Abstract
The unusual chemoorganoheterotrophic proteobacterial strain MWH-Nonnen-W8redT was isolated from a lake located in the Black Forest (Schwarzwald), Germany, by using the filtration-acclimatization method. Phylogenetic analyses based on the 16S rRNA gene sequence of the strain could not provide clear hints on classification of the strain in one of the current classes of Proteobacteria. Whole genome sequencing resulted in a genome size of 3.5 Mbp and revealed a quite low G+C content of 32.6 mol%. In-depth phylogenetic analyses based on alignments of 74 protein sequences of a phylogenetically broad range of taxa suggested assignment of the strain to a new order of the class Oligoflexia. These analyses also suggested that the order Bdellovibrionales should be transferred from the Deltaproteobacteria to the Oligoflexia, that this order should be split into two orders, and that the family Pseudobacteriovoracaceae should be transferred from the order Bdellovibrionales to the order Oligoflexiales. We propose to establish for strain MWH-Nonnen-W8redT (DSM 23856T =CCUG 58639T) the new species and genus Silvanigrella aquatica gen. nov., sp. nov. to be placed in the new family Silvanigrellaceae fam. nov. of the new order Silvanigrellales ord. nov.
Strain MWH-Nonnen-W8redT was isolated from a freshwater lake located in the Black Forest Mountains (Schwarzwald), Germany. Analyses of the strain’s 16S rRNA gene sequence indicated that this strain is only distantly related to any type strain. BLAST searches against sequences of type material revealed that the top hits (November 2016) represent type strains belonging to various classes of Proteobacteria. The best hit, Vulgatibacter incomptus DSM 27710T (Deltaproteobacteria), shared a 16S rRNA sequence similarity of 85%, while various type strains affiliated with the classes Gammaproteobacteria and Acidithiobacillia shared similarities of 81-82%. Inclusion of non-type-material in BLAST searches resulted in much higher sequence similarity values. Interestingly, the uncultured taxon ‘Spirobacillus cienkowskii’, a pathogen of water flea (Daphnia spp.), which was described by Élie Metchnikoff almost 130 years ago [1] and subsequently rediscovered a few years ago by Rodrigues and colleagues [2] shared a 16S rRNA similarity of 96%. Other taxonomically unclassified cultured and uncultured bacteria even share 97-99% 16S rRNA gene similarities [3–6]. According to Nakai and colleagues, who recently described the new class Oligoflexia of the phylum Proteobacteria, ‘Spirobacillus cienkowskii’ and related strains may represent a novel class of Proteobacteria [7].
We characterized strain MWH-Nonnen-W8redT by following the polyphasic approach and included genome sequencing and comparative analysis of the annotated genome sequence of the strain. Based on the obtained results, we propose that this strain represents a new species, genus, family, and order affiliated with the class Oligoflexia Nakai et al. 2014 [7] within the phylum Proteobacteria.
Strain MWH-Nonnen-W8redT was isolated by using the filtration-acclimatization method [8], which included filtration of a water sample through a filter with a pore size of 0.2 µm and stepwise acclimatization to higher substrate concentrations. Liquid and solidified (1.5% agar) NSY medium [8], which mainly consists of equal amounts of nutrient broth, soytone and yeast extract (all three from Difco, BD International) was used for isolation and maintenance of the strain. The isolate was stored at -70°C in NSY medium plus 15% (w/v) glycerol prior to deposition of the strain in public culture collections.
Strain MWH-Nonnen-W8redT was obtained from Lake Nonnenmattweiher located (geographic coordinates 47.795299°N and 7.798552°E) in the Black Forest Mountains (Schwarzwald), Germany, at an altitude of 926 m. The lake has a surface area of 71 ha and is characterized by a floating peat moss island. The lake is located at the site of a former glacial cirque lake, which was naturally infilled and replaced by a mire in the Middle Ages. The current lake was established by construction of an embankment dam in 1722, lost its water for a couple of years due to dam failure in 1922, and was re-established in the early 1930s. The current lake can be characterized as a shallow softwater lake influenced by a mire. Surface waters (about 10-20 cm depths) of the lake were sampled from the shore line by using a water sampling dipper. At the day of sampling (27 July 2008), the water temperature was 19.4°C, the pH was 6.7 and conductivity was 21.8 µS cm-1. The water was slightly stained by dissolved humic matter (absorption of 0.2µm-filtered water at a wavelength of 250 nm of 0.12).
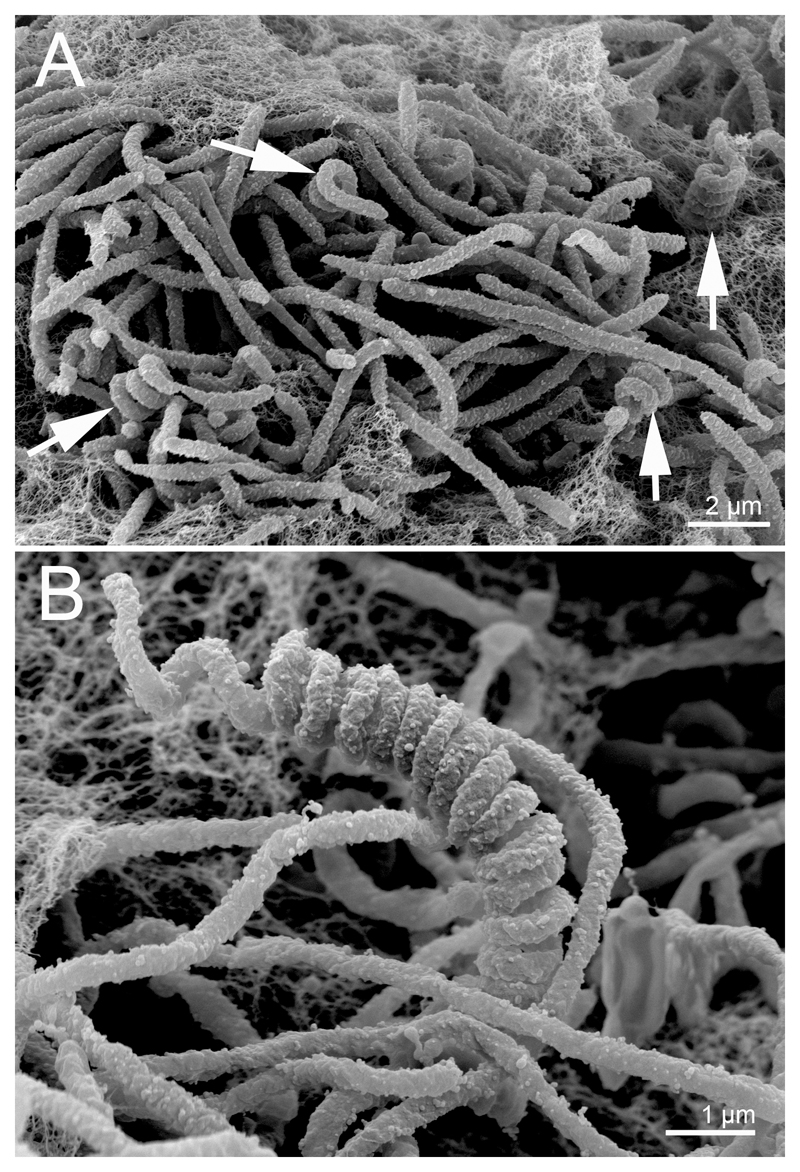
Strain MWH-Nonnen-W8redT could be grown on NSY or R2A medium [9]. Comparative tests with R2A medium of different strength suggested that dilution of the medium to half the standard concentration accelerated growth (turbidity after 2 days). The strain formed large convex, shiny, red pigmented colonies on NSY agar plates (1.5% agar), which reached at room temperature (about 23 °C) a diameter of 5 mm after 18 days of incubation. No pronounced subsequent increase of colony diameter was observed. In liquid NSY medium (3 g/L, pH 7.2), the strain grew at 20 °C with a rate of 0.11 ± 0.003 h-1 (average and SD of three parallels) equalling a generation time of 6.5 h and reached a maximum OD575nm of about 0.38. In the logarithmic growth phase the strain appeared with a rod-shaped morphology with cell length of 3 - 4 µm and cell widths of 0.6 µm. When the strain was cultivated in soft agar (1 g L-1 yeast extract, 0.1 g L-1 K2HPO4, 2.0 g L-1 agar) swarming colonies reached a diameter of 30 mm within three days. Upon storage at about 23° C for three weeks, the appearance of the culture changed from uniformly turbid to mycelia-like floccose in some spots. Light microscopic observation mainly revealed filamentous rods 0.3 µm wide, and rather rare twisted spirals. The spirals typically had 4 - 7 right-handed turns and a diameter of 1 - 1.2 µm. Transitional states, like filaments seemingly starting to curl, were also observed. Scanning electron microscopic pictures confirmed the impression found at the light microscope, i.e. that there were no constrictions or separations visible along the spirals (Fig. 1). Local addition of 100 µl soil extract (prepared in water as described in DSMZ medium 80, www.dsmz.de/?id=441) near the colony edge promoted the formation of these spirals.
