Abstract
Acute paraquat (PQ) poisoning is one of the most common forms of pesticide poisoning. Oxidative stress and inflammation are thought to be important mechanisms in PQ-induced acute lung injury (ALI). Selenium (Se) can scavenge intracellular free radicals directly or indirectly. In this study, we investigated whether porous Se@SiO2 nanospheres could alleviate oxidative stress and inflammation in PQ-induced ALI. Male Sprague Dawley rats and RLE-6TN cells were used in this study. Rats were categorized into 3 groups: control (n=6), PQ (n=18), and PQ + Se@SiO2 (n=18). The PQ and PQ + Se@SiO2 groups were randomly and evenly divided into 3 sub-groups according to different time points (24, 48 and 72 h) after PQ treatment. Porous Se@SiO2 nanospheres 1 mg/kg (in the PQ + Se@SiO2 group) were administered via intraperitoneal injection every 24 h. Expression levels of reduced glutathione, malondialdehyde, superoxide dismutase, reactive oxygen species (ROS), nuclear factor-κB (NF-κB), phosphorylated NF-κB (p-NF-κB), tumor necrosis factor-α and interleukin-1β were detected, and a histological analysis of rat lung tissues was performed. The results showed that the levels of ROS, malondialdehyde, NF-κB, p-NF-κB, tumor necrosis factor-α and interleukin-1β were markedly increased after PQ treatment. Glutathione and superoxide dismutase levels were reduced. However, treatment with porous Se@SiO2 nanospheres markedly alleviated PQ-induced oxidative stress and inflammation. Additionally, the results from histological examinations and wet-to-dry weight ratios of rat lung tissues showed that lung damage was reduced after porous Se@SiO2 nanosphere treatment. These data indicate that porous Se@SiO2 nanospheres may reduce NF-κB, p-NF-κB and inflammatory cytokine levels by inhibiting ROS in PQ-induced ALI. This study demonstrates that porous Se@SiO2 nanospheres may be a therapeutic method for use in the future for PQ poisoning.
Keywords: porous Se@SiO2 nanospheres, acute lung injury, paraquat poisoning, oxidative stress, inflammatory cytokines, ROS, NF-kappa B
Introduction
Paraquat (PQ) is an organic heterocyclic contact herbicide that is highly toxic to humans and animals. PQ can enter the human body through the skin, respiratory tract and digestive tract. Acute PQ poisoning is one of the most common forms of pesticide poisoning.1,2 PQ primarily accumulates in the lung after poisoning. The concentration of PQ in lung tissues can be up to 10 times the concentration in the blood. PQ-induced acute lung injury (ALI) involves early acute pulmonary edema, pulmonary congestion, acute respiratory distress syndrome and advanced progressive pulmonary fibrosis.3,4 The mortality rate of PQ poisoning is as high as 60%–80%, which is due to lack of an antidote for its specific effects.5 Thus, it is important to study the mechanism of PQ poisoning and identify a better therapeutic method to treat it.
Oxidative stress is thought to initiate and be the main mechanism of PQ-induced ALI. PQ can enter cells and participate in a series of redox reactions. In this process, PQ consumes nicotinamide adenine dinucleotide phosphate and cytochrome P450 reductase, increasing superoxide anion and hydrogen peroxide levels through the disproportionation of hydrogen peroxide to generate hydroxyl and other radicals, constituting a reactive oxygen species (ROS) system.6 ROS can activate lipid peroxidation and increase malondialdehyde (MDA) levels in this process. The level of MDA indirectly reflects the degree of peroxidation, and elevated MDA levels can also cause cell metabolism disorders.7 In addition, an increase in oxidized glutathione (GSH) and a decrease in reduced GSH levels have been observed after PQ poisoning, which could reduce the body’s antioxidant defenses.8,9 Superoxide dismutase (SOD) is one of the main endogenous antioxidants and can effectively remove the superoxide anion (O2−) and reduce the oxidative response and the subsequent acute inflammatory response.10
Nuclear factor-κB (NF-κB) is an important transcription factor in controlling the inflammatory response.11–13 NF-κB can also be a second messenger of oxygen radicals and can indirectly promote an increased inflammatory response. Studies have shown that NF-κB levels are increased and NF-κB continues to be expressed after PQ poisoning, which subsequently induces production of tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and other cytokines to promote inflammation. The mechanism behind this effect may be governed by the activation of a large number of oxygen free radicals that induce the production of such proteins as NF-κB, TNF-α, and IL-1β. Effective inhibition of NF-κB activity can inhibit the development of ALI.14,15 However, there are still no specific drugs used in the clinic in this regard.
