Abstract
Methylenetetrahydrofolate Reductase (MTHFR) is key enzyme in metabolism of homocysteine. Homozygotes for mutation (TT genotype) have hyperhomocysteinemia, risk factor for atherosclerosis development. The aim of the study was to find out distribution of genotype frequencies of C677T MTHFR among patients on maintenance hemodialysis. Possible association of alleles and genotypes of C677T polymorphism of the MTHFR gene with age of onset, duration of dialysis and cause of kidney failure was studied also. Cross-sectional study includes 80 patients from Clinic of Hemodialysis KUCS in Sarajevo. In order to perform genotyping, isolated DNA was analyzed by RFLP-PCR and gel-electrophoresis. From total of 80 patients, 42.5% (n=24) were female, 57.5% (n=46) were male, mean age 54.59±1.78 years and duration of dialysis 79.92±6.32 months. Genotype distribution was: CC 51.2% (n=41), CT 37.5% (n=30) and TT 11.2% (n=9). Patients with wild-type genotype have longer duration of dialysis in month (87.1 ± 63.93) comparing to TT genotype patients (67.06 ± 39.3), with no statistical significance. T allele frequency was significantly higher in group of vascular and congenital cause of kidney failure (Pearson X2 =6.049, P<0.05) comparing to inflammation etiology group. Genotype distribution results are within the results other studies in Europe. Obtained results indicate that C677T polymorphism is not associated with onset, duration and cause of kidney failure in our hemodialysis population. There is an association of T allele of the MTHFR gene and vascular and congenital cause kidney failure.
Keywords: Methylenetetrahydrofolate Reductase, C677T polymorphism of the MTHFR gene, hemodialysis
INTRODUCTION
Homocysteine (Hcy) lies at an important metabolic branch point of methionine metabolism, between the remethylation and transsulfuration pathways. These lead to the formation of methionine and cystathionine, respectively. Homocysteine causes endothelial cell dysfunction and damage via production of potent reactive oxygen species during its autooxidation. This metabolite increases tromboxane formation, enhances platelet aggregation and is potent mitogen for vascular smooth muscle cells. Also, Hcy is antagonist of nitric oxide. Those facts may explain why renal impairment patients have a frequent cardiovascular complication and higher overall mortality rate than general population (1). Methylenetetrahydrofolate reductase enzyme (MTHFR, EC 1.5.1.20) is an enzyme that exists in the cytoplasm of cells. It contains a flavin cofactor and uses NADH as the reducing agent. MTHFR is key enzyme in one-carbone metabolism. The enzyme catalyzes irreversibly reduction of 5,10-methylenetetrahydrofolate (5,10-methylTHF) to 5-methyltetrahydrofolate (5-methylTHF), predominate circulating form of folate. Methionine synthase (MS) catalyzes transfer of methyl group from 5-methylTHF to homocysteine. The product of this B12 dependent remethylation is methionine and tetrahydrofolate. Methionine serves as a methyl group donor for over 100 reactions of synthesis, regulation and detoxification. The most common mutation connected with impaired homocysteine metabolism and hyperhomocysteinemia had been described by Frosst B and coworkers(2). It is a point mutation on MTHFR gen, located at chromosome 1, at region 1p36.3 (3). MTHFR genetic polymorphism is a cytosine to thymidine transition at nucleotide 677 in the predicted catalytic domain of MTHFR gene. This 677C>T transition results in an alanine to valine substitution and has been associated with a thermolabile enzyme synthesis (4). Serum activity of MTHFR in individuals with two copies of this variant (homozygotes with T alleles) is reduced about 70%, while activity in individuals with one mutated allele (heterozygotes with TC alleles) is reduce 35%1. About half the general population carries at least one mutated allele and the frequency of the homozygous mutated genotype (TT) ranges from 1 to 20% depending on the population (5). There is substantial variability in the prevalence of this mutation among different ethnic groups with a prevalence of 0-2% in Africans, 12% in Whites and 20% in Asians. The 677TT genotype was particularly common in Australia (11,5%), northern China (20%), southern Italy (26%), and Mexico (32%) (6). Hyperhomocysteinemia is a major independent risk factor for atherothrombotic diseases and is common in hemodialysis patients. Homozygosity for the C677T allele is associated with elevated homocysteine levels, especially in individuals who have a poor folate status(7). Many studies have shown that homozygous mutant individuals have a significant higher level of serum tHcy comparing to CT and CC genotype persons. Patient on maintained hemodialysis bearing TT homozygosity have hyperhomocysteinemia in 40 up to 100% cases comparing to heterozygous (CT) and wild-type (CC) (8). Polymorphism at nucleotide position 677 CT within MTHFR gene is associated with earlier onset and duration of hemodialysis (9,1). However this finding was not confirmed in subsequent prospective study at larger scale. Aucella and coworkers claimed that genotypes were not different for time spent on dialysis or age at onset of dialysis (10). Aim of our study was to investigate the distribution of allele and genotype frequencies of C677T MTHFR among patients on maintenance hemodialysis at Clinic of Hemodialysis, KCUS, Bosnia and Herzegovina. Also, we wanted to investigate is there influence of MTHFR genotype on earlier onset and duration of hemodialysis in patients.
