Abstract
Objective
To compare the efficacy of axillary radiotherapy (ART) with that of completion axillary lymph node dissection (cALND) in clinically node-negative breast cancer patients with a positive sentinel lymph node.
Methods
A literature search was performed in PubMed, EMBASE and Cochrane Library by using the search terms “breast cancer”, “sentinel lymph node biopsy”, “axillary radiotherapy” or “regional node irradiation” for articles published between 2004 and 2016. Only randomized controlled trials that included patients with positive sentinel nodes were included in the meta-analysis.
Results
Two randomized controlled trials and three retrospective studies were identified. The reported overall survival rate (hazard ratio [HR] = 1.09, 95% confidence interval [CI]: 0.75–1.43, P = 0.365), disease-free survival rate (HR = 1.01, 95% CI: 0.58–1.45, P = 0.144), and axillary recurrence rate (1.2% and 0.4%, and 1.3% and 0.8%, respectively) were similar in both groups. The absence of knowledge on the extent of nodal involvement in the ART group appeared to have no major impact on the administration of adjuvant systemic therapy.
Conclusions
ART is not inferior to cALND in the patients with clinically node-negative breast cancer who had a positive sentinel lymph node. Information obtained by using cALND after SLNB may have no major impact on the administration of adjuvant systemic therapy.
Keywords: Breast cancer, Sentinel lymph node biopsy, Completion axillary lymph node dissection, Axillary radiotherapy, Meta-analysis
Introduction
Sentinel lymph node (SLN) biopsy (SLNB) is accepted as an alternative method to evaluate axillary lymph node status in clinically node-negative breast cancer.1 Completion axillary lymph node dissection (cALND) is the standard of care for patients with a positive SLNB. A cALND provides additional prognostic information, optimizes regional control and potentially improves overall survival (OS).2, 3 However, some patients do not need cALND because of a low risk of residual disease or recurrence. In 15–20% of cases, a cALND leads to long-term complications such as pain, paresthesia due to intercostobrachial nerve injury, impairment of shoulder function, or lymphedema.4, 5 The 2009 St. Gallen Consensus Panel did not recommend the use of cALND in patients with SLN-detected micrometastases or isolated tumor cells, and those who had small and well-differentiated tumors.6
Axillary radiotherapy (ART) is a possible alternative for cALND. Some retrospective studies examined the use of ART to decrease the rate of regional failure in node-positive disease.7, 8, 9 However, it is unclear if it could be used as a therapeutic substitute for cALND in patients with low burden of axillary disease. A prospective study was conducted at the Massachusetts General Hospital and Brigham and Women's Hospital in Boston between 2000 and 2004 to examine breast plus axillary radiotherapy after positive SLNB.10 Forty-eight of the 73 patients in the study had SLN macrometastasis, but only one had more than one positive SLN. With a median follow-up of 32 months, one patient had axillary failure 17 months after treatment; she was disease-free 2.5 years after salvage dissection. The AMAROS trial11 was a prospective randomized controlled trial (RCT) involving patients with cT1-2N0 breast cancer up to 5 cm and clinically node-negative axilla who underwent either breast conservation or mastectomy with SLNB. Of the patients with positive lymph nodes, 744 received cALND and 681 received ART. After 5 years of follow-up, the axillary recurrence rate (ARR) was lower in the cALND than in the ART group. No significant differences in disease-free survival (DFS) rate and OS rate were found between the two groups. The incidence rate of lymphedema in the cALND group was twice that in the ART group.12 The OTOASOR trial,13 another prospective RCT, conducted between August 2002 and June 2009, involved 244 patients who were randomized to undergo cALND and 230 patients who were randomized to undergo SLNB plus ART. The mean length of follow-up was 70 months, and the ARRs were 1.6% and 1.7% in the cALND and ART groups, respectively (P < 0.05). The 5.8-year OS rates were 84.9% and 91.2%, and the 5-year DFS rates were 79.9% and 85.6% in the cALND and ART groups, respectively. The 5-year follow-up data of the OTOASOR trial suggest that ART without cALND does not increase the risk of axillary failure in patients with positive SLNs.
The aim of this review was to compare the efficacy of ART with that of axillary lymph node dissection (ALND) in clinically node-negative breast cancer patients with a positive SLN.
