Abstract
Acute kidney injury (AKI) is a common clinical syndrome with a broad aetiological profile. It complicates about 5% of hospital admissions and 30% of admissions to intensive care units (ICU). During last 20 years has been a significant change in the spectrum of severe AKI such that it is no longer mostly a single organ phenomenon but rather a complex multisystem clinical problem. Despite great advances in renal replacement technique (RRT), mortality from AKI, when part of MOF, remains over 50%.
The changing nature of AKI requires a new approach using the new advanced technology. Clinicians can provide therapies tailored to time constraints (intermittent, continuous, or extended intermittent), haemodynamic, and metabolic requirements and aimed at molecules of variable molecular weight.
Peritoneal dialysis (PD) is technically the simplest form of RRT and is still commonly used worldwide. The problems include difficulty in maintaining dialysate flow, peritoneal infection, leakage, protein losses, and restricted ability to clear fluid and uraemic wastes. PD is the preferred treatment modality for AKI in pediatric practice. Patients that are hemodynamically stable can be managed with intermittent hemodyalisis (IHD), whereby relatively short (3 to 4 h) dialysis sessions may be performed every day or every other day. Patients who are haemodynamically unstable are best managed using continuous renal replacement therapies (CRRT), which allow for continuous fine-tuning of intravascular volume, easier correction of hypervolemia, better solute removal, more accurately correction of metabolic acidosis, and offers possibilities for unlimited energy support. Recently, “hybrid or sustained low-efficiency dialysis (SLED) was introduced as a method which combines the advantages of IHD with those of CRRT. In this technique, classic dialysis hardware is used at low blood and dialysate flow rates, for prolonged period of time (6 to 12 h/day). SLED offers more haemodynamic stability, better correstion of hypervolaemia, and more adequate solue removal, compared with IHD. In conclusion, AKI in the ICU is increasingly a component of sepsis and MSOF, and the development of rational strategies for initiation, dosing, and effective delivery of RRT in this setting is among the greatest challenges facing nephrologists and intensivists today.
Keywords: acute kidney injury, intensive care unit, peritoneal dialysis, intermittent hemodialysis, continuous renal replacement therapy, “hybrid”, dialysis, sustained low-efficiency dialysis
INTRODUCTION
Acute kidney injury (AKI) is defined as deterioration of renal function over hours, days to weeks. The mortality rate of AKI is 50-80% in intensive care unit (ICU) patients, and has not declined significantly since the initial marked benefit of acute dialysis therapy. Although multiple system organ failure (MSOF) and other comorbidities contribute to its high mortality rate, AKI independently increases morbidity and mortality. AKI-specific severity of illness scoring systems have been validated to predict prognosis in ICU AKI, accounting for both severity of renal failure and associated MSOF (1,2). A prospective multicenter ICU study of AKI found that subjects with septic AKI had a far higher mortality rate (74% vs 45%, p < 0.001) than those without sepsis (1). Septic AKI is the dominant problem in managing ICU patients requiring renal replacement therapy (RRT) (3).
When should RRT be started?
A number of the indications for RRT are uncontroversial, including uremic symptoms (anorexia, nausea, vomiting) or signs (bleeding, encephalopathy, uremic pericarditis), hyperkalemia refractory to medical management, volume overload unresponsive to fluid restriction and diuretics, metabolic acidosis that is severe or accompanied by volume overload (precluding adequate bicarbonate therapy), certain dialyzable intoxications (e.g., lithium, salicylate, toxic alcohols), some cases of hypocalcemia, hyperphosphatemia, or hypercalcemia, and anuric AKI unresponsive to acute interventions (reversal of prerenal factors or relief of obstruction). Modern practice is to initiate RRT sooner rather than later, for example, when the SCr concentration reaches 500-700 perhaps even earlier, unless there is clear evidence that renal function is about to recover (3).
Institution of RRT
The choice of the treatment will depend on the clinical practice, technical resources, and well-trained nurses of a given department, than on precise clinical indication. A proficient and accountable team, experienced in various dialytic modalities with a quality assurance education program would be ideal (3). If this is not possible, the best combination of simple and easy treatment schedules, which are functional and efficient with no significant increases in personnel demand or labour intensity must suffice.