PATIENTS AND METHODS
Patients
A total of 80 patients, both sex, were recruited from Clinic of Hemodialysis KUCS in Sarajevo, Bosnia and Herzegovina and were enrolled in cross-sectional study of MTHFR gene polymorphism. Exclusion criteria included severe malnutrition and patients with CV events. After performing MTH-FR genotyping, patients were separated into three groups: patients with TT genotype, patients with CT genotype, and patients with CC genotype.
Genetic Analysis
Approximately 5 ml of blood taken by cubital venipunction and stored in EDTA coated vacutainers (BD Biosciences) was used for genetic analysis. DNA was extracted in subsequent salting out procedure using DNA extraction kit (QIAamp blood kit, Qiagen). In order to perform genotyping, isolated DNA was analyzed by RFLP-PCR and gel-electrophoresis. In vitro amplification of MTHFR gene segment that contains polymorphism genomic C677T was carried out in 50 1 PCR reaction under following conditions: 10X PCR buffer, 3,5 mM MgCh, 0,2 mM of each of the dNTPs (Fermentas), 20 pmol of each of the primers (Sigma-Aldrich) forward 5’-TGAAGGAGAAGGTGTCTGCGGGA-3’ and reverse 5’AGGACGGTGCGGTGAGAGTG-3’, 100 ng of DNA template and 2 units of Taq DNA polymerase (Platinum Taq, Invitrogen). Cycling conditions used for amplification of 198 bp product were as follows: 35 cycles 95°C for 60 seconds, 62°C for 90 seconds, 72°C for 60 seconds. PCR products were evaluated for successful amplification on 1% agarose gels stained with ethidium bromide and then digested with Hinf I according to manufacturer’s protocol (Pure Extreme, Fermentas). Hinf I recognizes the sequence that contains substitution of T for C nucleotide and is useful for genotype discrimination in this matter. Digested fragments were loaded on 3% agarose gels stained with ethidium bromide and documented and evaluated as transilluminated image on KODAK Edas software.
PICTURE 1.
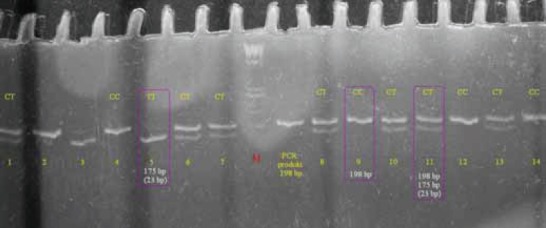
Amplified DNA of MTHFR gene with C677T mutation after digestion with Hin I 3% agarose gel electrophoresis shows the different genotypes of MTHFR mutation. Lane M: 100 base pairs (bp) ladder marker. Lanes 4,9,12 and 14: wild type CC genotype; only one band DNA fragment of 198 bp is presented. Lanes 1,6,7,8,10,11 and 13: heterozygous CT genotype; two DNA fragments of 198 bp and 175 bp presented. Lane 5: homozygous TT genotype; only one band DNA fragment of 175 bp presented. Negative control was not shown.