Fig. 1.
Scanning electron microscopy images. Most visible bacteria show a filamentous morphology when growing in soft agar while some bacteria form spirals (arrows in A) with no visible constrictions or separations along the spirals (B).
The spirals had high similarity to those observed in the aerial mycelium of Streptomyces species (Actinobacteria). In order to exclude the possibility that slowly growing streptomycetes were co-isolated with strain MWH-Nonnen-W8redT, the very cultures and spots from which the figures were taken, were inoculated to media typically used for streptomycetes (DSMZ media 65 (GYM Streptomyces Medium) and 987 (ISP2 Medium)), were subjected to Gram staining (Suppl. Mat. Fig. S1), and its 16S rRNA gene was sequenced. The filaments and spirals stained Gram-negative, the cultures did not grow on these media when incubated at 28°C for 7 days, and the 16S rDNA sequence did not show any difference to the sequences obtained by genome sequencing or previous Sanger sequencing of the gene. These results confirmed that the spirals are truly a morphological, and possibly a developmental state of strain MWH-Nonnen-W8redT.
Similar spirals were reported previously from uncultured ‘Spirobacillus cienkowskii’ [1, 2], from Oligoflexus tunisiensis [7, 10] and from Bdellovibrio bacteriovorus [11], however, the spirals observed in O. tunisiensuis and B. bacteriovorus were not as densely packed as in strain MWH-Nonnen-W8redT and in ‘S. cienkowskii’.
The further phenotypic characterizations (Table 1) were performed as described previously [12, 13]. Assimilation of particular substances was tested by comparing growth on media with and without test substance [12]. Substrate-specific growth was determined by comparison of OD at 575 nm established in liquid one tenth-strength NSY medium (0.3 g L-1) with and without 0.5 g L-1 test substrate, respectively. Differences of < 10 %, 10–50% and >50% of the OD obtained in the test treatments compared to the OD obtained without test substrate (i.e. in 0.3 g L-1 NSY medium) were scored after 10 days of growth as no utilization (-), weak utilization (w) and good utilization (+), respectively. The strain utilized D-mannose, D-glucose, L-proline, L-glutamate, and L-alanine. Three other tested substances were only weakly assimilated (Table 1). These assimilation experiments recorded growth of the strain based on optical density measurements. Additional substrate utilization tests were performed with BIOLOG GN2 MicroPlates (BIOLOG, Hayward CA, USA), which detects utilization of substrates as electron donor by the subsequent reduction of a tetrazolium redox dye. These tests were performed as follows. Cells were suspended in distilled water, because initial tests revealed that this treatment gave higher scores compared to inoculation in a 0.17% NaCl solution (the BIOLOG manual even suggests a 0.85% NaCl solution). The plates were read after 48 h incubation at 28°C. A threshold of 100 counts was set to evaluate a response as positive. The highest observed count was 232. These tests suggested that the strain is able to use alpha-D-glucose, alpha-ketobutyric acid, alpha-ketovaleric acid, succinic acid, L-asparagine, L-aspartic acid, L-hydroxyproline, L-proline, L-serine, L-threonine and inosine as electron donors. Note that these BIOLOG results and assimilation experiments based on growth of the tested strain are in partial contradiction regarding utilization of some substances. In addition to experiments by these two methods, substrate utilization tests with API20NE strips (bioMérieux, Lyon, France) were performed according to the recommendations by the manufacturer. Interestingly, no substrate utilization nor any enzymatic reactions were detected with the API20NE strips.
Table 1.
Traits that characterize strain MWH-Nonnen-W8redT. The assimilation data represent results of growth experiments performed as described previously [12].
-, negative; +, positive; w, weakly positive
| Characteristic | MWH-Nonnen-W8redT |
|---|---|
| Cell morphology | pleomorphic |
| Cell length of rods (µm) | 3.6 |
| Cell width of rods (µm) | 0.6 |
| Motility (soft agar) | + |
| Catalase/oxidase | w/- |
| Temperature range of growth (°C) | 10(w) – 32(w) |
| NaCl tolerance (%NaCl, w/v) | 0 - 1.0(w) |
| Anaerobic growth: | |
| NSY medium | - |
| NSY enriched with nitrate | - |
| Assimilation of: | |
| Glyoxylic acid | - |
| Glycolic acid | - |
| Acetic acid | w |
| Propionic acid | - |
| Oxaloacetic acid | - |
| Malonic acid | - |
| DL-Lactate | - |
| Fumaric acid | w |
| Citric acid | - |
| D-Xylose | - |
| D-Mannose | + |
| D-Glucose | + |
| D-Fucose | - |
| D-Sorbitol | - |
| Glycine | w |
| L-Proline | + |
| L-Glutamate | + |
| L-Alanine | + |
| L-Methionine | - |
| Betaine | - |
Enzymatic activities of strain MWH-Nonnen-W8redT were tested by using API Zym strips (bioMérieux, Lyon, France) incubated for four hours at 37°C. These experiments showed strong reactions for alkaline and acid phosphatases and intermediate reactions for C4-esterase, esterase-lipase and leucine arylamidase. Test for oxidase and catalase activity performed as described previously [12] suggested that strain MWH-Nonnen-W8redT was oxidase negative and weakly catalase positive (Table 1).
It was observed that the phenotypic responses of the strain in growth experiments testing substrate utilization, temperature range of growth, and salinity tolerance were not reliable. Repetition of experiments yielded in some cases contradicting results. For instance, growth at 15°C was in two experiments negative but positive in a third experiment. In all three experiments controls incubated at room temperature (about 23 °C), which was also the incubation temperature of the culture used for inoculation of the experiments, were clearly positive, respectively. This lack of phenotypic reliability has to be considered in future comparative investigations including this strain.
The chemotaxonomic characterization of the strain included analyses of composition of whole cell fatty acids, polar lipids and quinones, as well as analysis of the peptidoglycan structure. The whole cell fatty acid composition was analyzed after growth at 28°C on R2A and on NSY agar, respectively, by using an Agilent Technologies 6890N instrument and the Microbial Identification System (MIDI) Sherlock version 6.1 (results were evaluated against the TSBA 40 peak-naming table database) as described by Sasser [14]. Main compounds were iso-C15:0, C16:0, feature 3 including C16:1 ω7c, anteiso-C15:0, and C17:0, however the composition differed between biomass grown on the two different media (Suppl. Mat. Table S4). A high number of 3-hydroxylated fatty acids were noticeable. In general, the fatty acid composition of strain MWH-Nonnen-W8redT differed significantly from that given for Oligoflexus tunisiensis [7], in which C16:1 ω5c and C16:0 constituted 93 % of the detected cellular fatty acids.
Polar lipids were extracted and analyzed as described by Tindall [15, 16] based on the method by Bligh & Dyer [17]. This analysis revealed phosphatidylethanolamine and phosphatidylglycerol as the main components and a smaller proportion of an unknown lipid (Suppl. Mat. Fig. S2). Extraction and analyses of respiratory quinones were also performed as described in Tindall [15, 16], however this analysis could not identify the present quinones. During development of the thin layer chromatogram the extracted compounds showed an ascending height (rate) in between those of ubiquinones and menaquinones. The HPLC separation resulted in four peaks, but their retention time did not corresponded to those of known ubiquinones or menaquinones. Thus, the quinones of the strain could neither being identified as ubi- nor menaquinones. The peptidoglycan structure of the strain was analyzed according to Schumann [18]. After preparation and hydrolysis of the peptidoglycan, meso-diaminopimelinic acid was detected by GC/MS as expected in Gram negatively staining bacteria.
The genome of strain MWH-Nonnen-W8redT was sequenced and annotated. DNA used for genome sequencing was extracted from biomass grown in liquid NSY medium as described previously [19]. Two libraries were sequenced by an Illumina and a Roche system, respectively. A Long Jumping Distance (LJD) library of 8 kb fragment size was mate pair sequenced on an Illumina MiSeq instrument, which resulted in 271,499 filtered reads with a mean length of 112 nt. Paired–end sequencing of a shotgun library on a GS FLX instrument by using Titanium chemistry resulted in 161,591 filtered reads with a mean length of 453 nt. A de novo hybrid assembly was conducted using an in-house pipeline (Eurofins Genomics) that incorporates the software tool newbler 2.9. This resulted in five scaffolds consisting of 41 contigs. Gap closure was performed by in silico analyses and by PCR amplification of gap regions and subsequent Sanger sequencing of amplicons. Thirteen gaps could be closed. The obtained genome sequence has a length of 3.51 Mbp and a G+C content of 32.63 mol% and is characterized by a coverage of about 30x (Table 2). The resulting genome sequence was annotated using the IMG/ER annotation pipeline [20]. Additionally, the genome was annotated by using the NCBI pipeline for prokaryotic genomes and deposited in DDBJ/EMBL/GenBank under the Accession Numbers CP017834 - CP017838.
Table 2.
