oxidation-specific biomarkers, lipoprotein(a), and risk of fatal and nonfatal coronary events. J Am Coll Cardiol. 2010;56:946–955. doi: 10.1016/j.jacc.2010.04.048. [DOI] [PubMed] [Google Scholar]
- 22.Drogan D., Weikert C., Dierkes J. Plasma gamma-glutamyltransferase, cysteinyl-glycine, and oxidized low-density lipoprotein: a pathway associated with myocardial infarction risk? Arterioscler Thromb Vasc Biol. 2010;30:2053–2058. doi: 10.1161/ATVBAHA.110.209346. [DOI] [PubMed] [Google Scholar]
- 23.Gómez M., Valle V., Arós F. Oxidized LDL, lipoprotein (a) and other emergent risk factors in acute myocardial infarction (FORTIAM study) Rev Esp Cardiol. 2009;62:373–382. doi: 10.1016/s1885-5857(09)71664-0. [DOI] [PubMed] [Google Scholar]
- 24.Lu C., Gao Y., Zhou H., Tian H. The relationships between PON1 activity as well as oxLDL levels and coronary artery lesions in CHD patients with diabetes mellitus or impaired fasting glucose. Coron Artery Dis. 2008;19:565–573. doi: 10.1097/MCA.0b013e3283109206. [DOI] [PubMed] [Google Scholar]
- 25.Kiechl S., Willeit J., Mayr M. Oxidized phospholipids, lipoprotein(a), lipoprotein-associated phospholipase A2 activity, and 10-year cardiovascular outcomes: prospective results from the Bruneck study. Arterioscler Thromb Vasc Biol. 2007;27:1788–1795. doi: 10.1161/ATVBAHA.107.145805. [DOI] [PubMed] [Google Scholar]
- 26.Wu T., Willett W.C., Rifai N., Shai I., Manson J.E., Rimm E.B. Is plasma oxidized low-density lipoprotein, measured with the widely used antibody 4E6, an independent predictor of coronary heart disease among U.S. men and women? J Am Coll Cardiol. 2006;48:973–979. doi: 10.1016/j.jacc.2006.03.057. [DOI] [PubMed] [Google Scholar]
- 27.Tsimikas S., Kiechl S., Willeit J. Oxidized phospholipids predict the presence and progression of carotid and femoral atherosclerosis and symptomatic cardiovascular disease: five-year prospective results from the Bruneck study. J Am Coll Cardiol. 2006;47:2219–2228. doi: 10.1016/j.jacc.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 28.Johnston N., Jernberg T., Lagerqvist B., Siegbahn A., Wallentin L. Oxidized low-density lipoprotein as a predictor of outcome in patients with unstable coronary artery disease. Int J Cardiol. 2006;113:167–173. doi: 10.1016/j.ijcard.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 29.Anselmi M., Garbin U., Agostoni P. Plasma levels of oxidized-low-density lipoproteins are higher in patients with unstable angina and correlated with angiographic coronary complex plaques. Atherosclerosis. 2006;185:114–120. doi: 10.1016/j.atherosclerosis.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 30.Meisinger C., Baumert J., Khuseyinova N., Loewel H., Koenig W. Plasma oxidized low-density lipoprotein, a strong predictor for acute coronary heart disease events in apparently healthy, middle-aged men from the general population. Circulation. 2005;112:651–657. doi: 10.1161/CIRCULATIONAHA.104.529297. [DOI] [PubMed] [Google Scholar]
- 31.Dominguez-Rodriguez A., Abreu-Gonzalez P., Garcia-Gonzalez M., Ferrer-Hita J., Vargas M., Reiter R.J. Elevated levels of oxidized low-density lipoprotein and impaired nocturnal synthesis of melatonin in patients with myocardial infarction. Atherosclerosis. 2005;180:101–105. doi: 10.1016/j.atherosclerosis.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 32.Braun S., Ndrepepa G., von Beckerath N. Lack of association between circulating levels of plasma oxidized low-density lipoproteins and clinical outcome after coronary stenting. Am Heart J. 2005;150:550–556. doi: 10.1016/j.ahj.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 33.Shimada K., Mokuno H., Matsunaga E. Circulating oxidized low-density lipoprotein is an independent predictor for cardiac event in patients with coronary artery disease. Atherosclerosis. 2004;174:343–347. doi: 10.1016/j.atherosclerosis.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 34.Weinbrenner T., Cladellas M., Isabel Covas M. High oxidative stress in patients with stable coronary heart disease. Atherosclerosis. 2003;168:99–106. doi: 10.1016/s0021-9150(03)00053-4. [DOI] [PubMed] [Google Scholar]
- 35.Kugiyama K., Sugiyama S., Soejima H. Increase in plasma levels of oxidized low-density lipoproteins in patients with coronary spastic angina. Atherosclerosis. 2001;154:463–467. doi: 10.1016/s0021-9150(00)00494-9. [DOI] [PubMed] [Google Scholar]