Methods
Literature search
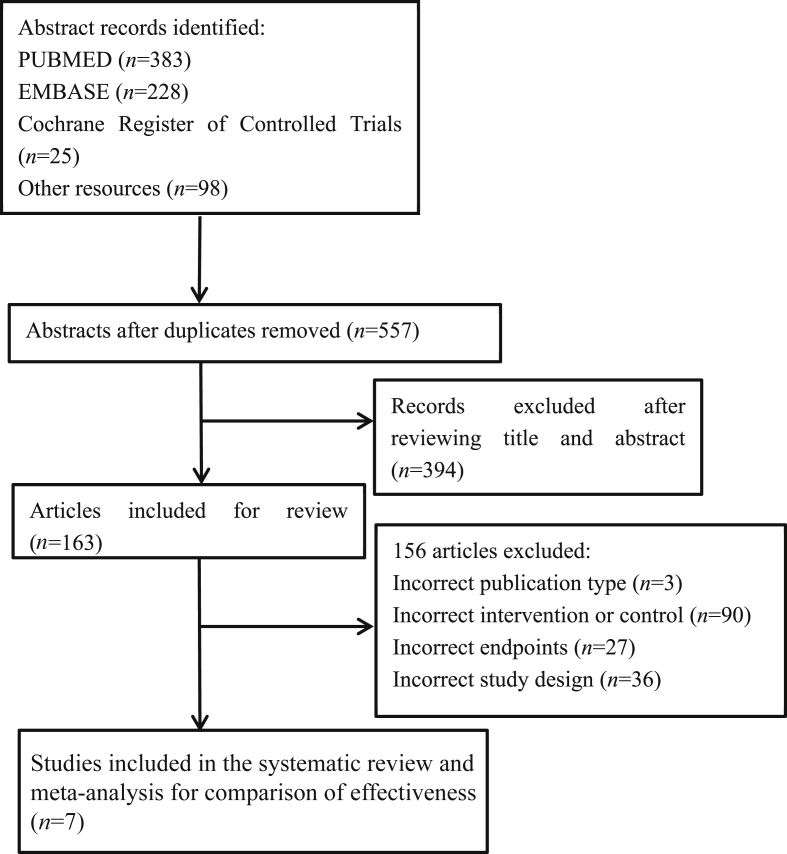
A literature search was performed in PubMed, EMBASE, and the Cochrane Library by using the search terms “breast cancer”, “sentinel lymph node biopsy”, and “axillary radiotherapy or regional node irradiation” from 2004 to 2016. Articles published in English alone were considered. The complete search strategy is presented in Fig. 1 according to the PRISMA statement.14 The search was performed independently by two reviewers who selected potentially relevant papers based on the title and abstract.
Fig. 1.
Flow diagram according to PRISMA statement.
Review inclusion and exclusion criteria
Two persons independently reviewed abstracts and full-text articles. Eligibility criteria for the studies were defined a priori and are presented in Table 1. Studies whose populations had positive SLNs and those that compared ART with cALND were included. Only RCTs were included in the meta-analysis. A secondary analysis included some observational studies. Studies that included negative SLNs or SLNB alone were excluded from this study. Several studies such as the ACOSOG Z0011 trial, which failed to determine how the positive SLNB was impacting radiation practice patterns, were excluded.
Table 1.
Eligibility criteria for the studies included in the meta-analysis.
| Items | Eligibility |
|---|---|
| Population | Women with invasive breast cancer and positive sentinel lymph node who underwent breast-conserving therapy or mastectomy |
| Intervention | SLNB plus ART |
| Control | SLNB plus cALND |
| Outcomes | Disease-free survival and overall survival were the primary outcomes. The secondary outcomes were axillary recurrence rates and systemic treatment |
| Timing | For effectiveness: study duration of at least 1 year |
| Study design | RCTs, comparative observational studies, and systematic reviews with meta-analysis |
SLNB: sentinel lymph node biopsy; ART: axillary radiotherapy; cALND: completion axillary lymph node dissection; RCTs: randomized controlled trials.
Study selection and quality assessment
The RCTs were assessed with a score assigned for each item identified according to the Consolidated Standards of Reporting Trials (CONSORT) checklist.15 The studies were assessed for risk of bias according to the Cochrane Handbook for Systematic Reviews of Interventions16 (Table 2).
Table 2.
Study quality.
| Author | Random Sequence generation | Allocation concealment | Blinding | Blinding of outcome | Incomplete outcome data | Selective reporting | CONSORT score |
|---|---|---|---|---|---|---|---|
| Donker et al12 | Yes | No | No | No | No | Yes | 24 |
| Sávolt et al17 | Yes | No | No | No | No | Yes | 24 |
CONSORT: Consolidated Standards of Reporting Trials.
Outcome measures
The primary outcome measures for this study were OS and DFS, reported as hazard ratios (HRs) with confidence intervals (CIs), and overall percentage. The HRs for DFS and OS in the two studies by Donker et al12 and Sávolt et al17 were estimated by using a method described by Tierney et al.18
Statistical analyses
The statistical software Stata 12.0 (StataCorp, College Station, TX, USA) was used for data analysis. The outcomes of OS and DFS were included in the meta-analysis. For each meta-analysis, we conducted a test of heterogeneity and applied a random or a fixed-effects model.
Heterogeneity was assessed by using the following methods: (1) observation of forest plots to ensure overlap of CIs. (2) Q examination: when the P value was >0.1, heterogeneity was assessed as insignificant and combining studies were deemed acceptable. (3) Examination of I2 value: I2 > 50% was considered to represent moderate heterogeneity and a random-effects model was applied.
Results are presented with forest plots, in which the estimates of the HRs of all single studies and their combined estimates are visualized. Horizontal bars indicate the amount of variation (95% CIs of the parameter estimates).