Genome characteristics of strain MWH-Nonnen-W8redT.
| Scaffold | Type | Accession Number | Size | No. Genes | GC (Mol%) |
|---|---|---|---|---|---|
| 1 | Chromosome | CP017834 | 3.34 Mbp | 2896 | 33 |
| 2 | Putative conjugative plasmid | CP017835 | 42.2 Kbp | 48 | 30 |
| 3 | Putative prophage | CP017837 | 41.8 Kbp | 58 | 31 |
| 4 | Putative prophage | CP017838 | 43.3 Kbp | 58 | 36 |
| 5 | Putative conjugative plasmid | CP017836 | 37.0 Kbp | 42 | 29 |
Mbp, mega base pairs; Kbp, kilo base pairs.
The genome of strain MWH-Nonnen-W8redT putatively encodes 3049 protein and 53 RNA genes. It consists of five scaffolds representing one chromosome, two putative conjugative plasmids and two putative prophages. The four smaller scaffolds share a small size of about 40 kbp each. The putative conjugative plasmids both encode a relaxase, a type IV coupling protein, a type IV secretion system putatively involved in transfers of the two conjugative plasmids, respectively, and a DNA topoisomerase. The putative prophages both encode terminases and oligoribonucleases, however, only one of the two putative prophages encodes a substantial number of genes annotated as putative phage genes.
The chromosome of the strain encodes five copies of ribosomal operons. These operons could be assembled but contain gaps of unknown sequences located downstream of the 16S rRNA genes, respectively. Genes 2653193881-2653193883 (IMG Gene ID) encode a putative non-ribosomal peptide synthetase/polyketide synthase system. Annotations of these three genes hint on synthesis of products with putative antimicrobial activity (lichenysin-like substances). Two other putative non-ribosomal peptide synthetases are encoded by genes 2653195819 and 2653195162 (IMG Gene ID), which are both annotated as a bacitracin synthase. All these genes encode large proteins of 700 - 2021 amino acids. At all three loci with putative non-ribosomal synthetase genes, open reading frames encoding putative drug/metabolite transporters are present nearby. Furthermore, the genome encodes four giant genes of > 10000 bp, which are all annotated as fibronectin type 3 domain-containing protein (IMG Gene IDs 2653194608, 2653194165, 2653195410, and 2653194609).
Besides the above mentioned two plasmid-encoded type IV secretion systems the genome encodes on its chromosome a Sec-pathway (general secretion route) and a twin-arginine translocation pathway, which both mediate the secretion of proteins across the cytoplasmic membrane. Some other chromosomally encoded genes seem to belong to a type II secretion system, however, it seems that the set of genes necessary for synthesis of a functional type II system is incomplete. No genes potentially contributing to type III or type VI secretion systems were annotated. Regarding presence and absence of chromosomal genes encoding secretion systems, the gene content of strain MWH-Nonnen-W8redT is quite similar to Bdellovibrio bacteriovorus HD 100T and Halobacteriovorax marinus SJT, but the latter two lack plasmid-related type IV systems. By contrast, Myxococcus strains usually encode both a type III and a type VI secretion system. See below for the phylogenetic relationships of the mentioned taxa to strain MWH-Nonnen-W8redT.
Relating to the below suggested phylogenetic relationships between strain MWH-Nonnen-W8redT, Oligoflexus and the order Bdellovibrionales, it is interesting that the former two organisms do not possess the majority of the 59 genes present in all genome-sequenced members of the Bdellovibrionales but lacking in other previously investigated bacteria [21]. For instance, BLASTp searches resulted in the genome of strain MWH-Nonnen-W8redT only in nine of the 59 query protein sequences in hits, however all the resulting alignments were characterized by identity values of ≤ 32% and E values of ≥ e-14. Interestingly, the genome of strain MWH-Nonnen-W8redT lacks homologues of the hit locus, which is a conserved region in Bdellovibrio and Halobacteriovorax genomes known to encode functions involved in the predatory lifestyle of these bacteria [22].
Regarding the above mentioned motility of the strains and the below discussed potential virulence (water flea) of the strain, it is worth to mention that its genome contains genes putatively encoding the synthesis and use of flagella, as well as putative chemotaxis genes.
Genome comparisons based on average nucleotide identity (ANI) analyses [23] of the MWH-Nonnen-W8redT genome with the closest related type strains available (see below), i.e. with Oligoflexus tunisiensis Shr3T [24], Bdellovibrio spp. [25, 26], Halobacteriovorax marinus SJT [22], and ‘Bacteriovorax’ spp. [21], resulted in quite low values of 66-69% ANI, which suggests only distant phylogenetic relationship between strain MWH-Nonnen-W8redT and these taxa. In all three comparisons, these results obtained by using the IMG system [20] are based on alignment fractions of only about 1-2% of the genome sequences. Two way average amino acid identity (AAI) values calculated with the AAI calculator [27] for those genomes resulted in AAI values of about 35-38%, respectively. These results are based on alignments of >40% of the proteins encoded by the genome of MWH-Nonnen-W8redT. ANI and AAI results both suggest only distant phylogenetic relationships of strain MWH-Nonnen-W8redT to the compared taxa. It should also be mentioned that strain MWH-Nonnen-W8redT and the reference taxon with the most similar 16S rRNA gene sequence, i.e. Vulgatibacter incomptus DSM 27710T, even share an ANI value of 79.6% but the alignment fraction is less than 0.1% of the genome sequences.
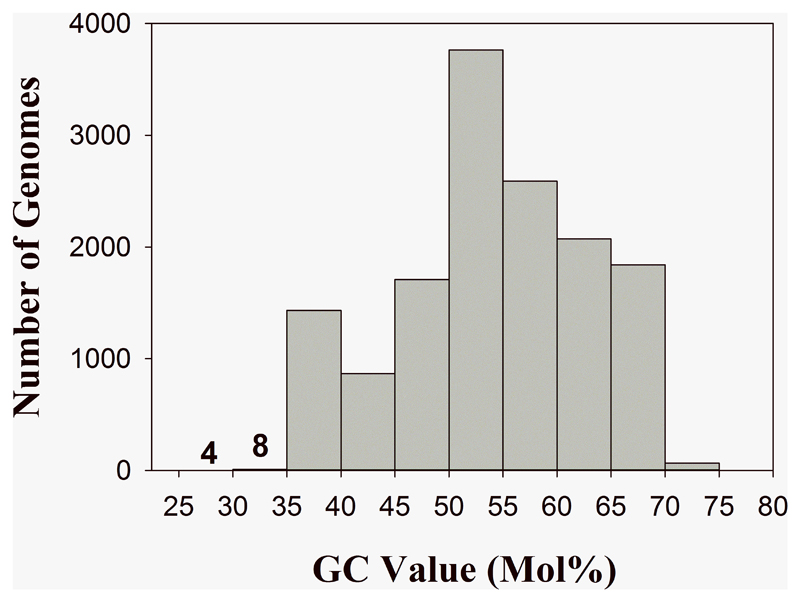
The G+C value of the MWH-Nonnen-W8redT genome of 32.6 mol% is exceptionally low for a proteobacterium with a genome size of > 3 Mbp (Suppl. Mat. Fig. S4). Among the 14,351 genomes of cultured Proteobacteria (environmental genomes were excluded) available in the IMG system [20] at the time of analysis (June 2016) characterized by genome sizes ≥ 3 Mbp were only 11 genomes found with G+C contents less than 35% (Fig. 2). Among these 11 taxa, no member of the current order Bdellovibrionales or the current class Oligoflexia are found. Interestingly, six genomes currently classified by the IMG system as ‘Bacteriovorax’ strains (including Halobacteriovorax marinus SJT), which should be considered as members of the below proposed new order Bacteriovoracales ord. nov., possess G+C values in the range of 35-40%. However, the G+C values of the genomes of other members of the current order Bdellovibrionales, e.g. of Bdellovibrio spp., as well as of Oligoflexus tunisiensis Shr3T, are higher than 40%. Thus, a G+C content of less than 40% is no common feature of the below proposed revised class Oligoflexia. Genomes with sizes smaller than 3 Mbp were excluded from these analyses, because genomes shaped by reductive genome evolution usually possess reduced G+C values. However, of the 3002 proteobacterial genomes with sizes of less than 3 Mbp, 75% possess G+C values higher than the value of MWH-Nonnen-W8redT.
Fig. 2.
Frequency distribution of G+C values of Proteobacteria genomes available in the Integrated Microbial Genomes (IMG) system characterized by genome sizes of ≥ 3 Mbp. Only four and eight (including MWH-Nonnen-W8redT) genomes possess G+C values in the range of 25-30 and 30-35 mol%, respectively.