Statistical Analysis
Difference in baseline characteristic, possible association of alleles and genotypes of C677T polymorphism with age of onset, duration of dialysis and cause of kidney failure was analyzed using SPSS software (version 12.0). Data are presented as mean ± SD. Allelic and genotype frequencies among hemodialysis patients were calculated using MedCalc ver 10.0.0. and tested for statistical significance of allele frequency distribution between comparison groups (Chi square and Fisher’s Exact test). A value of p < 0.05 was considered statistically significant.
RESULTS
Patient characteristics
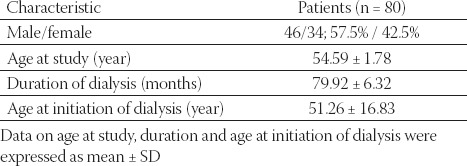
Mean age of hemodialysis patients (n=80) was 54.59 ± 1.78 years (Table 1.) Among them 46 or 57.5% were male and 34 or 42.5% were female. The average duration of dialysis was 79.92 ± 6.32 months. Age of patients at the initiation of dialysis was 51.26 ± 16.83 years.
TABLE 1.
Baseline characteristics of patients on dialysis
Genetic Analysis
Picture 1. shows an agarose gel that illustrates different genotypes of C677T mutation. When the C of the 677 nucleotide is replaced by T, restriction site for Hinf I is synthesized. Only one fragment of 198 bp long is presented in lines loaded with wild type C alleles DNA. The 175 bp fragment is regarded as mutant type T allele and DNA fragments with CT alleles have two 198 and 175 bp fragments. Lanes with TT genotype has single 175 bp fragment.
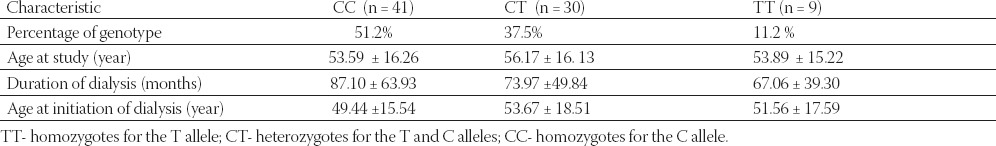
The distribution of the genotypes in the patients was as follows: 11.2 % for TT genotype. 37.5% of CT genotype and 51.2% for CC genotype (Table 2.). Mean age of patients according to MTHFR genotype was 53.89 ± 15.22 for TT genotype, 56.17 ± 16.13 for CT genotype and 53.59 ± 16.26 for CC genotype. Data on duration of dialysis shows the difference between genotype groups: 67.06 ± 39.30 for TT, 73.97 ± 49.84 for CT and 87.10 ± 63.93 months for CC genotype. Wild type genotype patients spend more months on maintained hemodialysis then heterozygous or homozygous patients. but the difference is not significant. No difference in the age at initiation of dialysis was found between three different genotypes groups 51.56 ± 17.59, 53.67 ± 18.51 and 49.44 ±15.54 (TT, CT and CC group respectively).
TABLE 2.
Profile of patients according to MTHFR genotype
No significant difference in the genotype distribution was found between male and female patients (Table 3.) There were predominant number of male patients in CT and TT genotype groups compare to female patients (66.7% v. 33.3% males v. females) but the gender difference was not statistically significant.
TABLE 3.
Gender characteristic of the patients according to MTHFR genotype
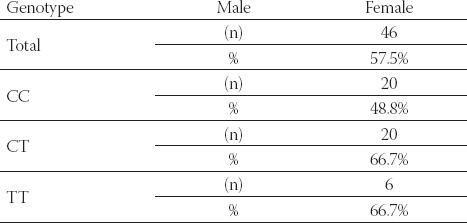
Distribution of the genotypes among male and female patients on hemodialysis. Results of gender difference in three different MTHFR genotype groups are expressed as general number and percentage.