Results
In the literature search, 557 relevant abstracts were identified. We retrieved 7 full-text articles for a more detailed examination. Fig. 1 depicts the results of the search and the study selection process. Two RCTs met our eligibility criteria for meta-analysis.12, 17 For the meta-analysis, 1899 breast cancer patients from two RCTs were identified. The patients' characteristics and outcomes in the two trials are shown in Table 3, Table 4. Radiotherapy target areas in the ART group included all 3 levels of the axilla and the supraclavicular fossa in both RCTs. We identified three additional retrospective studies (Table 5) to determine the ARR,19, 21 and two articles from the two RCTs were examined for the administration of systemic treatment (Table 6, Table 7).
Table 3.
Characteristics of the included randomized trials.
| Study | Study design | Recruitment | Median follow-up (months) | n (ART/cALND) | ART group mean age (years) | cALND group mean age (years) | Outcomes | Randomized method | Adjuvant treatment |
|---|---|---|---|---|---|---|---|---|---|
| Donker et al12 | RCT | 2001–2010 | 73.2 | 681/744 | 55 | 56 | OS, DFS, ARR | Computer generated allocation schedule | Most received systemic therapy |
| Sávolt et al17 | RCT | 2002–2009 | 43.3 | 230/244 | 55.2 | 54.7 | OS, DFS, ARR | Not mentioned | All received systemic therapy |
ART: axillary radiotherapy; cALND: completion axillary lymph node dissection; RCT: randomized controlled trial; OS: overall survival; DFS: disease-free survival; ARR: axillary recurrence rate.
Table 4.
Summary of differences in outcomes of ART vs. cALND.
| Study | OS | DFS | ARR | Lymphedema |
|---|---|---|---|---|
| Donker et al12 | 92.5% vs. 93.3% P = 0.34 |
82.7% vs. 86.9% P = 0.18 |
1.2% vs. 0.4% P = 0.09 |
10.8% vs. 23.2% P < 0.0001 |
| Sávolt et al17 | 97.0% vs. 94.3% P > 0.05 |
91.3% vs. 86.1% P > 0.05 |
1.3% vs. 0.8% P > 0.05 |
Not assessed |
ART: axillary radiotherapy; cALND: completion axillary lymph node dissection; OS: overall survival; DFS: disease-free survival; ARR: axillary recurrence rate.
Table 5.
Treatment and outcome data for retrospective studies included in this review.
Table 6.
Administration of adjuvant therapy according to treatment groups, n (%).
| Therapy | Straver et al22 |
Sávolt et al23 |
||||
|---|---|---|---|---|---|---|
| cALND (n = 300) | ART (n = 266) | P | cALND (n = 244) | ART (n = 230) | P | |
| CT | 175 (58.3) | 162 (60.9) | 0.296 | 190 (77.9) | 159 (69.1) | 0.020 |
| ET | 235 (78.3) | 203 (76.3) | 0.318 | 213 (87.3) | 204 (88.7) | 0.372 |
| CT + ET | 140 (46.7) | 123 (46.2) | 0.434 | 159 (65.2) | 133 (57.8) | 0.061 |
| Trastuzumab | – | – | – | 6 (2.5) | 13 (5.7) | 0.061 |
| RT (breast/chest wall) | 257 (85.7) | 237 (89.1) | 0.136 | 232 (95.1) | 208 (90.4) | 0.115 |
| RT (axillary/supraclavicular) | 15 (5.0) | 266 (100) | 0.000 | 76 (31.1) | 230 (100) | 0.000 |
cALND: complete axillary lymph node dissection; ART: axillary radiotherapy; CT: chemotherapy; ET: endocrine therapy; RT: radiotherapy. –: not applicable.
Note: In the study of Straver et al,22 information about the adjuvant treatment is missing in 7 patients; 23 and 24 patients did not receive CT or ET in the cALND and ART group, respectively.
Table 7.
Use of adjuvant chemotherapy according to menopausal status and tumor size in the two treatment groups.
| Characteristics | Straver et al22 |
Sávolt et al23 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| cALND |
ART |
P | cALND |
ART |
P | |||||
| Total number of patients | Number of patients treated with CT (%) | Total number of patients | Number of patients treated with CT (%) | Total number of patients | Number of patients treated with CT (%) | Total number of patients | Number of patients treated with CT (%) | |||
| Total | 293 | 175 (59.7) | 266 | 162 (60.9) | 0.296 | 244 | 190 (77.9) | 230 | 159 (69.1) | 0.020 |
| Menopausal status | ||||||||||
| pre | 86 | 77 (89.5) | 93 | 84 (90.3) | 0.352 | 83 | 76 (91.6) | 62 | 55 (88.7) | 0.806 |
| peri | 19 | 13 (68.4) | 20 | 15 (75.0) | 0.460 | – | – | – | – | – |
| post | 172 | 72 (41.9) | 142 | 55 (38.7) | 0.328 | 161 | 114 (70.8) | 168 | 104 (61.9) | 0.056 |
| pT status | ||||||||||
| pT1 | 187 | 101 (54.0) | 174 | 95 (54.6) | 0.498 | 105 | 73 (69.5) | 138 | 83 (60.1) | 0.084 |
| pT2 | 105 | 73 (69.5) | 91 | 66 (72.5) | 0.381 | 123 | 100 (81.3) | 87 | 72 (82.8) | 0.468 |
| pT3 | 1 | 1 (100) | 1 | 1 (100) | NS | 16 | 16 (100) | 5 | 5 (100) | NS |
cALND: complete axillary lymph node dissection; ART: axillary radiotherapy; CT: chemotherapy; pT: tumor size on pathology; NS: not significant. –: not applicable.