We searched for other exceptional genomic or genetic traits of strain MWH-Nonnen-W8redT. An interesting feature is the lack of the diagnostic amino acid sequence GGKH in the alanyl-tRNA synthetase, which is assumed to be present in this protein in all Proteobacteria [28]. A systematic screening of 6212 proteobacterial genomes available in the IMG system revealed that strain MWH-Nonnen-W8redT is quite exceptional in this trait. For this screening all genomes assigned to the classes Alpha-, Beta-, Delta-, Epsilon-, ‘Zetaproteobacteria’, Acidithiobacillia and Oligoflexia, respectively, were considered but due to the large number of genomes assigned to the Gammaproteobacteria only those of this class with the status finished were included in the analysis. After exclusion of low quality genomes completely lacking an annotation of the alanyl-tRNA synthetase gene or putatively containing only an incomplete gene, 5808 genomes remained for the further analysis. Of these genomes, 34 encoded alanyl-tRNA synthetases with substitutions in the signature sequence and in nine genomes the gene possessed a complete deletion of the four amino acid signature sequence. Interestingly, all those nine genomes are classified by the IMG system as ‘Bacteriovorax’, which includes again Halobacteriovorax marinus SJT. By contrast, the signature sequence was found among all other genome-sequenced strains currently classified as Bdellovibrionales, as well as in Oligoflexus tunisiensis Shr3T. Twenty-two of the 34 genomes with substitutions in the signature sequence are currently classified as Deltaproteobacteria, which also includes the obviously misclassified Vampirovibrio chlorellavorus [29].
Furthermore, we tested if those taxa currently classified as Deltaproteobacteria, but proposed by us to be assigned to the class Oligoflexia (see below), could be distinguished from the other Deltaproteobacteria by the copy number of the ribosomal protein S1 gene. Karlin et al. [30] suggested that deltaproteobacterial genomes encode two ‘giant’ S1 ribosomal protein genes, while other bacteria encode only a single copy. We analysed 183 genomes of bacteria classified at the time of investigation (spring 2016) as Deltaproteobacteria by using the IMG system. Genomes of strains not classified at the species level, as well as ‘environmental genomes’ (metagenomic assemblies and single cell genomes) were excluded. Of the investigated Deltaproteobacteria 46.4% encoded one, 53.0% encoded two, and 0.6% encoded three genes annotated as ribosomal protein S1 genes (COG 0539). Strain MWH-Nonnen-W8redT, as well as none of the genomes currently representing the order Bdellovibrionales encoded two copies of the gene, however, Oligoflexus tunisiensis Shr3Tencodes two non-identical genes with this annotation. Obviously, the copy number of the ribosomal protein S1 gene is not a homogenous feature among bacteria currently classified as Deltaproteobacteria and is also not suitable for distinguishing those from other Proteobacteria.
For gaining a first hint on the phylogeny of strain MWH-Nonnen-W8redT comparative analyses of the 16S rRNA gene were performed. The strain encodes five copies of this ribosomal gene, all sharing identical sequences. Blast searches revealed that type strains with most similar genes belong to various classes of Proteobacteria. Surprisingly, no type strains sharing a 16S rRNA gene similarity higher than 85% with the new isolate could be found. However, inclusion of non-type-material in analyses resulted in much higher sequence similarity values. ‘Spirobacillus cienkowskii’, an uncultured pathogen of water flea (Daphnia spp.), which was described by Élie Metchnikoff almost 130 years ago [1] and rediscovered a few years ago by Rodrigues and colleagues [2], shares a 16S rRNA similarity of 96%. The morphology [1, 2] of this so far uncultured bacterium, as well as its 16S rRNA and gyrase B subunit gene sequences [2] have been described.
For reconstruction of the phylogenetic position of the strain multilocus protein trees with a large set of proteins extracted from a phylogenetically broad set of reference taxa were calculated. Overall, our phylogenetic analyses followed the strategy by Williams and Kelly [31]. We selected a set of genome-sequenced reference strains representing the whole phylogenetic widths of the phylum Proteobacteria, as well as a couple of representatives of other phyla. We tried to optimize the set of reference strains for high proportions of type strains, high proportions of high quality genomes, and a balanced taxonomic distribution across the phylum Proteobacteria. We also included the genome of Oligoflexus tunisiensis Shr3T [24], which represents the latest described class of Proteobacteria [7]. We screened each selected genome for the presence of the 98 protein families used in the analyses by Williams and Kelly [31] previously. If a family was lacking in one or more genomes, we rejected either the genome (if genome sequences of close relatives were available) or the protein family from the further analyses. These analyses were performed by using the IMG/ER (Integrated Microbial Genomes/Expert Review) system [20]. Protein families were identified by their COG (Clusters of Orthologous Groups) classification and families represented by more than one similar gene from the same genome in its COG category were usually rejected. Finally, the set of reference strains consisted of 84 strains (basic reference set, Suppl. Mat. Table S2) and the set of protein families consisted of 74 COGs (Suppl. Mat. Table S1). In a second analysis step, we enriched the set of reference strains for members of the phylum Acidobacteria and strains affiliated with the deltaproteobacterial order Bdellovibrionales to a total number of 93 reference strains (extended taxon set; Suppl. Mat. Tables S2 and S3).
Protein sequences were extracted from the genomes and separate alignments were established for each COG by using MUSCLE [32] implemented in the software MEGA7 [33]. Alignments were trimmed and protein sequences of each reference strain were concatenated. These alignments were masked with Gblocks V0.91b [34] in order to reduce phylogenetic noise potentially caused by unreliable aligned regions. Masking was optimized by stepwise relaxation of the masking and comparison of bootstrap results of trees calculated by RAxML [35] with the differently masked alignments. Four different masking settings were tested, which resulted in alignments consisting of 32 to 66% of the positions in the primary alignment. The average bootstrap values of the particular RAxML trees increased with increasing relaxation of the masking criteria. Finally, in contrast to the analyses performed by Williams and Kelly [31], quite relaxed criteria for masking were selected, which included, for instance, a high gap tolerance (setting ‘all’). The protein alignment used for construction of phylogenetic trees consisted of 20,950 alignment positions. Treeing was performed with the RAxML, MrBayes (version 3.2.1, [36]) and Neighbour-Joining (MEGA7) algorithms.
The trees calculated with these three different algorithms placed strain MWH-Nonnen-W8redT consistently in a branch formed by Bdellovibrio spp., Halobacteriovorax marinus and Oligoflexus tunisiensis (Fig. 3). These trees confirm the status of Oligoflexus tunisiensis Shr3T as the type of an own class of Proteobacteria [7]. However, these trees also suggest that the included representatives of the Bdellovibrionales are, in contrast to their current classification, not affiliated with the class Deltaproteobacteria. In order to test the phylogenetic position of the Bdellovibrionales, the set of reference strains was expanded by addition of four more strains currently classified as members of this order, as well as addition of some more taxa affiliated with the phylum Acidobacteria. This expansion of the taxon set did not change the formation of a well bootstrap-supported branch consisting of Oligoflexus, MWH-Nonnen-W8redT, and members of the order Bdellovibrionales (Suppl. Mat. Fig. S3) except Vampirovibrio chlorellavorus [37, 38]. The reconstructed phylogenetic position of V. chlorellavorus confirms that this strain does neither belong to the order Bdellovibrionales nor to the phylum Proteobacteria, but is affiliated with the Candidatus phylum Melainabacteria [39]. This candidatus phylum represents a sibling phylum to the Cyanobacteria [39, 40].
Fig. 3.
RAxML tree calculated with an alignment of 74 protein sequences extracted from 85 genome sequences. Taxa affiliated with the phylum Proteobacteria are shown by using colour codes. All proteobacterial classes (including ‘Zetaproteobacteria’) but Deltaproteobacteria are shown by colour-coded fonts. Deltaproteobacterial orders are highlighted by colour-coded areas. Furthermore, the two representatives of the phylum Acidobacteria are highlighted by red fonts. Bootstrap values obtained by the RAxML, MrBayes and Neighbour-Joining method are depicted. The coloured dots indicate nodes supported by different treeing methods.
In contrast to the tree based on the primary taxon set, the tree based on the extended set places the monophyletic lineage formed by Oligoflexus, MWH-Nonnen-W8redT, and the order Bdellovibrionales within the class Deltaproteobacteria (Suppl. Mat. Fig. S3). Importantly, the affiliation of this lineage with the Deltaproteobacteria lacks any bootstrap support, while the separate phylogenetic positioning suggested by the previous multi-protein tree (Fig. 3) and 16S rRNA trees ([7], Suppl. Mat. Figs. S5 and S6) clearly suggest a phylogenetic position outside of the class Deltaproteobacteria.
Another major difference between the two multi-protein trees is the position of the branch representing the phylum Acidobacteria. This group appeared in the first analysis between the Alphaproteobacteria and the major branch of the Deltaproteobacteria but the extension of the taxon set shifted the position between the Deltaproteobacteria and the Epsilonproteobacteria (Suppl. Mat. Fig. S3).