Biocompatibility
Two major studies found that use of biocompatible membranes improved these outcomes in AKI (5,6). Several subsequent studies have failed to confirm the benefits of biocompatibility in AKI, but have lacked the statistical power to definitively exclude any effect (7). Use of a biocompatible dialyzer that also has higher “flux” (permeability) than the bioincompatible dialyzer may have influenced outcome in one study (5). In light of a number of conflicting studies, the clinical relevance of this aspect of the acute dialysis prescription remains unproven, despite the fact that this strategy has already become standard in many centers (8).
Dose of dialysis
The lack of a standardized measurement of RRT dose in AKI is a major deficiency in the field. Clearance of urea is the most commonly studied marker of adequate uremic detoxification by IHD. The term Kt/V is a measure of the volume of plasma cleared of urea during an HD session (Kt) divided by the urea distribution volume (V, assumed to be total body water: 0.5-0.6 L/kg). The larger Kt/V values signify greater HD dose. Until further data is available, a reasonable recommendation offered by the Acute Dialysis Quality Initiative is to deliver a dose of HD in the acute setting that is at least equal to what is considered acceptable in the chronic end-stage renal disease (ESRD) population. This is a Kt/V >1.2 per treatment if IHD is provided three-times per week. Alternatively, one can attempt to deliver a Kt/V >1.0 per treatment on a dialysis schedule of at least six days a week. Paganini et al.(9) demonstrated by retrospective analysis of a prospectively gathered database that delivered dialysis dose was predictive of mortality in critically ill ARI patients treated with RRT, if they had moderate-range severity of illness, but mortality of the most and least severely ill patients was independent of dialysis dose .
Choice of RRT
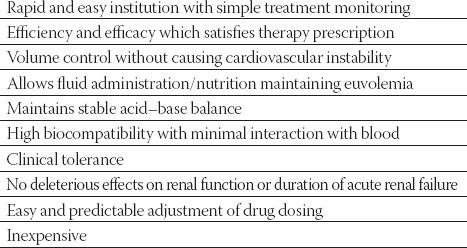
There are now many available options of RRT which may be divided into 4 groups: 1. peritoneal dialysis (PD), 2. IHD, 3. continuous therapies (CRRT), and 4. hybrid therapies (SLED). The ideal RRT should mimic the functions and physiological mechanisms of the native organ, ensuring qualitative and quantitative blood purification, be free of complications, have good clinical tolerance and restore and maintain homeostasis, thus favouring organ recovery (Table 1). These 4 groups of RRT differ in the method of delivery, efficiency, and their clinical tolerability. There are certain clinical situations in which only a particular therapy is indicated (e.g., CRRT in critically ill patients with cardiovascular instability) (10). Distinguishing whether AKI is a result of single organ dysfunction or part of MOF is a key factor. These two groups of subjects differ substantially and should be treated differently. AKI without MOF is less complex, can be managed outside ICU and the same RRT techniques used for the treatment of chronic renal failure may be applied. AKI associated with MOF is a more complex condition and requires more flexible RRT (3,11).
TABLE 1.
Technical and clinical requirements for an optimal renal replacement therapy in acute renal injury
Peritoneal dialysis
Acute PD remains a viable option for the treatment of selected patients with AKI, particularly pediatric population, and those who are hemodynamically compromised, have severe coagulation abnormalities, difficulty in obtaining blood access, removal of high molecular weight toxins (>10 kD), and clinically significant hypothermia and hyperthermia. There are very few absolute contraindications for acute PD, most of the following conditions are only relative contraindications to this modality (3). One of the important determinants of a successful acute PD procedure is a reliable peritoneal access, which can easily be obtained by inserting a semirigid acute catheter or a single cuff Tenckhoff catheter. Acute PD can be performed intermittently or continuously, and either manually or via an automated device. Complications of acute PD are numerous and potentially serious, but preventable, and include peritonitis, hyperglycemia, substantial protein loss, disturbance of respiratory mechanics, and visceral perforation while inserting the catheter (12). Most studies have shown that the mortality and incidence of renal recovery with acute PD was at least comparable to IHD (3,12).