- 36.Calmarza P., Trejo J.M., Lapresta C., López P. LDL oxidation and its association with carotid artery intima-media thickness and other cardiovascular risk factors in a sample of Spanish general population. Angiology. 2014;65:357–362. doi: 10.1177/0003319713488639. [DOI] [PubMed] [Google Scholar]
- 37.Kampus P., Kals J., Ristimäe T. Augmentation index and carotid intima-media thickness are differently related to age, C-reactive protein and oxidized low-density lipoprotein. J Hypertens. 2007;25:819–825. doi: 10.1097/HJH.0b013e328014952b. [DOI] [PubMed] [Google Scholar]
- 38.Wallenfeldt K., Fagerberg B., Wikstrand J., Hulthe J. Oxidized low-density lipoprotein in plasma is a prognostic marker of subclinical atherosclerosis development in clinically healthy men. J Intern Med. 2004;256:413–420. doi: 10.1111/j.1365-2796.2004.01402.x. [DOI] [PubMed] [Google Scholar]
- 39.Metso S., Loimaala A., Mercuri M.F. Circulating oxidized low-density lipoprotein and common carotid artery intima-media thickness in a random sample of middle-aged men. J Biomed Sci. 2004;11:356–361. doi: 10.1007/BF02254440. [DOI] [PubMed] [Google Scholar]
- 40.Nakhjavani M., Khalilzadeh O., Khajeali L. Serum oxidized-LDL is associated with diabetes duration independent of maintaining optimized levels of LDL-cholesterol. Lipids. 2010;45:321–327. doi: 10.1007/s11745-010-3401-8. [DOI] [PubMed] [Google Scholar]
- 41.Njajou O.T., Kanaya A.M., Holvoet P. Association between oxidized LDL, obesity and type 2 diabetes in a population-based cohort, the Health, Aging and Body Composition Study. Diabetes Metab Res Rev. 2009;25:733–739. doi: 10.1002/dmrr.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holvoet P., Lee D.H., Steffes M., Gross M., Jacobs D.R., Jr. Association between circulating oxidized low-density lipoprotein and incidence of the metabolic syndrome. JAMA. 2008;299:2287–2293. doi: 10.1001/jama.299.19.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaikkonen J.E., Kresanov P., Ahotupa M. Longitudinal study of circulating oxidized LDL and HDL and fatty liver: the Cardiovascular Risk in Young Finns Study. Free Radic Res. 2016;50:396–404. doi: 10.3109/10715762.2015.1133906. [DOI] [PubMed] [Google Scholar]
- 44.Park J.H., Park H., Lim S.T., Park J.K. Effects of a 12-week healthy-life exercise program on oxidized low-density lipoprotein cholesterol and carotid intima-media thickness in obese elderly women. J Phys Ther Sci. 2015;27:1435–1439. doi: 10.1589/jpts.27.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tavridou A., Efthimiadis A., Efthimiadis I., Paschalidou H. Antioxidant effects of simvastatin in primary and secondary prevention of coronary heart disease. Eur J Clin Pharmacol. 2006;62:485–489. doi: 10.1007/s00228-006-0097-z. [DOI] [PubMed] [Google Scholar]
- 46.Aydin M.U., Aygul N., Altunkeser B.B., Unlu A., Taner A. Comparative effects of high-dose atorvastatin versus moderate-dose rosuvastatin on lipid parameters, oxidized-LDL and inflammatory markers in ST elevation myocardial infarction. Atherosclerosis. 2015;239:439–443. doi: 10.1016/j.atherosclerosis.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 47.Ndrepepa G., Braun S., von Beckerath N. Oxidized low density lipoproteins, statin therapy and severity of coronary artery disease. Clin Chim Acta. 2005;360:178–186. doi: 10.1016/j.cccn.2005.04.032. [DOI] [PubMed] [Google Scholar]
- 48.Tsai N.W., Lee L.H., Huang C.R. Statin therapy reduces oxidized low density lipoprotein level, a risk factor for stroke outcome. Crit Care. 2014;18:R16. doi: 10.1186/cc13695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kei A., Tellis C., Liberopoulos E., Tselepis A., Elisaf M. Effect of switch to the highest dose of rosuvastatin versus add-on-statin fenofibrate versus add-on-statin nicotinic acid/laropiprant on oxidative stress markers in patients with mixed dyslipidemia. Cardiovasc Ther. 2014;32:139–146. doi: 10.1111/1755-5922.12072. [DOI] [PubMed] [Google Scholar]
- 50.Yoshimoto R., Fujita Y., Kakino A., Iwamoto S., Takaya T., Sawamura T. The discovery of LOX-1, its ligands and clinical significance. Cardiovasc Drugs Ther. 2011;25:379–391. doi: 10.1007/s10557-011-6324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]