The results of the performed phylogenetic analyses have diverse taxonomic implications. They confirm the previously revealed paraphyletic nature of the phylum Proteobacteria. Independently established trees suggest that taxa representing the class Epsilonproteobacteria are more closely related to taxa affiliated to other phyla than Proteobacteria [31, 41–43] (Fig. 3; Suppl. Mat. Fig. S3). Epsilonproteobacteria and the deltaproteobacterial order Desulfurellales appear to be more distantly related to the major part of the Proteobacteria than the phylum Acidobacteria ([31], Fig. 3). Besides the currently single species class Oligoflexia, which cannot be evaluated for monophyly, all other proteobacterial classes but Deltaproteobacteria appear to be monophyletic clades. Interestingly, the polyphyletic nature of the Deltaproteobacteria was also suggested by analyses based on 16S rRNA gene sequences [26, 44]. Furthermore, it is obvious that the current class Deltaproteobacteria differs from all other classes of Proteobacteria in its phylogenetic breath. Obviously, revisions of the classes Deltaproteobacteria and Epsilonproteobacteria are required regarding membership of subgroups or taxonomic rank, however, these tasks are beyond the scope of this study. Nonetheless, the appropriate classification of strain MWH-Nonnen-W8redT proposed below requires a revision of the classification of the order Bdellovibrionales. Furthermore a reclassification of the species V. chlorellavorus in a new Candidatus phylum or class ‘Melainabacteria’ [29], would be advisable. However, since no viable culture of the type strain is available [29] and no cultured representative of the ‘Melainabacteria’ is available yet, a revision of the classification of V. chlorellavorus has to be postponed until a member of this clade can be cultivated.
Does MWH-Nonnen-W8redT represent ‘Spirobacillus cienkowskii’? Strain MWH-Nonnen-W8redT is regarding cell morphology, morphological variability and pigmentation very similar to ‘Spirobacillus cienkowskii’ characterized by Metchnikoff in 1889 [1]. Metchnikoff observed and described the life cycle of ‘S. cienkowskii’ in infected Daphnia. He reported various morphological forms, including rods, spirillae, and filaments. Appearance of such ‘S. cienkowskii’ morphotypes in infected Daphnia spp. was confirmed by Rodrigues and colleagues [2]. By using fluorescent in situ hybridization (FISH) probes specific for ‘S. cienkowskii’ they demonstrated that all these morphotypes belong to this taxon. We observed the same morphotypes in cultures of MWH-Nonnen-W8redT including the unusual densely coiled spirals (compare Fig. 1 and Fig 2D in [2]), however formation of spirals occurred only under specific cultivation conditions. Obviously, both taxa share quite unusual morphologic features and a morphological plasticity. Importantly, very similar morphologies including spirillae, filaments, curved rods and spherical cells were observed for the type strain of Oligoflexus tunisiensis [7, 10], which is much more distantly related to strain MWH-Nonnen-W8redT as compared to ‘S. cienkowskii’ (Fig. 4). By contrast, only the latter two taxa seem to share pigmentation by a red or pink-red carotenoid. Green detected a carotenoid in ‘S. cienkowskii’ [45] and the genome of MWH-Nonnen-W8redT encodes genes putatively enabling this organism to synthesise at least the red pigmented carotenoid lycopene (Suppl. Mat. Table S5). These phenotypical similarities between strain MWH-Nonnen-W8redT and ‘S. cienkowskii’ are contrasted by differences in 16S rRNA and gyrB sequence. The 16S rRNA sequence of ‘S. cienkowskii’ determined by Rodrigues et al. [2] and the sequence of MWH-Nonnen-W8redT share a similarity of only 95.9% (57 bp different), and importantly, are also distinct by five nucleotide insertions at two sites of the ‘S. cienkowskii’ gene. The gyrB gene of the two taxa shared only a nucleotide sequence similarity of 82% (protein identity 92%) but the gyrB genes of both taxa share very similar G+C contents of about 36%. Interestingly, Rodrigues and colleagues found no differences in the 16S rRNA genes across a couple of European ‘S. cienkowskii’ populations but a sequences difference of 1% between European and North American populations [2]. All these sequences were obtained from infected Daphnia spp. including at least three different host species. The very high sequence similarity among ‘S. cienkowskii’ populations across host species and continents makes it unlikely that MWH-Nonnen-W8redT represents an ‘S. cienkowskii’-like pathogen of Daphnia spp.
Fig. 4.
Revised taxonomy of the class Oligoflexia. The shown Neighbour-Joining tree was calculated with almost complete 16S rRNA sequences of bacteria proposed to be classified in the previously monotypic class Oligoflexia. Alignment positions with gaps in any sequence were completely omitted for the tree calculation, which resulted in an alignment length of 1343 positions. Phylogenetic distances were calculated by using the Tamura 3-parameter substitution model. Sequences of taxa not affiliated with the phylum Proteobacteria were used as outgroup (not shown). Bootstrap values obtained with the neighbour joining, the maximum likelihood and the maximum parsimony methods (1000, 100, 100 replications, respectively) are indicated. Note that this 16S rRNA tree differs regarding the position of the revised Bdellovibrionales from the calculated multi-protein trees (Fig. 4 and Suppl. Mat. Fig. S3), however the responsible node is only weakly supported in the 16S rRNA tree. The branching order of the calculated NJ and ML tree is identical.
Other 16S rRNA sequences sharing similarities > 96% with the gene of strain MWH-Nonnen-W8redT (Suppl. Mat. Figs. S5 and S6) mainly represent aquatic bacteria of unknown lifestyle [3–6]. Some of these organisms dwelled in surface freshwater habitats like Yellowstone Lake [6], while other sequences were obtained from a peat bog [4] or a subsurface water pool [5]. Because it is unlikely that in all those habitats daphnids are present, it can be assumed that at least some organisms sharing with ‘S. cienkowskii’ 16S rRNA sequence similarities ≥ 96% do not represent obligate pathogens of Daphnia spp.
Two experiments were performed in order to test if MWH-Nonnen-W8redT is able to infect Daphnia cf. pulex. The species D. pulex was reported to be susceptible to infections by ‘S. cienkowskii’ [2]. In a first experiment daphnids were challenged with 1.5 x 106 MWH-Nonnen-W8redT cells mL-1. Besides the added bacteria, the daphnids also received algae (Cryptomonas sp. 26.80) as food. The batch cultures (40 ml, six replicates) containing the daphnids were fed every 2-3 days with a mixture containing algae and the tested bacteria. Throughout the experiment, which lasted for 23 days, the added food cocktail contained 100 times more bacterial carbon (MWH-Nonnen-W8redT) than algal carbon (Cryptomonas sp.). Two controls were included in the experiment, both with six replicates (40 mL each). The first control (Cryptomonas only) received no bacteria but the same amount of algal carbon as the test treatments. The second control received instead MWH-Nonnen-W8redT the terrestrial bacterium Cupriavidus basilensis DSM 11853T [46] and the algal food. The total amount of carbon, as well as the carbon ratio of algae to bacteria (1:100) was identical in the two treatments receiving bacteria. The total number of daphnids and the number of off springs were counted during the experiment at 18 days. Special attention was paid to the appearance of red coloured daphnids and dead daphnids. Interestingly, the daphnids grew better in the control treatment without added bacteria, however, in the treatment, which received strain MWH-Nonnen-W8redT the daphnids grew better than in the treatment with C. basilensis DSM 11853T (Suppl. Mat. Fig. S7). Red pigmented daphnids or other hints on a bacterial infection were not observed in any replicate of all three treatments. A second experiment challenging daphnids with higher concentrations of MWH-Nonnen-W8redT cells was conducted in order to test if infections occur at higher doses. Four parallel treatments receiving 3, 6, 9, and 12 x 106 MWH-Nonnen-W8redT cells mL-1 were established. All treatments received the same amount of algal food and were fed in the same way during the experiment. Again, infected daphnids were not observed in any of the four treatments.
In general, the two experiments did not result in any hint on a pathogenic potential of strain MWH-Nonnen-W8redT regarding Daphnia cf. pulex, however, we cannot really exclude that the strain is able to infect daphnids if an appropriate host or appropriate infection conditions would be given. We note that a pathogenic potential of strain MWH-Nonnen-W8redT could not be demonstrated so far.
Proposal of the new species Silvanigrella aquatica gen. nov., sp. nov. and required taxonomic revisions
The large phylogenetic distance of strain MWH-Nonnen-W8redT to any described species does not leave any doubt that this strain represents a new species. According to the obtained phylogenetic trees, Oligoflexus tunisiensis and members of the order Bdellovibrionales represent the closest related described species. We propose to establish for the investigated strain the new genus and species Silvanigrella aquatica gen. nov., sp. nov. and to place it in the class Oligoflexia [7] of the phylum Proteobacteria [47]. The currently available characterization of ‘S. cienkowskii’ is too superficial to provide hints if strain MWH-Nonnen-W8redT and those pathogens of daphnids should be placed in the same genus. Because of lack of evidence for pathogenicity in strain MWH-Nonnen-W8redT and because of the inappropriateness of the name “bacillus” for a proteobacterium, we refrain from proposing ‘Spirobacillus’ as genus name for the new strain.