Selenium (Se) is a necessary trace element in the human body and has a wide range of biological functions.16 Se, which is an ingredient of GSH peroxidase, can directly or indirectly scavenge intracellular free radicals. However, Se can also damage cellular components by catalyzing the oxidation of thiols and simultaneously generating superoxide (O⋅−).17 Nanotechnology may lead to extensive awareness of applied science and technology that is necessary to organize matter on the atomic and molecular scales.18,19 Thus, we speculated that porous Se@SiO2 nanospheres may be used as a cytoprotectant to reduce ROS after PQ poisoning.
The aim of this study was to research whether porous Se@SiO2 nanospheres could significantly alleviate oxidative stress and the inflammatory response in PQ-induced ALI.
Methods and materials
Synthesis of porous Se@SiO2 core-shell nanospheres
The porous Se@SiO2 nanospheres were synthesized according to our previously described method. The detailed synthesis procedures were performed as in our previous study.20
Animal experiments
Male 6–8-week-old Sprague Dawley (SD) rats were obtained from Shanghai Jiao Tong University. Forty-two SD rats were evenly and randomly divided into a control group, a PQ group and a PQ + porous Se@SiO2 nanospheres group (PQ + Se@SiO2 group). The PQ group and PQ + Se@SiO2 group were treated once with an intragastric infusion of a 20% PQ solution (50 mg/kg), and the control group received the same volume of saline. The PQ + Se@SiO2 group was also treated with 1 mg/kg porous Se@SiO2 nanospheres via intraperitoneal injection every 24 h. The concentration of porous Se@SiO2 nanospheres was selected according to our previous research.21 The PQ group and PQ + Se@SiO2 group were randomly divided into 3 sub-groups according to differing times of examination (24, 48 and 72 h) after PQ treatment. The rats were executed at the corresponding time points after injection of sodium pentobarbital (50 mg/kg). Lung tissues were collected in liquid nitrogen or neutral formalin solution for subsequent analyses. The animal experimental protocol was in accordance with the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and was approved by the Ethics Committee of Shanghai General Hospital.
Cell culture
Rat type II alveolar cells (RLE-6TN) were obtained from American Type Culture Collection (Rockville, MD, USA). RLE-6TN cells were grown in DMEM/F-12 (HyClone, Logan City, UT, USA) with 10% fetal bovine serum (Gibco, Grand Island, NY, USA) and 1% antibiotics. The cells were cultured at 37°C in a 5% carbon dioxide incubator.
Cell viability assays
The influence of porous Se@SiO2 nanospheres on cell viability was detected with a Cell Counting Kit-8 (CCK-8; Dojindo, Kumamoto, Japan). RLE-6TN cells (8×103/well) were grown and treated with porous Se@SiO2 nanospheres for 24 h in 96-well plates. The concentrations of porous Se@SiO2 nanospheres used on the RLE-6TN cells were 0, 10, 20, 40, 80, 160 and 320 μg/mL. After 24 h, 10 μL of CCK-8 solution was added to each well, and the plates were incubated for 1 h. The absorbance at 450 nm was measured using a Multimode Reader.
Assay of intracellular ROS production
RLE-6TN cells (2×105/well) were seeded into 6-well plates 24 h before being treated. Cells from the PQ group and the PQ + Se@SiO2 group were treated with PQ (the final concentration was 160 μmol/L), the concentration of which was determined in our previous study.22 Cells from the PQ + Se@SiO2 group were treated with porous Se@SiO2 nanospheres at a final concentration based on the results above. The levels of ROS were later detected with an ROS assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s instructions. The fluorescence signal was observed with a fluorescence microscope (Leica DMi8, Leica, Heidelberg, Germany).
Assay of oxidative biochemical parameters in lung tissues and plasma
Blood was obtained from the abdominal aorta after the rats were anesthetized with sodium pentobarbital. Then, plasma was obtained after the blood was centrifuged for 15 min at a speed of 2,500 rpm. The levels of GSH, SOD and MDA were detected with a GSH assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) (spectrophotometric method), an SOD assay kit (Nanjing Jiancheng Bioengineering Institute) (WST-1 method) and an MDA assay kit (TBA method) (Nanjing Jiancheng Bioengineering Institute), respectively. The detailed methods were executed according to the protocols of these assays.
Lung wet-to-dry weight ratio
The anterior lobe and middle lobe of the right lungs were excised and weighed immediately to obtain the wet weight. Next, the lungs were heated at 70°C for 48 h until constant weight to measure the dry weight. The wet-to-dry weight ratio was calculated by dividing the wet weight by the dry weight.
Enzyme-linked immunosorbent assay (ELISA)
The rat IL-1β ELISA Kit and rat TNF-α ELISA Kit were purchased from Neobioscience (Shenzhen, China). The expression levels of IL-1β and TNF-α in the cell culture supernatants, the plasma and the lung tissues were detected according to the relevant manufacturer’s protocol. All experiments were performed in triplicate.