Comparison of OS rates between ART and cALND
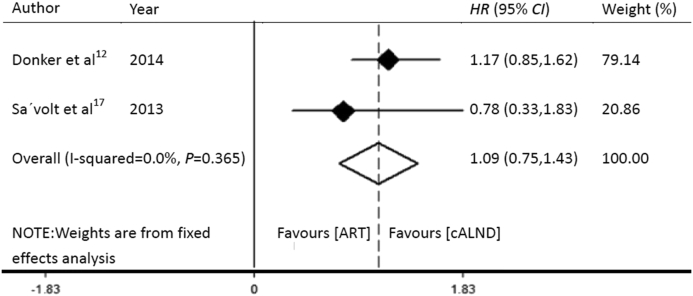
The OS rates of the patients who received ART and cALND in the AMAROS12 and OTOASOR17 trials were 92.5% and 93.3%, and 97.0% and 94.3%, respectively (Table 4). As the two trials reported OS by using survival curves, an estimate of HR was derived by using a method described by Tierney et al.18
A P value of 0.365, which was >0.1, suggested that this was not significant. The overall I2 statistical value was 0, which was <50%, so we applied a fixed-effects model. The HR for OS was 1.09 (95% CI: 0.75–1.43). cALND had no significant benefit over ART (Fig. 2).
Fig. 2.
Forest plot showing the pooled effect of overall survival with ART compared to that with cALND for the patients with SLN-positive breast cancer. HR: hazard ratio; CI: confidence interval; ART: axillary radiotherapy; cALND: completion axillary lymph node dissection.
Comparison of DFS between ART and cALND
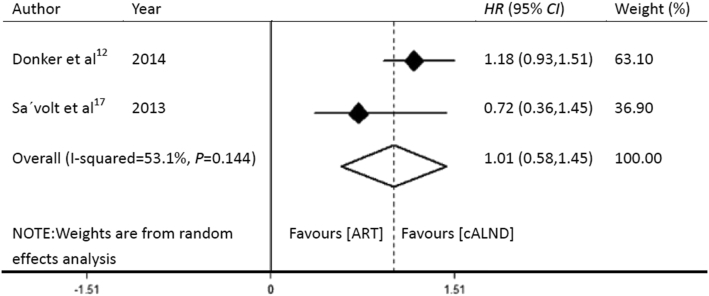
DFS rates of the patients who received ART and cALND in the AMAROS12 and OTOASOR17 trials were 82.7% and 86.9%, and 91.3% and 86.1%, respectively (Table 4). As the two trials reported DFS by using survival curves, an estimate of HR was derived by using a method described by Tierney et al.18
A P value of 0.144, which was >0.1, suggested that this was not significant. However, the overall I2 statistical value was 53.1%, which was >50%, and indicated moderate heterogeneity. Therefore, a random-effects model was applied. The HR for the pooled effect of DFS was 1.01 (95% CI: 0.58–1.45). cALND had no significant benefit over ART (Fig. 3).
Fig. 3.
Forest plot showing the pooled effect of disease-free survival with ART compared to that with cALND for the patients with SLN-positive breast cancer. HR: hazard ratio; CI: confidence interval; ART: axillary radiotherapy; cALND: completion axillary lymph node dissection.
ARR in the ART and cALND groups
The ARR of the patients who received ART and cALND in the AMAROS12 and OTOASOR trials17 were 1.2% and 0.4%, and 1.3% and 0.8%, respectively (Table 4). No significant difference in ARR was found between the two groups in both studies.
The ART group was a subgroup in the three retrospective studies. The three studies reported on smaller cohorts, so the number of patients treated with ART ranged from 16 to 68. The ARRs in the ART group of the three retrospective studies were all 0 (Table 5). No significant difference in ARR was found among the three studies.
Influence on the recommendation for adjuvant treatment
No significant difference in the administration of adjuvant systemic therapy was found in the AMAROS trial22 (Table 6). In the cALND and ART arms, 58.3% (175/300) and 60.9% (162/266) of the patients (P = 0.296) received chemotherapy, respectively. Endocrine therapy was administered to 78.3% (235/300) and 76.3% (203/266) of the patients in the cALND and ART arms, respectively (P = 0.318). The extent of nodal involvement seems to have no major impact on the administration of adjuvant systemic therapy. In the OTOASOR trial,23 77.9% (190/244) and 69.1% (159/230) of the patients received chemotherapy in the cALND and ART arms, respectively (P = 0.020). Endocrine therapy was administered to 87.3% (213/244) and 88.7% (204/230) of the patients in the cALND and ART arms, respectively (P = 0.372). Six (2.5%) and 13 patients (5.7%) in the cALND and ART arms received adjuvant trastuzumab treatment, respectively (P = 0.061). The results of the subgroup analyses showed that more frequent administration of adjuvant chemotherapy in the cALND arm might be associated with a higher percentage of premenopausal patients and patients with larger (pT2-3) tumors (Table 7).