The 16S rRNA sequence similarity value of less than 82% between Oligoflexus tunisiensis ShrT, the sole type strain in the class, and strain MWH-Nonnen-W8redT, provides strong evidence for placement of the two strains in distinct orders [43]. Therefore, we propose to establish for Silvanigrella aquatica gen. nov., sp. nov. the new family Silvanigrellaceae fam. nov. to be placed in the new order Silvanigrellales ord. nov. of the class Oligoflexia. Furthermore, the multi-protein (Fig. 3) and the 16S rRNA phylogenies (Fig. 4) presented here, suggest the transfer of the order Bdellovibrionales from the class Deltaproteobacteria to the class Oligoflexia. A rather isolated position of the genus Bdellovibrio or the Bdellovibrionales within the Deltaproteobacteria was shown previously [22, 48] and lack of bootstrap support for placement in this class was shown previously [22]. Interestingly, the multi-protein tree calculated by Williams and Kelly [31], which did not include Oligoflexus, and the multi-protein tree presented here (Fig. 3) differ in bootstrap support for the class Deltaproteobacteria. While the tree lacking Oligoflexus, integrated Bdellovibrio in the Deltaproteobacteria but lacked bootstrap support for this class (39%), our tree excludes the Bdellovibrionales from the Deltaproteobacteria and supports the remaining class with high bootstrap supports in trees calculated with two out of three algorithms (Fig. 3, Suppl. Mat. Fig. S3). Only the NJ algorithm did not result in a sufficient bootstrap support. Based on phylogenetic analyses of multi-protein alignments, the transfer of the order Bdellovibrionales [49] from the class Deltaproteobacteria [50] to the class Oligoflexia [7] is proposed.
According to phylogeny and because none of the type species of the genera Bacteriovorax, Peredibacter and Halobacteriovorax share 16S rRNA sequence similarities of more than 82% with the type species of Bdellovibrio we propose to establish the new order Bacteriovoracales ord. nov. for those three genera (Fig. 4). Finally, based on phylogenetic analyses of 16S rRNA genes (Fig. 4), the transfer of the family Pseudobacteriovoracaceae McCauley et al. 2015 [44] from the order Bdellovibrionales Garrity et al. 2006 [51] to the order Oligoflexiales Nakai et al. 2014 [7] is proposed. The description of the family remains as given by McCauley et al. (2015) [44].
Description of Silvanigrella gen nov.
Silvanigrella (Sil.va.ni.grel’la. N.L. fem. dim. n. Silvanigrella named after Silva nigra the Latin geographic name of the Schwarzwald (Black Forest) mountains located in the South-West of Germany).
The description of the genus is based on the polyphasic characterization of the type strain of the sole species proposed to be affiliated currently with this genus. Features probably characterizing other strains affiliated with this genus are as follows: Gram-negative, pleomorphic cell morphology, aerobic chemoorganoheterophs, red pigmentation.
The genus is a member of the class Oligoflexia [7] of the phylum Proteobacteria [47]. The type species is Silvanigrella aquatica sp. nov.
Description of Silvanigrella aquatica sp. nov.
Silvanigrella aquatica (a.qua’ti.ca. L. fem. adj. aquatica living, growing, or found in the water, aquatic).
The type strain is MWH-Nonnen-W8redT (DSM 23856T =CCUG 58639T), which is the only strain investigated so far. Apart from the characters given for the genus, the species is characterized as follows: catalase weakly positive, oxidase negative, cells are motile, cell morphology is pleomorphic, ranging from short and large rod-shaped cells to filamentous morphology and formation of densely coiled spirals. Red pigmentation. Aerobic chemoorganoheterotroph, anaerobic growth was neither observed on standard NSY medium nor NSY medium enriched with nitrate. Temperature range is 10 °C to 32 °C, and the salt tolerance is up to 1.0 % NaCl (w/v), however, growth at 1.0% salinity was quite weak. Assimilates D-mannose, D-glucose, L-proline, L-glutamate, and L-alanine. Weak assimilation of acetate, fumarate and glycine. No assimilation of glyoxylate, glycolate, propionate, oxaloacetate, malonate, lactate, citrate, D-xylose, D-fucose, D-sorbitole, L-methionine, and betaine. Main fatty acids are iso-C15:0, anteiso-C15:0, feature 3 including C16:1 ω7c and iso-C15:0 2-OH, C16:0, C17:1 ω8c and C17:0. Main polar lipids are phosphatidylethanolamine and phosphatidylglycerol. Contains unidentified quinones, known ubi- and menaquinones could not be detected.
The type strain was isolated from a water sample obtained from a freshwater lake located in the Black Forest Mountains, Germany. The genome of the type strain has a size of about 3.5 Mbp and a G+C content of 32.6 mol%. The genome sequence was deposited in DDBJ/EMBL/GenBank under the Accession Numbers CP017834 - CP017838.
Description of Silvanigrellaceae fam. nov.
Silvanigrellaceae (Sil.va.ni.grel.la.ce’ae. N.L. fem. dim. n. Silvanigrella type genus of the family; suff. -aceae ending to denote a family; N.L. fem. pl. n. Silvanigrellaceae the family of the genus Silvanigrella).
The description is the same as for the genus Silvanigrella. The type genus is Silvanigrella gen. nov.
Description of Silvanigrellales ord. nov.
Silvanigrellales (Sil.va.ni.grel.la’les. N.L. fem. dim. n. Silvanigrella type genus of the order; suff. -ales ending to denote an order; N.L. fem. pl. n. Silvanigrellales the order of the genus Silvanigrella).
The description is based on phylogenetic analyses of 16S rRNA gene sequences. Includes the family Silvanigrellaceae fam. nov. and Candidatus Turabacter [52], as well as undescribed or not validly described taxa, which were predominantly found in freshwater systems. This includes strains isolated from the skin of an amphibian [3], the pathogen of water flea ‘Spirobacillus cienkowskii’ [2], the non-aquatic isolate GLA1 (accession number KF246685) from a human lymph node aspirate (Humrighouse, Whitney, and McQuiston, Genbank deposition), uncultured bacteria found in surface freshwater systems like natural [6] and artificial lakes [53], wetlands like a peat bog system Kip [4], or a subsurface epiphreatic pool in a karst cave [5]. The type genus is Silvanigrella gen. nov.
Description of Bacteriovoracales ord. nov.
Bacteriovoracales (Bac.te.ri.o.vo.ra.ca’les. N.L. masc. n. Bacteriovorax, type genus of the family; suff. -ales, ending to denote an order; N.L. fem. pl. n. Bacteriovoracales, the order of the genus Bacteriovorax).
Encompasses the families Bacteriovoracaceae Davidov and Jurkevitch 2004 [54] and Halobacteriovoraceae Koval et al. 2015 [55]. The description of the order is based on the descriptions of the included families. This order is composed of Gram-negative, vibroid bacteria. They are obligate or facultative predators of various Gram-negative bacteria. The type genus is Bacteriovorax [56]. The order belongs to the class Oligoflexia.
Emended description of the order Bdellovibrionales Garrity et al., 2005a
Bdellovibrionales (Bdel.lo.vib.ri.o.na'les. N.L. masc. n. Bdellovibrio, type genus of the order; suff. -ales, ending denoting an order; N.L. fem. pl. n. Bdellovibrionales, the order of the genus Bdellovibrio).
Includes solely the genera Bdellovibrio Stolp and Starr 1963 [57], Micavibrio Lambina et al. 1982 [58] and Vampirivibrio Gromov and Mamkayeva 1980 [38], i.e. members of the illegitimate family ‘Bdellovibrionaceae’ [59]. The description of the order Bdellovibrionales remains as given by Garrity et al. [49] except for the exclusion of the families Bacteriovoracaceae, Halobacteriovoraceae, and Pseudobacteriovoracaceae. The type genus is Bdellovibrio Stolp and Starr 1963 [57].
Emended description of the order Oligoflexiales Nakai et al., 2014
Oligoflexales (O.li.go.fle.xa'les. N.L. masc. n. Oligoflexus type genus of the order; suff. -ales ending to denote an order; N.L. fem. pl. n. Oligoflexales the order of the genus Oligoflexus).
Encompasses the families Oligoflexiaceae and Pseudobacteriovoracaceae. The description is based on the descriptions of the genera Oligoflexus [7] and Pseudobacteriovorax [44]. Gram negative, chemoorganoheterotrophs, obligate aerobes, pleomorphic including filamentous stages. The type genus is Oligoflexus.
Emended description of the class Oligoflexia Nakai et al., 2014
Oligoflexia (O.li.go.fle’xi.a. N.L. masc. n. Oligoflexus type genus of the type order of the class; suff. -ia ending to denote a class; N.L. fem. pl. n. Oligoflexia the class of the order Oligoflexales).
The class is described on the basis of a phylogenetic analysis of 16S rRNA gene sequences presented by Nakai et al. [7], and additionally includes the monophyletic lineage formed by ‘Spirobacillus cienkowskii’ (Accession number of the 16S rRNA gene EU220836) and related cultured and uncultured bacteria (compare Fig. 3 in [7]). Includes the orders Oligoflexiales, Bdellovibrionales, Bacteriovoracales ord. nov., and Silvanigrellales ord. nov.. The type order is Oligoflexiales.