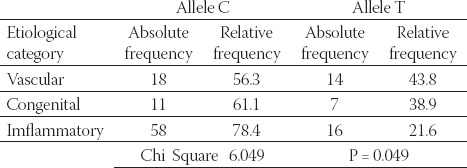
Data in Table 4 present results about alleles C and T frequencies and allele frequency distribution between three comparison groups, colloquial etiological categories: vascular, congenital and inflammatory (based on primary pathology of end stage renal disease). We observed that minor allele T occurs more frequently in a group of vascular and congenital rather than inflammatory type of end stage renal disease (chi square 6.049, P =0.049; Fisher’s P=0.0494).
TABLE 4.
Allele frequencies distribution among three colloquial cathegories
DISCUSSION
The results of this study revealed that genotype distribution results for patients receiving hemodialysis are within the results of other studies in Europe. Lim11 published results of distribution: 58% for CC, 35.4% for CT and 6.6% for TT genotype patients. Kimura’s1 results were 36.5% for CC, 46.1% for CT and 17.4% for TT genotype patients. Distribution of MTHFR genotypes obtained for 161 hemodialysis patients done by Tremblay12 was as follows: 42% had no mutation (CC); 42% were heterozygotes (CT), and 16% were homozygotes (TT). If we compare our results of distribution (51.2% for CC, 37.5% for CT and 11.2 % for TT) with above mentioned ones, no difference is observable (P=0.14). According to the work of Kimura1 and his associates, we presumed to find a less prevalence of the TT genotype in older age patients on maintained hemodialysis in Bosnia and Herzegovina region. We also, expected that TT genotype patients are significantly younger at initiation of hemodialysis comparing to other two genotype bearing patients. Similar results showed Elizabeth Wrone8. She had found that mutant allele frequency and percentage of homozygotes were lower in group of patients with dialysis period longer than one year. We didn’t find an association between C677T polymorphism and onset and duration of hemodialysis. However, we found that patients with wild-type genotype had a longer duration of dialysis in month (87.1 ± 63.93) comparing to CT (73.97 ± 49.84) and TT genotype patients (67.06 ± 39.3), with no statistical significance (P>0.05). Possible cause of our findings may be in the difference in number of patients included in investigation. Kimura1 and Wrone9 had over 450 patients in their investigation, but our cross-sectional study included 80 patients and it is obviously less number of examined patients.
Well known fact is that hemodialysis patients have a much higher mortality rate than general population. Cardiovascular diseases are major cause of the mortality of these patients. Recently, hyperhomocysteinemia is recognized as a cardiovascular risk factor. MTHFR gene polymorphism determinates level of homocysteine among other factors and may be considered as one of the cardiovascular risk factor. It is important to determine T allele for prediction of Hcy serum concentration and possible prevention or postponing complication and mortality by taking Hcy lowering therapy. Concerning our result that T allele is associated with vascular and congenital cause of kidney failure, we believe that mutation on MTHFR gene may be one of the risk factors for kidney failure or factor that may aggravate pathological changes.
CONCLUSION
In summary, we have shown in this work that genotype distribution is similar to results of other studies in Europe. Obtained results indicate that C677T polymorphism is not associated with onset, duration and cause of kidney failure in our hemodialysis population. But, we found an association of T allele of the MTHFR gene and vascular and congenital cause kidney failure. For precise conclusion about influence of MTHFR polymorphism on age of onset, duration of hemodialysis and cardiovascular complication of patients on dialysis in Bosnia and Herzegovina, large prospective study that includes specific clinical features and biochemical markers with the application of novel simultaneous genetic risk profiling is necessary.