Discussion
This is the first meta-analysis that compared the effects of ART with those of cALND in SLN-positive patients. In this review, the OS rates, DFS rates, and ARRs in the two RCTs and three retrospective studies showed that ART was not inferior to cALND. In the AMAROS trial,12 the incidence of lymphedema was higher (23.2%) in the cALND group than in the ART group (10.8%).
Before SLNB was introduced, the NSABP B-04 trial, conducted from 1971 to 1974, included patients who were randomized to undergo total mastectomy, total mastectomy plus nodal irradiation, or total mastectomy plus axillary dissection. The axillary failure rates in the latter two approaches were 3% and 1%, respectively.24 A 25-year follow-up study showed no significant difference in total nodal failure rate (4% vs. 4%), distant DFS rate, or OS between the two arms.25 Spruit et al26 conducted a study that included two groups, a regional radiotherapy (RT) group and an ALND group. The treatment groups were comparable, except for age. The patients in the RT group were significantly older than those in the ALND group. The median follow-up period was 7.2 years. The 5-year regional relapse rates were low and equal in both treatment groups (1.1% in the RT group and 1.5% in ALND group). The 5-year OS rates were also similar (92% vs. 90%). DFS was significantly better in the RT group, with an HR of 0.4 (95% CI: 0.3–0.8, P = 0.003) in the univariate analysis. Between 1982 and 1987, Louis-Sylvestre et al27 conducted a randomized study with 15 years of follow-up, involving 658 patients with breast carcinoma <3 cm in diameter and clinically uninvolved lymph nodes; patients were randomly assigned to either axillary dissection or ART. The 10- and 15-year survival rates were identical in both groups (73.8% vs. 75.5% at 15 years). ARR was less frequent in the axillary dissection group at 15 years (1% vs. 3%; P = 0.04). No significant differences in recurrence rates in the breast or supraclavicular lymph nodes and distant metastases were found between the two groups. These randomized trials,25, 27 along with non-randomized studies, suggest that ART might be as effective as ALND for axillary control but less toxic,28, 29, 30 thus providing the rationale for the AMAROS and OTOASOR trials. The limitations of this review were that only two RCTs were included in the meta-analysis, and the data available from the OTOASOR trial were the early results. A 2014 update of the OTOASOR trial13 indicated that the mean length of follow-up was 70 months, and that the ARRs were 1.6% and 1.7%, the 5.8-year OS rates, 84.9% and 91.2%; and the 5-year DFS rates, 79.9% and 85.6% for cALND and ART, respectively (P > 0.05). The 5-year follow-up data from the OTOASOR trial suggested that ART without cALND did not decrease OS and DFS, or increase the risk of axillary recurrence in SLN-positive patients. In addition, the results may be biased because of the inclusion of a small number of RCTs.
Several reviews and studies compared the effects of SLNB alone and cALND. The Z0011 trial,31 which is the only RCT that compared SLNB alone with cALND in patients with 1 or 2 positive SLNs, showed similar outcomes in women with clinical T1–T2 invasive breast cancer among the 445 patients randomized to ALND and 446 randomized to SLNB alone. At a median follow-up of 6.3 years, the 5-year OS rate was 91.8% with ALND and 92.5% with SLNB alone, and the 5-year DFS rate was 82.2% with ALND and 83.9% with SLNB alone. No significant difference in local recurrence (3.6% vs. 1.9%) or regional recurrence (0.5% vs. 0.9%) was found between the ALND and non-ALND arms. Among the patients with limited SLN metastatic breast cancer treated with breast conservation and systemic therapy, the use of SLNB alone did not result in inferior survival when compared with ALND. Positive axillary nodes were not removed in approximately 27% of the patients in the non-ALND arm in the Z0011 trial, but only 0.9% of the patients developed axillary recurrence. One reason for this better-than-expected result was that systemic therapy, which most of the patients in the Z0011 trial received, may have played a significant role. The results of studies that included patients with positive SLNs implied that approximately 25% of patients who were treated with chemotherapy had complete eradication of nodal disease.32, 33 The long-term use of endocrine therapy may also contribute to this result. The other reason is the choice of radiotherapy patterns. Although the Z0011 trial did not involve a choice between the supraclavicular or axillary fields, Haffty et al34 suggested that radiation of the lower axillary nodes with high tangents to the breast may have contributed to the low ARR. However, Jagsi et al35 summarized