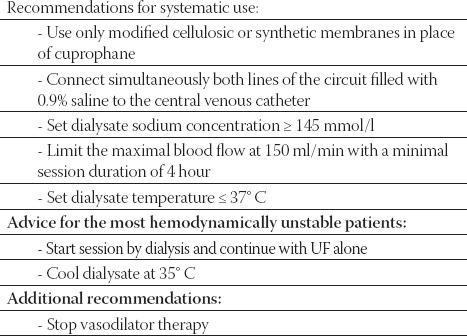
Intermittent hemodialysis
These technique require good vascular access, special equipment and specially trained physicians and nurses to carry out the dialysis. Patients that are hemodynamically stable can be managed with IHD techniques. Maintaining hemodynamic stability is probably one of the most important aspects of dialysis technique as well as one of the most difficult challenges. To improve hemodynamic tolerance of IHD, specific guidelines were implemented into practice (Table 2). The practice guidelines are based on dialysis strategies experienced in chronic HD patients suffering from cardiovascular insufficiency. However, the targets for adequate solute clearance in AKI remain unknown, and the importance of removing middle and large uremic toxins in the setting of AKI remains to be determined. Consequently, physicians should consider empirical increases in dialytic time and IHD frequency and judicious use of anticoagulation to improve dialysis adequacy.
TABLE 2.
Intermittent hemodialysis practice quidelines
Continuous renal replacement therapies
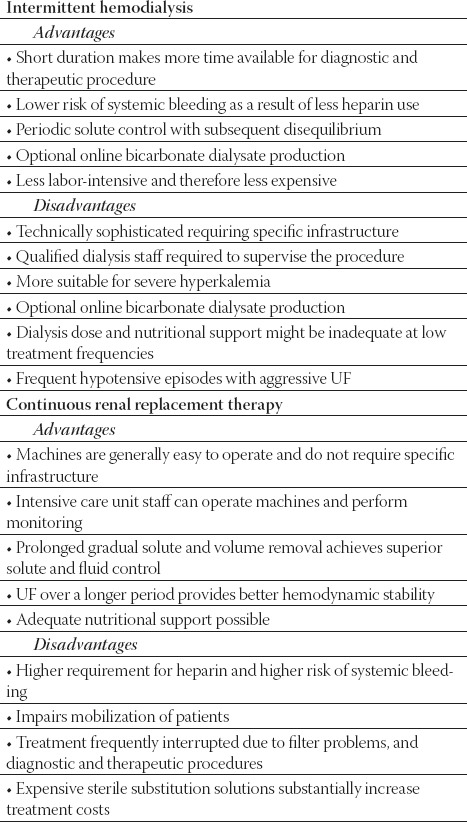
With CRRT, the continuous regulation of volume homeostasis could lessen the hourly rate of required UF, thereby improving hemodynamic stability compared with IHD. Control of azotemia with modern veno-venous CRRT is at least equivalent to alternate-day IHD, and superior to daily IHD in large or hypercatabolic patients. Clinical data suggest that CRRT should be strongly considered for patients with severe hyperphosphatemia (tumor lysis syndrome, rhabdomyolysis), elevated intracranial pressure (ICP), cerebral edema complicating acute liver failure, might be a useful component of therapy for lithium intoxication, and because of continuous nature of process prevents the post-dialytic “rebound” elevation of plasma concentration of uremic toxins typically seen with IHD. The clinical benefits of CRRT have also been reported for cardiac surgery patients. CRRT appears to have beneficial effects on hemodynamics in SIRS, sepsis or septic shock (Figure 1). Although much attention has been focused on perceived benefits of CRRT compared with IHD, comparatively less attention has been focused on the potential for increased risks with CRRT therapy (Table 3). In AKI patients stable enough to tolerate IHD, this benefit should be balanced against aspects of CRRT that might adversely affect outcome, such as continuous anticoagulation, prolonged membrane exposure, hypothermia, and nonselective removal of nutrients, inflammatory mediators, and drugs. Despite apparent advantages over IHD in unstable patients, superiority of CRRT with respect to mortality or recovery of renal function has not been demonstrated. In patients stable enough to tolerate IHD, this benefit should be balanced against aspects of CRRT that might adversely affect outcome. IHD and CRRT should be regarded as complementary techniques, which should both be available in institutions that care for critically ill patients, allowing RRT to be individualized to the needs of the complex patients who develop AKI in the ICU. AKI in the ICU patients is increasingly a component of SIRS, sepsis or septic shock, and the development of rational strategies for initiation, dosing, and effective delivery of RRT in this setting is among the greatest challenges facing nephrologists and intensivists today (11). The use of CRRT is increasing in children and even newborns, and includes several modalities (CVVH, CV-VHD, and CVVHDF). In patients treated with CRRT, the rate of fluid and solute removal is slow and continuous. As a result, CRRT is better tolerated than IHD in patients who are hemodynamically unstable. The removal of solutes over the course of 24 to 48 hours is as efficient as conventional hemodialysis. In addition, some prefer this technique in patients with sepsis or MOF, as it may enhance the removal of cytokines.