Histological analysis of lung tissues
Lung tissues from rats of each group were fixed in neutral formalin solution and were processed for paraffin sectioning. Sections ~5 μm in thickness were stained with hematoxylin and eosin to observe under a light microscope (Leica DM5500 B).
Western blotting analysis
RLE-6TN cells and rat lung tissues were collected and lysed with radioimmunoprecipitation assay lysis buffer (Beyotime, Shanghai, China). The concentrations of proteins were detected by a bicinchoninic acid protein assay kit (Beyotime). The protein samples were fractionated by 8% sodium dodecyl sulfate polyacrylamide gel electrophoresis and were transferred to polyvinylidene difluoride membranes (Bio-Rad, Richmond, CA, USA). Membranes were later blocked with 5% nonfat milk in TBS-Tween-20 (TBST) for 1.5 h at room temperature and were blotted with the appropriate primary antibody (NF-κB, 1:500; glyceraldehyde 3-phosphate dehydrogenase [GAPDH], 1:1,000) (Cell Signaling Technology, Boston, MA, USA) (p-NF-κB, 1:500) (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA). GAPDH was used as a loading control. After being washed 3 times with TBST, the membranes were incubated with a horseradish peroxidase-conjugated anti-rabbit or anti-mouse secondary antibody (Beyotime) for 2 h. The proteins were visualized using enhanced chemiluminescence (Thermo Fisher Scientific, Waltham, MA, USA).
Statistical analysis
All of the values are expressed as the mean ± standard error of mean (n=6). Significant differences between the groups were determined with GraphPad Prism 5 (Graphpad Software, San Diego, CA, USA) using a Student's t-test. The difference was considered to be significant at values of p<0.05.
Results
Structure of porous Se@SiO2 nanospheres
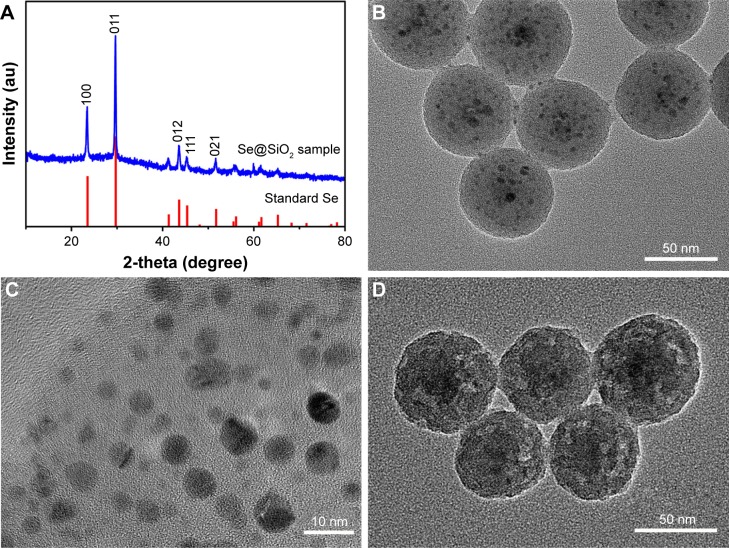
The structure of porous Se@SiO2 nanospheres was detected by an X-ray diffractometer (XRD) pattern (Figure 1A). Several well-defined characteristic peaks, such as (100), (011), (110) and (012), indicate the hexagonal phase, referenced as the standard Se phase (JCPDS card no 65–1,876). Moreover, due to the amorphous silica coating, the XRD pattern of porous Se@SiO2 nanospheres showed a steady increase in the low angle region. The diameter of homogeneous nanospheres waŝ55 nm, with many small nanoparticles (<5 nm) interspersed from the center to the surface (Figure 1B). The porous Se@SiO2 nanospheres formed porous structures after being treated with hot water (Figure 1C and D).
Figure 1.
Identification of the porous Se@SiO2 nanospheres.
Notes: (A) XRD pattern of the porous Se@SiO2 nanospheres and standard hexagonal phase of selenium (JCPDS and no 65–1,876). (B) TEM of the porous Se@SiO2 nanospheres. (C and D) Low-magnified and medium-magnified images of the porous Se@SiO2 nanospheres.
Abbreviations: TEM, transmission electron microscopy; XRD, X-ray diffractometer.