REFERENCES
- 1.Kimura H, Gejyo F, Suzuki S, Takeda T, Miyazaki R, Yoshida H. A C677T mutation in the methylenetetrahydrofolate reductase gene modifies serum cysteine in dialysis patients. Am. J. Kidney Dis. 2000;36(5):925–933. doi: 10.1053/ajkd.2000.19085. [DOI] [PubMed] [Google Scholar]
- 2.Frosst P, Blom H.J, Milos R, Goyette P, Sheppard C.A, Matthews R.G, Boers G.J, den Heijer M, Kluijtmans LA, van den Heuvel L.P, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat. Genet. 1995;10(1):111–113. doi: 10.1038/ng0595-111. [DOI] [PubMed] [Google Scholar]
- 3.Botto N, Andreassi M.G, Manfredi S, Masetti S, Cocci F, Colombo M.G, Storti S, Rizza A, Biagini A. Genetic polymorphisms in folate and homocysteine metabolism as risk factors for DNA damage. Eur. J. Hum. Genet. 2003;11(9):671–678. doi: 10.1038/sj.ejhg.5201024. [DOI] [PubMed] [Google Scholar]
- 4.Sunder-Plassmann G, Födinger M. Genetic determinants of the homocysteine level. Kidney Int. Suppl. 2003;84:S141–S144. doi: 10.1046/j.1523-1755.63.s84.52.x. [DOI] [PubMed] [Google Scholar]
- 5.Wilcken B, Bamforth F, Li Z, Zhu H, Ritvanen A, Renlund M, Stoll C, et al. Geographical and ethnic variation of the 677C> T allele of 5,10 methylenetetrahydrofolate reductase (MTHFR): findings from over 7000 newborns from 16 areas world wide. J. Med. Genet. 2003;40(8):619–625. doi: 10.1136/jmg.40.8.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cortese C, Motti C. MTHFR gene polymorphism, homocysteine and cardiovascular disease. Public Health Nutr. 2001;4(2B):493–497. doi: 10.1079/phn2001159. [DOI] [PubMed] [Google Scholar]
- 7.Siva A, De Lange M, Clayton D, Monteith S, Spector T, Brown M.J. The heritability of plasma homocysteine, and the influence of genetic variation in the homocysteine methylation pathway. QJM. 2007;100(8):495–499. doi: 10.1093/qjmed/hcm054. [DOI] [PubMed] [Google Scholar]
- 8.Födinger M, Mannhalter C, Wölfl G, Pabinger I, Müller E, Schmid R, Hörl W.H, Sunder-Plassmann G. Mutation (677 C to T) in the methylenetetrahydrofolate reductase gene aggravates hyperhomocysteinemia in hemodialysis patients. Kidney Int. 9. 1997;52(2):517–523. doi: 10.1038/ki.1997.362. [DOI] [PubMed] [Google Scholar]
- 9.Wrone E.M, Zehnder J.L, Hornberger J.M, McCann L.M, Coplon N.S, Fortmann S.P. An MTHFR variant, homocysteine, and cardiovascular comorbidity in renal disease. Kidney Int. 2001;60(3):1106–1113. doi: 10.1046/j.1523-1755.2001.0600031106.x. [DOI] [PubMed] [Google Scholar]
- 10.Aucella F, Margaglione M, Grandone E, Vigilante M, Gatta G, Forcella M, Ktena M, De Min A, Salatino G, Procaccini D.A, Stallone C, Gesualdo L. Genetic Polymorphisms in Dialysis Study Group. The C677T methylenetetrahydrofolate reductase gene mutation does not influence cardiovascular risk in the dialysis population: results of a multicentre prospective study. Nephrol. Dial. Transplant. 2005;20(2):382–386. doi: 10.1093/ndt/gfh620. [DOI] [PubMed] [Google Scholar]
- 11.Lim P.S, Hung W.R, Wei Y.H. Polymorphism in methylenetet-rahydrofolate reductase gene: its impact on plasma homocysteine levels and carotid atherosclerosis in ESRD patients receiving hemodialysis. Nephron. 2001;87(3):249–256. doi: 10.1159/000045922. [DOI] [PubMed] [Google Scholar]
- 12.Tremblay R, Bonnardeaux A, Geadah D, Busque L, Lebrun M, Ouimet D, Leblanc M. Hype rhomo cysteine mia in hemodialysis patients: effects of 12-month supplementation with hydrosoluble vitamins. Kidney Int. 2000;58(2):851–858. doi: 10.1046/j.1523-1755.2000.00234.x. [DOI] [PubMed] [Google Scholar]