FIGURE 1.
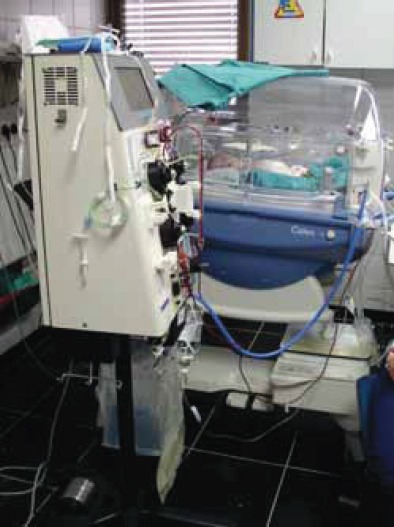
The use of continuous renal replacement therapy in newborn with acute kidney injury - Department for Dialysis, University Hospital Centre Zagreb, Zagreb, Croatia
TABLE 3.
Comparison of intermittent and continuous renal replacement therapy
Hybrid’ renal replacement therapies
This technique utilizes equipmen t originally developed for treatment of patients with chronic renal failure and does not require industrially produced substitution fluid. The term “sustained low-efficiency dialysis” (SLED) is the most widely used. This means of RRT combines several advantages of both IHD and CRRT, most notably excellent detoxification and cardiovascular tolerability akin to that associated with CVVH. Machines for hybrid therapy should ideally have the characteristics like flexible options for dialysate flow (QD) (allowing for low flows should the clinical situation mandate low solute clearance and UFR), flexible options for hybrid treatment duration (allowing prolonged or even continuous treatments), clear interface with the nurse managing the treatment preferably via a dedicated hybrid therapy screen, and standard procedures for changing between IHD and hybrid therapy (allowing either modality to be conveniently chosen at treatment commencement without any resultant delay) (3) (Figure 2). Different combinations of dry and liquid concentrates can be mixed allowing treatments to be tailored to the needs of individual patients. The extremely flexible, yet highly efficient, SLED treatment modality fulfills all ICU requirements: it offers immediate, highly effective dialysis therapy for acute hyperkalemia, whereas for less-urgent indications, treatment durations can be extended up to 18 h. Sustained low-efficiency dialysis offers several advantages over CRRT, including less cumbersome technique, patient mobility, and decreased requirements for anticoagulation, while providing similar hemodynamic stability and volume control. Several economic evaluations have shown SLED to be less expensive than CRRT. The Genius system consists of a single-pass batch dialysis machine that provides up to 75 L of an ultrapure germ- and endotoxin-free pure bicarbonate dialysate per dialysis session and a station for automated production of dialysate and filling of the machine. The technically simple machine has one roller pump in which both blood and dialysate tubing is inserted and that pumps blood and dialysate countercurrently with a ratio fixed at 1:1 or 2:1, depending on the diameter of the blood and dialysis tubing used. Thus, blood flow determines duration of treatment, ie, the time in which the 75 L dialysate tank is used up. Treatment time can be varied simply by changing the speed of the blood pump from 4 hours (conventional IHD with a 1:1 system and blood flow of 300 mL/min) to as much as 24 hours (extended dialysis with a 2:1 system and blood flow of 100 mL/min). The blood flow and treatment time may be modified within treatment session.
FIGURE 2.