the association between treatment arm and various patient characteristics with the use of a high-tangent field within the subgroup with evaluable tangents. Treatment arm was not associated with the use of high-tangent RT in either the univariable or multivariable analysis. Modern computed tomography (CT)-guided treatment planning allows for identification of the axillary nodes and treatment of at least part of the axilla by adjusting the superior and deep tangent borders. Reznik et al36 used CT to estimate the proportion of the prescription dose to the breast that was given to the axilla based on standard breast tangents (66% to level I, 44% to level II, and 31% to level III) in comparison with “high” breast tangents (86%, 71%, and 73%, respectively), and recommended high tangents for axillary prophylaxis. Whelan et al37 reported on the MA.20 trial, in which patients with high-risk node-negative or 1–3 node-positive breast cancer were randomly assigned to whole breast irradiation (WBI) with or without regional node irradiation (RNI). Among the patients, 85% who had 1–3 positive nodes overlapped to some extent with the Z0011 population, of whom at least 19% had received RNI.35 Compared with WBI alone, WBI plus RNI was associated with an improvement in isolated locoregional DFS (HR = 0.59, P = 0.02, 5-year risks: 96.8% and 94.5%, respectively), distant DFS (HR = 0.64, P = 0.002, 5-year risks: 92.4% and 87.0%, respectively), DFS (HR = 0.68, P = 0.003, 5-year risk: 89.7% and 84.0%, respectively), and OS (HR = 0.76, P = 0.07, 5-year risks: 92.3% and 90.7%, respectively). Results from the MA.20 trial indicate that additional RNI reduces the risks of locoregional and distant recurrences, and improves DFS with a trend of improved OS. Although outcomes from limited prospective randomized trials need more discussion, ART is an effective method when compared with axillary lymph node clearance for patients with breast cancer who have positive SLNs. Ram et al38 reviewed the cases of patients with a clinically negative axilla and micrometastasis in the SLN and showed that SLNB alone was not inferior to cALND. They combined the Z0011 trial39 with multicenter IBCSG 23-01 trial40 and AATRM trial,41 which included patients with micrometastasis in the SLN. The HR of the pooled effect of OS was 0.83 (95% CI: 0.60–1.14), and that of DFS was 0.94 (95% CI: 0.79–1.13). The reported rates for locoregional recurrence were similar in the SLNB-alone and ALND groups. Surgical morbidity was found to be higher in the ALND group than in the SLNB-alone group. The 2009 St. Gallen Consensus Panel recommended that ALND be avoided in patients with SLN micrometastases or isolated tumor cells, and those who had small and well-differentiated tumors.6 The AMAROS12 and OTOASOR17 trials also included a certain percentage of patients with SLN micrometastases. Additional clinical trials (adjuvant systemic therapy alone vs. adjuvant systemic therapy plus clearance or ART) are needed to define the role of axillary intervention in patients with macrometastases in the SLNs before avoidance of further axillary intervention in this patient group can be routinely recommended.
Assessment of axillary nodal status is essential in the staging and determination of adjuvant treatment. One controversial issue is whether the staging information on the number of positive SLNs obtained from cALND would have an impact on the administration of adjuvant systemic therapy. The AMAROS trial22 evaluated the role of axillary clearance after SLNB in the administration of adjuvant therapy. No significant difference was found in the administration of adjuvant systemic therapy, and the authors concluded that the absence of knowledge regarding the extent of nodal involvement seemed to have no major impact on the administration of chemotherapy and hormonal therapy. In the OTOASOR trial,23 significantly more patients (77.9% vs. 69.1%) received chemotherapy in the cALND group. However, the authors implied that this difference may be associated with the significantly larger number of premenopausal patients and larger tumors in the cALND group. Therefore, the results of the two RCTs support the notion that the absence of knowledge regarding the extent of nodal involvement in the ART group appeared to have no major impact on the administration of adjuvant therapy.
Conclusion
In short, ART is not inferior to cALND in patients with clinically negative early-stage breast cancer with a positive SLN. Information obtained by using cALND after SLNB seems to have no major impact on the administration of adjuvant systemic therapy.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 81372811) and Science and Technology Agency of Liaoning Province (No. 2013225049).