Emended description of the class Deltaproteobacteria Kuever et al., 2006
Deltaproteobacteria (Del.ta.pro.te.o.bac.te' ri.a. Gr. n. delta, name of the fourth letter of Greek alphabet; Gr. or L. n. Proteus, Greek god of the sea, capable of assuming many different shapes; N.L. n. bacter, a rod; suff. -ia, ending to denote a class; N.L. neut. pl. n. Deltaproteobacteria).
The description of the class Deltaproteobacteria remains as given by [50] with the exception that the order Bdellovibrionales with its current taxa ‘Bdellovibrionaceae’, Bacteriovoracaeae, Halobacteriovoraceae and Pseudobacteriovoracaceae are excluded from the classis.
Supplementary Material
Acknowledgements
We thank P. Luijckx and D. Ebert (University of Basel, Switzerland) for conduction of infection experiments on Daphnia spp. not reported here. We are thankful to B. Tindall who analyzed the quinones and P. Schumann who carried out the peptidoglycan analysis. The excellent technical assistance of G. Pötter, A. Frühling and C. Berg is acknowledged.
Funding Information
This study was supported by the Austrian Science Fund (FWF) project I482-B09 and the European Science Foundation (ESF) project FREDI.
Footnotes
Conflicts of Interest
The authors declare the absence of any conflict of interest.
Ethical Statement
The presented study does not include any experimental work with humans or vertebrates.
References
- 1.Metchnikoff E. Contributions á l'etude du pleomorphisme des bacteriens. Annales de l'Institut Pasteur. 1889;3:61–8. [Google Scholar]
- 2.Rodrigues JL, Duffy MA, Tessier AJ, Ebert D, Mouton L, Schmidt TM. Phylogenetic characterization and prevalence of "Spirobacillus cienkowskii", a red-pigmented, spiral-shaped bacterial pathogen of freshwater Daphnia species. Appl Environ Microbiol. 2008;74(5):1575–82. doi: 10.1128/AEM.02438-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walke JB, Becker MH, Hughey MC, Swartwout MC, Jensen RV, Belden LK. Most of the dominant members of amphibian skin bacterial communities can be readily cultured. Appl Environ Microbiol. 2015;81(19):6589–600. doi: 10.1128/AEM.01486-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kip N, Fritz C, Langelaan ES, Pan Y, Bodrossy L, Pancotto V, et al. Methanotrophic activity and diversity in different Sphagnum magellanicum dominated habitats in the southernmost peat bogs of Patagonia. Biogeosciences. 2012;9(1):47–55. [Google Scholar]
- 5.Shabarova T, Widmer F, Pernthaler J. Mass effects meet species sorting: transformations of microbial assemblages in epiphreatic subsurface karst water pools. Environ Microbiol. 2013;15(9):2476–88. doi: 10.1111/1462-2920.12124. [DOI] [PubMed] [Google Scholar]
- 6.Clingenpeel S, Macur RE, Kan J, Inskeep WP, Lovalvo D, Varley J, et al. Yellowstone Lake: high-energy geochemistry and rich bacterial diversity. Environ Microbiol. 2011;13(8):2172–85. doi: 10.1111/j.1462-2920.2011.02466.x. [DOI] [PubMed] [Google Scholar]
- 7.Nakai R, Nishijima M, Tazato N, Handa Y, Karray F, Sayadi S, et al. Oligoflexus tunisiensis gen. nov., sp. nov., a Gram-negative, aerobic, filamentous bacterium of a novel proteobacterial lineage, and description of Oligoflexaceae fam. nov., Oligoflexales ord. nov. and Oligoflexia classis nov. Int J Syst Evol Microbiol. 2014;64(Pt 10):3353–9. doi: 10.1099/ijs.0.060798-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hahn MW, Stadler P, Wu QL, Pockl M. The filtration-acclimatization method for isolation of an important fraction of the not readily cultivable bacteria. J Microbiol Methods. 2004;57(3):379–90. doi: 10.1016/j.mimet.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Reasoner DJ, Geldreich EE. A new medium for the enumeration and subculture of bacteria from potable water. Appl Environ Microbiol. 1985;49(1):1–7. doi: 10.1128/aem.49.1.1-7.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakai R, Naganuma T. Oligoflexia, the newest class of the phylum Proteobacteria, consisting of only one cultured species and uncultured bacterial phylotypes from diverse habitats. Journal of Phylogenetics and Evolutionary Biology. 2015;3(1):141. [Google Scholar]
- 11.Marbach A, Shilo M. Dependence of marine bdellovibrios on potassium, calcium, and magnesium ions. Appl Environ Microbiol. 1978;36(1):169–77. doi: 10.1128/aem.36.1.169-177.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hahn MW, Lang E, Brandt U, Wu QL, Scheuerl T. Emended description of the genus Polynucleobacter and the species Polynucleobacter necessarius and proposal of two subspecies, P. necessarius subsp. necessarius subsp. nov. and P. necessarius subsp. asymbioticus subsp. nov. Int J Syst Evol Microbiol. 2009;59:2002–9. doi: 10.1099/ijs.0.005801-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hahn MW, Schmidt J, Pitt A, Taipale SJ, Lang E. Reclassification of four Polynucleobacter necessarius strains as Polynucleobacter asymbioticus comb. nov., Polynucleobacter duraquae sp. nov., Polynucleobacter yangtzensis sp. nov., and Polynucleobacter sinensis sp. nov., and emended description of the species Polynucleobacter necessarius. Int J Syst Evol Microbiol. 2016;66:2883–92. doi: 10.1099/ijsem.0.001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sasser M. Identification of bacteria by gas chromatography of cellular fatty acids. USFCC Newsl. 1990;20:16. [Google Scholar]
- 15.Tindall BJ. Lipid composition of Halobacterium lacusprofundi. FEMS Microbiol Lett. 1990;66(1–3):199–202. [Google Scholar]
- 16.Tindall BJ. A comparative study of the lipid composition of Halobacterium saccharovorum from various sources. Syst Appl Microbiol. 1990;13(2):128–30. [Google Scholar]
- 17.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Canadian journal of biochemistry and physiology. 1959;37(8):911–7. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 18.Schumann P. Peptidoglycan structure. In: Rainey F, Oren A, editors. Methods in Microbiology. Vol. 38. pp. 101–29. Taxonomy of Prokaryotes. Methods in Microbiology, 382011. [Google Scholar]
- 19.Meincke L, Copeland A, Lapidus A, Lucas S, Berry KW, Del Rio TG, et al. Complete genome sequence of Polynucleobacter necessarius subsp. asymbioticus type strain (QLW-P1DMWA-1T) Stand Genomic Sci. 2012;6(1):74–83. doi: 10.4056/sigs.2395367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Markowitz VM, Chen IMA, Palaniappan K, Chu K, Szeto E, Grechkin Y, et al. IMG: the integrated microbial genomes database and comparative analysis system. Nucleic Acids Res. 2012;40(D1):D115–D22. doi: 10.1093/nar/gkr1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen H, Brinkac LM, Mishra P, Li N, Lymperopoulou DS, Dickerson TL, et al. Draft genome sequences for the obligate bacterial predators Bacteriovorax spp. of four phylogenetic clusters. Stand Genomic Sci. 2015;10(1):11. doi: 10.1186/1944-3277-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crossman LC, Chen H, Cerdeno-Tarraga AM, Brooks K, Quail MA, Pineiro SA, et al. A small predatory core genome in the divergent marine Bacteriovorax marinus SJ and the terrestrial Bdellovibrio bacteriovorus. ISME J. 2013;7(1):148–60. doi: 10.1038/ismej.2012.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Konstantinidis KT, Tiedje JM. Genomic insights that advance the species definition for prokaryotes. Proc Natl Acad Sci U S A. 2005;102(7):2567–72. doi: 10.1073/pnas.0409727102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakai R, Fujisawa T, Nakamura Y, Baba T, Nishijima M, Karray F, et al. Genome sequence and overview of Oligoflexus tunisiensis Shr3T in the eighth class Oligoflexia of the phylum Proteobacteria. Stand Genomic Sci. 2016;11:90. doi: 10.1186/s40793-016-0210-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rendulic S, Jagtap P, Rosinus A, Eppinger M, Baar C, Lanz C, et al. A predator unmasked: life cycle of Bdellovibrio bacteriovorus from a genomic perspective. Science. 2004;303(5658):689–92. doi: 10.1126/science.1093027. [DOI] [PubMed] [Google Scholar]
- 26.Pasternak Z, Pietrokovski S, Rotem O, Gophna U, Lurie-Weinberger MN, Jurkevitch E. By their genes ye shall know them: genomic signatures of predatory bacteria. ISME J. 2013;7(4):756–69. doi: 10.1038/ismej.2012.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodriguez-R L, Konstantinidis K. Bypassing cultivation to identify bacterial species. Microbe Magazine. 2014;9(3):111–8. [Google Scholar]