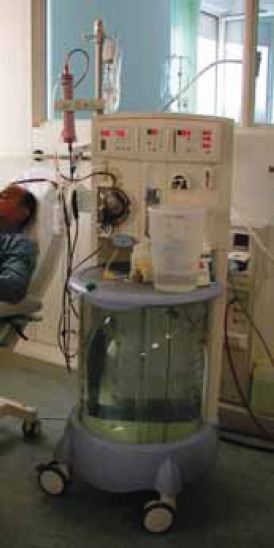
“Hybrid” renal replacement therapy - The Genius single-pass dialysis machine
The impact of dialytic modality on mortality and renal recovery
Currently, it has been found no difference in mortality or renal recovery between hybrid RRT, CRRT or IHD for critically ill patients with AKI. However, future investigations should collect detailed information on long-term costs and the relative likelihood of renal recovery associated with dialysis modality. Until information from such a trial is available, a systematic review such as this may constitute the best possible evidence
LIST OF ABBREVIATIONS
PD - peritoneal dialysis
IH - intermittent hemodialysis
AKI - acute kidney injury
CRRT - continuous renal replacement therapy
ICU - intensive care unit
REFERENCES
- 1.Neveu HD, Kleinknecht F, Brivet Ph, Loirat P. Landais and the French Study Group on Acute Renal Failure. Prognostic factors in acute renal failure due to sepsis: results of a prospective multi centre study. Nephrol. Dial. Transplant. 1996;11:293–299. doi: 10.1093/oxfordjournals.ndt.a027256. [DOI] [PubMed] [Google Scholar]
- 2.Silvester W. Outcome studies of continuous renal replacement therapies in the intensive care unit. Kidney Int. 1998;53(Suppl. 66):S138–S41. [PubMed] [Google Scholar]
- 3.Kes P, BašićJukić N. New experiences with the therapy of acute kidney injury. Contributions. Sec. Biol. Med. Sci. 2008;29:119–153. [PubMed] [Google Scholar]
- 4.Kes P. Continuous renal replacement therapy. Acta Clin. Croat. 2000;39:99–116. [Google Scholar]
- 5.Schiffl H, Lang S.M, Konig A, Strasser T, Haider M.C, Held E. Biocompatible membranes in acute renal failure: prospective case-controlled study. Lancet. 1994;344:570–572. doi: 10.1016/s0140-6736(94)91964-x. [DOI] [PubMed] [Google Scholar]
- 6.Hakim R.M, Wingard R.L, Parker R.A. Effect of dialysis membrane in the treatment of patients with acute renal failure. NEJM. 1994;331:1338–1342. doi: 10.1056/NEJM199411173312003. [DOI] [PubMed] [Google Scholar]
- 7.Jorres A, Gahl G.M, Dobis C, et al. Hemodialysis-membrane biocompatibility and mortality of patients with dialysis-dependent acute renal failure: a prospective randomised multicentre trial. Lancet. 1999;354:1337–1341. doi: 10.1016/s0140-6736(99)01213-1. [DOI] [PubMed] [Google Scholar]
- 8.Kes P, Šefer S. Utjecaj doze dijalize i biokompatibilnosti membrane dijalizatora na ishod liječenja bolesnika s akutnim zatajenjem bubrega. Liječ. Vjesn. 1999;121:326–328. [PubMed] [Google Scholar]
- 9.Paganini E. P, Tapolyai M, Goormastic M, et al. Establishing a dialysis therapy/patient outcome link in intensive care unit acute dialysis for patients with acute renal failure. Am. J. Kidney Dis. 1996;28(Suppl. 3):S81–S89. [Google Scholar]
- 10.Brunetta B, Bašić-Jukić N, Kes P. Premoštenje razdoblja do transplantacije srca kontinuiranom venovenskom hemofiltracijom u bolesnika sa završnim stupnjem zatajenja srca i akutnim zatajenjem bubrega. Acta Med. Croat. 2003;57:319–322. [PubMed] [Google Scholar]
- 11.Kes P, Ljutić D, Bašić-Jukić N, Brunetta B. Indikacije za kontinuirano nadomještanje bubrežne funkcije. Acta Med. Croat. 2003;57:69–73. [PubMed] [Google Scholar]
- 12.Ash S.R. Peritoneal dialysis in acute renal failure of adults: the safe, effective, and low-cost modality. In: Ronco C, Bellomo R, La Greca G, editors. Blood purification in intensive care. Vol. 132. Karger: Contrib Nephrol, Basel; 2001. pp. 210–221. [DOI] [PubMed] [Google Scholar]