Edited by Pei-Fang Wei
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.NIH consensus conference Treatment of early-stage breast cancer. JAMA. 1991;265:391–395. [PubMed] [Google Scholar]
- 2.Barkley C., Burstein H., Smith B. Can axillary node dissection be omitted in a subset of patients with low local and regional failure rates? Breast J. 2012;18:23–27. doi: 10.1111/j.1524-4741.2011.01178.x. [DOI] [PubMed] [Google Scholar]
- 3.Lyman G.H., Giuliano A.E., Somerfield M.R. American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol. 2005;23:7703–7720. doi: 10.1200/JCO.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Del B.P., Zavagno G., Burelli P. Morbidity comparison of sentinel lymph node biopsy versus conventional axillary lymph node dissection for breast cancer patients: results of the sentinella-GIVOM Italian randomised clinical trial. Eur J Surg Oncol. 2008;34:508–513. doi: 10.1016/j.ejso.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 5.Yeoh E.K., Denham J.W., Davies S.A., Spittle M.F. Primary breast cancer. Complications of axillary management. Acta Radiol Oncol. 1986;25:105–108. doi: 10.3109/02841868609136386. [DOI] [PubMed] [Google Scholar]
- 6.Jörger M., Senn H.J., Thürlimann B. St. Gallen 2009 recommendations on the treatment of early breast cancer: consensus and controversy. MEMO. 2009;2:229–231. [Google Scholar]
- 7.Sarvi M., Mehta P., Vallow L., Dowlat K., Griem K.L. Is nodal irradiation necessary in breast cancer patients with positive sentinel node biopsy without axillary dissection. Int J Radiat Oncol Biol Phys. 2002;54(2 suppl):232–233. [Google Scholar]
- 8.Guenther J.M., Hansen N.M., DiFronzo L.A. Axillary dissection is not required for all patients with breast cancer and positive sentinel nodes. Arch Surg. 2003;138:52–56. doi: 10.1001/archsurg.138.1.52. [DOI] [PubMed] [Google Scholar]
- 9.Tjan-Heijnen V.C., Pepels M.J., de Boer M. Impact of omission of completion axillary lymph node dissection (cALND) or axillary radiotherapy (ax RT) in breast cancer patients with micrometastases (pN1mi) or isolated tumor cells (pN0[i+]) in the sentinel lymph node (SN): results from the MIRROR study. J Clin Oncol. 2009;27(18S):CRA506. [Google Scholar]
- 10.Gadd M., Harris J., Taghian A. Prospective Study of Axillary Radiation Without Axillary Dissection for Breast Cancer Patients with a Positive Sentinel Node. Breast Cancer Res Treat. 2005;94(Suppl 1):S13. [Google Scholar]
- 11.Straver M.E., Meijnen P., van Tienhoven G. Sentinel node identification rate and nodal involvement in the EORTC 10981-22023 AMAROS trial. Ann Surg Oncol. 2010;17:1854–1861. doi: 10.1245/s10434-010-0945-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donker M., van Tienhoven G., Straver M.E. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol. 2014;15:1303–1310. doi: 10.1016/S1470-2045(14)70460-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sávolt A., Musonda P., Mátrai Z. Optimal treatment of the axilla after positive sentinel lymph node biopsy in primary invasive breast cancer patients (surgery versus radiotherapy)-OTOASOR trial: 5 years follow-up of a randomized clinical trial. Eur J Surg Oncol. 2014;40:S37–S38. [Google Scholar]
- 14.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med. 2009;3:e123–e130. [PMC free article] [PubMed] [Google Scholar]
- 15.Schulz K.F., Altman D.G., Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010;152:726–732. doi: 10.7326/0003-4819-152-11-201006010-00232. [DOI] [PubMed] [Google Scholar]
- 16.Higgins J.P.T., Altman D.G., Sterne A.C. Chapter 8: Assessing risk of bias in included studies. In: Higgins J.P.T., Green S., editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Chichester: The Cochrane Collaboration. 2011. http://www.cochrane-handbook.org Accessed January 1, 2015. [Google Scholar]
- 17.Sávolt A., Musonda P., Mátrai Z. Optimal treatment of the axilla after positive sentinel lymph node biopsy in early invasive breast cancer. Early results of the OTOASOR trial. Orv Hetil. 2013;154:1934–1942. doi: 10.1556/OH.2013.29765. [DOI] [PubMed] [Google Scholar]
- 18.Tierney J.F., Stewart L.A., Ghersi D., Burdett S., Sydes M.R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takei H., Suemasu K., Kurosumi M. Recurrence after sentinel lymph node biopsy with or without axillary lymph node dissection in patients with breast cancer. Breast Cancer. 2007;14:16–24. doi: 10.2325/jbcs.14.16. [DOI] [PubMed] [Google Scholar]
- 20.Fu Y., Chung D., Cao M.A., Apple S., Chang H. Is axillary lymph node dissection necessary after sentinel lymph node biopsy in patients with mastectomy and pathological N1 breast cancer. Ann Surg Oncol. 2014;21:4109–4123. doi: 10.1245/s10434-014-3814-3. [DOI] [PubMed] [Google Scholar]
- 21.Pejavar S., Wilson L.D., Haffty B.G. Regional nodal recurrence in breast cancer patients treated with conservative surgery and radiation therapy (BCS+RT) Int J Radiat Oncol Biol Phys. 2006;66:1320–1327. doi: 10.1016/j.ijrobp.2006.07.1379. [DOI] [PubMed] [Google Scholar]