- 28.Gupta RS. The phylogeny of proteobacteria: relationships to other eubacterial phyla and eukaryotes. FEMS Microbiol Rev. 2000;24(4):367–402. doi: 10.1111/j.1574-6976.2000.tb00547.x. [DOI] [PubMed] [Google Scholar]
- 29.Soo RM, Woodcroft BJ, Parks DH, Tyson GW, Hugenholtz P. Back from the dead; the curious tale of the predatory cyanobacterium Vampirovibrio chlorellavorus. PeerJ. 2015;3:e968. doi: 10.7717/peerj.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karlin S, Brocchieri L, Mrazek J, Kaiser D. Distinguishing features of delta-proteobacterial genomes. Proc Natl Acad Sci U S A. 2006;103(30):11352–7. doi: 10.1073/pnas.0604311103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams KP, Kelly DP. Proposal for a new class within the phylum Proteobacteria, Acidithiobacillia classis nov., with the type order Acidithiobacillales, and emended description of the class Gammaproteobacteria. Int J Syst Evol Microbiol. 2013;63(Pt 8):2901–6. doi: 10.1099/ijs.0.049270-0. [DOI] [PubMed] [Google Scholar]
- 32.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32(5):1792–7. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar S, Stecher G, Tamura K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:1870–4. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Talavera G, Castresana J. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst Biol. 2007;56(4):564–77. doi: 10.1080/10635150701472164. [DOI] [PubMed] [Google Scholar]
- 35.Stamatakis A, Hoover P, Rougemont J. A rapid bootstrap algorithm for the RAxML web servers. Syst Biol. 2008;57(5):758–71. doi: 10.1080/10635150802429642. [DOI] [PubMed] [Google Scholar]
- 36.Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, et al. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 2012;61(3):539–42. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gromov BV, Mamkaeva KA. [Electron microscopic study of parasitism by Bdellovibrio chlorellavorus bacteria on cells of the green alga Chlorella vulgaris] Tsitologiia. 1972;14(2):256–60. [PubMed] [Google Scholar]
- 38.Gromov BV, Mamkaeva KA. [New genus of bacteria, Vampirovibrio, parasitizing chlorella and previously assigned to the genus Bdellovibrio] Mikrobiologiia. 1980;49(1):165–7. [PubMed] [Google Scholar]
- 39.Di Rienzi SC, Sharon I, Wrighton KC, Koren O, Hug LA, Thomas BC, et al. The human gut and groundwater harbor non-photosynthetic bacteria belonging to a new candidate phylum sibling to Cyanobacteria. eLife. 2013;2:e01102. doi: 10.7554/eLife.01102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soo RM, Skennerton CT, Sekiguchi Y, Imelfort M, Paech SJ, Dennis PG, et al. An expanded genomic representation of the phylum Cyanobacteria. Genome biology and evolution. 2014;6(5):1031–45. doi: 10.1093/gbe/evu073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yeoh YK, Sekiguchi Y, Parks DH, Hugenholtz P. Comparative genomics of Candidate phylum TM6 suggests that parasitism is widespread and ancestral in this lineage. Mol Biol Evol. 2016;33(4):915–27. doi: 10.1093/molbev/msv281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hug LA, Baker BJ, Anantharaman K, Brown CT, Probst AJ, Castelle CJ, et al. A new view of the tree of life. Nature Microbiology. 2016;1:16048. doi: 10.1038/nmicrobiol.2016.48. [DOI] [PubMed] [Google Scholar]
- 43.Yarza P, Yilmaz P, Pruesse E, Glockner FO, Ludwig W, Schleifer K-H, et al. Uniting the classification of cultured and uncultured bacteria and archaea using 16S rRNA gene sequences. Nat Rev Micro. 2014;12(9):635–45. doi: 10.1038/nrmicro3330. [DOI] [PubMed] [Google Scholar]
- 44.McCauley EP, Haltli B, Kerr RG. Description of Pseudobacteriovorax antillogorgiicola gen. nov., sp. nov., a bacterium isolated from the gorgonian octocoral Antillogorgia elisabethae, belonging to the family Pseudobacteriovoracaceae fam. nov., within the order Bdellovibrionales. Int J Syst Evol Microbiol. 2015;65(Pt 2):522–30. doi: 10.1099/ijs.0.066266-0. [DOI] [PubMed] [Google Scholar]
- 45.Green J. Carotenoid pigment in Spirobacillus cienkowskii Metchnikoff, a pathogen of Cladocera. Nature. 1959;183(4653):56–7. doi: 10.1038/183056a0. [DOI] [PubMed] [Google Scholar]
- 46.Steinle P, Stucki G, Stettler R, Hanselmann KW. Aerobic mineralization of 2,6-dichlorophenol by Ralstonia sp. strain RK1. Appl Environ Microbiol. 1998;64(7):2566–71. doi: 10.1128/aem.64.7.2566-2571.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stackebrandt E, Murray RGE, Trüper HG. Proteobacteria classis nov., a name for the phylogenetic taxon that includes the “purple bacteria and their relatives”. Int J Syst Evol Microbiol. 1988;38(3):321–5. [Google Scholar]
- 48.Singer E, Emerson D, Webb EA, Barco RA, Kuenen JG, Nelson WC, et al. Mariprofundus ferrooxydans PV-1 the first genome of a marine Fe(II) oxidizing Zetaproteobacterium. PLoS ONE. 2011;6(9):e25386. doi: 10.1371/journal.pone.0025386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garrity GM, Bell JA, Lilburn T. Order VII. Bdellovibrionales ord. nov. In: Brenner DJ, Krieg NR, Staley JT, Garrity GM, editors. Bergeys Manual of Systematic Bacteriology. second edition. 2005. p. 1040. 2 (The Proteobacteria), part C (The Alpha-, Beta-, Delta-, and Epsilonproteobacteria) [Google Scholar]
- 50.Kuever J, Rainey FA, Widdel F. Class VI. Deltaproteobacteria class nov. In: Brenner DJ, Krieg NR, Staley JT, Garrity GM, editors. Bergey’s Manual of Systematic Bacteriology. Vol. 2. New York: Springer; 2005. part C. [Google Scholar]
- 51.Garrity GM, Bell JA, Lilburn T. Order VII. Bdellovibrionales ord. nov. In: Brenner DJ, Krieg NR, Staley JT, Garrity GM, editors. Bergey’s Manual of Systematic Bacteriology. Vol. 2. New York: Springer; 2005. Part C. [Google Scholar]
- 52.Dirren S, Posch T. Promiscuous and specific bacterial symbiont acquisition in the amoeboid genus Nuclearia (Opisthokonta) FEMS Microbiol Ecol. 2016;92(8) doi: 10.1093/femsec/fiw105. [DOI] [PubMed] [Google Scholar]
- 53.Shaw AK, Halpern AL, Beeson K, Tran B, Venter JC, Martiny JBH. It's all relative: ranking the diversity of aquatic bacterial communities. Environ Microbiol. 2008;10(9):2200–10. doi: 10.1111/j.1462-2920.2008.01626.x. [DOI] [PubMed] [Google Scholar]
- 54.Davidov Y, Jurkevitch E. Diversity and evolution of Bdellovibrio-and-like organisms (BALOs), reclassification of Bacteriovorax starrii as Peredibacter starrii gen. nov., comb. nov., and description of the Bacteriovorax–Peredibacter clade as Bacteriovoracaceae fam. nov. Int J Syst Evol Microbiol. 2004;54(5):1439–52. doi: 10.1099/ijs.0.02978-0. [DOI] [PubMed] [Google Scholar]
- 55.Koval SF, Williams HN, Stine OC. Reclassification of Bacteriovorax marinus as Halobacteriovorax marinus gen. nov., comb. nov. and Bacteriovorax litoralis as Halobacteriovorax litoralis comb. nov.; description of Halobacteriovoraceae fam. nov. in the class Deltaproteobacteria. Int J Syst Evol Microbiol. 2015;65(2):593–7. doi: 10.1099/ijs.0.070201-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baer ML, Ravel J, Chun J, Hill RT, Williams HN. A proposal for the reclassification of Bdellovibrio stolpii and Bdellovibrio starrii into a new genus, Bacteriovorax gen. nov. as Bacteriovorax stolpii comb. nov. and Bacteriovorax starrii comb. nov., respectively. Int J Syst Evol Microbiol. 2000;50(1):219–24. doi: 10.1099/00207713-50-1-219. [DOI] [PubMed] [Google Scholar]
- 57.Stolp H, Starr MP. Bdellovibrio bacteriovorus gen. et sp. n., a predatory, ectoparasitic, and bacteriolytic microorganism. Antonie Van Leeuwenhoek. 1963;29(1):217–48. doi: 10.1007/BF02046064. [DOI] [PubMed] [Google Scholar]
- 58.Lambina VA, Afinogenova AV, Romai Penabad S, Konovalova SM, Pushkareva AP. [Micavibrio admirandus gen. et sp. nov] Mikrobiologiia. 1982;51(1):114–7. [PubMed] [Google Scholar]
- 59.Garrity GM, Bell JA, Lilburn T. Family I. Bdellovibrionaceae fam. nov. In: Brenner DJ, K N, Staley JT, Garrity GM, editors. Bergey’s Manual of Systematic Bacteriology. Vol. 2. New York: Springer; 2005. pp. 1040–1. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.