- 22.Straver M.E., Meijnen P., van Tienhoven G. Role of axillary clearance after a tumor-positive sentinel node in the administration of adjuvant therapy in early breast cancer. J Clin Oncol. 2010;28:731–737. doi: 10.1200/JCO.2008.21.7554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sávolt A., Polgár C., Musonda P. Does the result of completion axillary lymph node dissection influence the recommendation for adjuvant treatment in sentinel lymph node-positive patients. Clin Breast Cancer. 2013;13:364–370. doi: 10.1016/j.clbc.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 24.Fisher B., Redmond C., Fisher E.R. Ten-year results of a randomized clinical trial comparing radical mastectomy and total mastectomy with or without radiation. N Engl J Med. 1985;312:674–681. doi: 10.1056/NEJM198503143121102. [DOI] [PubMed] [Google Scholar]
- 25.Fisher B., Jeong J.H., Anderson S., Bryant J., Fisher E.R., Wolmark N. Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N Engl J Med. 2002;347:567–575. doi: 10.1056/NEJMoa020128. [DOI] [PubMed] [Google Scholar]
- 26.Spruit P.H., Siesling S., Elferink M.A., Vonk E.J., Hoekstra C.J. Regional radiotherapy versus an axillary lymph node dissection after lumpectomy: a safe alternative for an axillary lymph node dissection in a clinically uninvolved axilla in breast cancer. A case control study with 10 years follow up. Radiat Oncol. 2007;2:40. doi: 10.1186/1748-717X-2-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Louis-Sylvestre C., Clough K., Asselain B. Axillary treatment in conservative management of operable breast cancer: dissection or radiotherapy? Results of a randomized study with 15 years of follow-up. J Clin Oncol. 2004;22:97–101. doi: 10.1200/JCO.2004.12.108. [DOI] [PubMed] [Google Scholar]
- 28.Hoebers F.J., Borger J.H., Hart A.A., Peterse J.L., Th E.J., Lebesque J.V. Primary axillary radiotherapy as axillary treatment in breast-conserving therapy for patients with breast carcinoma and clinically negative axillary lymph nodes. Cancer. 2000;88:1633–1642. doi: 10.1002/(sici)1097-0142(20000401)88:7<1633::aid-cncr18>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 29.O'Connell R.L., Rusby J.E., Stamp G.F. Long term results of treatment of breast cancer without axillary surgery – predicting a SOUND approach. Eur J Surg Oncol. 2016;42:942–948. doi: 10.1016/j.ejso.2016.03.027. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi R., Shiraishi K., Iwase S., Ohtomo K., Nakagawa K. Omission of axillary lymph node dissection for clinically node negative early-stage breast cancer patients. Breast Cancer. 2015;22:657–663. doi: 10.1007/s12282-014-0532-4. [DOI] [PubMed] [Google Scholar]
- 31.Giuliano A.E., Hunt K.K., Ballman K.V. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305:569–575. doi: 10.1001/jama.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gralow J.R., Burstein H.J., Wood W. Preoperative therapy in invasive breast cancer: pathologic assessment and systemic therapy issues in operable disease. J Clin Oncol. 2008;26:814–819. doi: 10.1200/JCO.2007.15.3510. [DOI] [PubMed] [Google Scholar]
- 33.Gralow J.R., Zujewski J.A., Winer E. Preoperative therapy in invasive breast cancer: reviewing the state of the science and exploring new research directions. J Clin Oncol. 2008;26:696–697. doi: 10.1200/JCO.2007.15.9459. [DOI] [PubMed] [Google Scholar]
- 34.Haffty B.G., Hunt K.K., Harris J.R., Buchholz T.A. Positive sentinel nodes without axillary dissection: implications for the radiation oncologist. J Clin Oncol. 2011;29:4479–4481. doi: 10.1200/JCO.2011.36.1667. [DOI] [PubMed] [Google Scholar]
- 35.Jagsi R., Chadha M., Moni J. Radiation field design in the ACOSOG Z0011 (Alliance) trial. J Clin Oncol. 2014;32:3600–3606. doi: 10.1200/JCO.2014.56.5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reznik J., Cicchetti M.G., Degaspe B., Fitzgerald T.J. Analysis of axillary coverage during tangential radiation therapy to the breast. Int J Radiat Oncol Biol Phys. 2005;61:163–168. doi: 10.1016/j.ijrobp.2004.04.065. [DOI] [PubMed] [Google Scholar]
- 37.Whelan T., Olivotto I., Ackerman I. NCIC-CTG MA. 20: an intergroup trial of regional nodal irradiation in early breast cancer. J Clin Oncol. 2011;29(18 suppl):LBA1003. [Google Scholar]
- 38.Ram R., Singh J., McCaig E. Sentinel node biopsy alone versus completion axillary node dissection in node positive breast cancer: systematic review and meta-analysis. Int J Breast Cancer. 2014;2014:513780. doi: 10.1155/2014/513780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giuliano A.E., Hunt K.K., Ballman K.V. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305:569–575. doi: 10.1001/jama.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Galimberti V., Cole B.F., Zurrida S. Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23-01): a phase 3 randomised controlled trial. Lancet Oncol. 2013;14:297–305. doi: 10.1016/S1470-2045(13)70035-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Solá M., Alberro J.A., Fraile M. Complete axillary lymph node dissection versus clinical follow-up in breast cancer patients with sentinel node micrometastasis: final results from the multicenter clinical trial AATRM 048/13/2000. Ann Surg Oncol. 2013;20:120–127. doi: 10.1245/s10434-012-2569-y. [DOI] [PubMed] [Google Scholar]