Porous Se@SiO2 nanospheres could reduce production of ROS in vitro
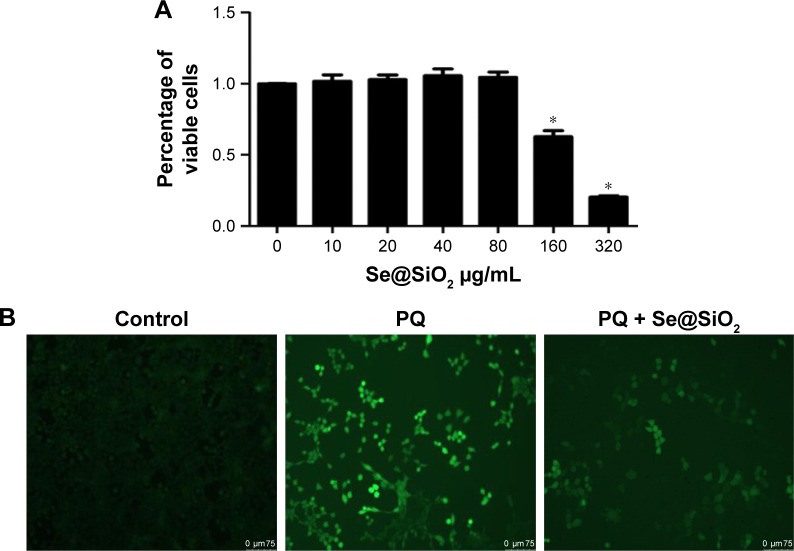
First, we determined an appropriate concentration of porous Se@SiO2 nanospheres to treat RLE-6TN cells. The data showed that there was no observable change in cell viability at the concentration of 80 μg/mL (Figure 2A). After PQ treatment, ROS were significantly increased in the RLE-6TN cells. ROS were reduced in the PQ + Se@SiO2 group compared with the PQ group (Figure 2B).
Figure 2.
Porous Se@SiO2 nanospheres reduced PQ-induced ROS in vitro.
Notes: (A) The influence of porous Se@SiO2 nanospheres on the viability of RLE-6TN cells was detected by a Cell Counting Kit-8. *p<0.05, versus control group. (B) Changes in ROS levels in RLE-6TN cells after treatment with PQ or PQ + Se@SiO2.
Abbreviations: PQ, paraquat; RLE-6TN, rat type II alveolar cells; ROS, reactive oxygen species.
MDA, GSH and SOD in rat lung tissues and plasma
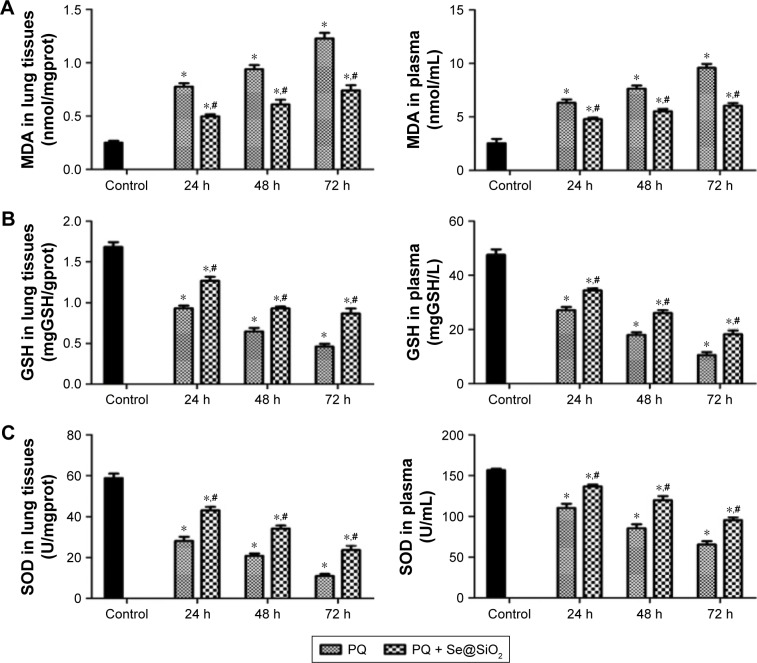
After PQ treatment, the levels of MDA in the rat lung tissues and plasma were increased. With the addition of porous Se@SiO2 nanospheres, MDA was significantly decreased in the PQ + Se@SiO2 group compared with the PQ group (Figure 3A). The levels of GSH and SOD in the rat lung tissues and plasma were reduced in the PQ group compared with the control group. In the PQ + Se@SiO2 group, the levels of GSH and SOD were markedly increased compared with those of the PQ group (Figure 3B and C).
Figure 3.
MDA, GSH and SOD in rat lung tissues and plasma.
Notes: (A, B and C) The levels of MDA, GSH and SOD in the rat lung tissues and plasma were detected with an MDA assay kit (TBA method), a reduced GSH assay kit (spectrophotometric method) and a superoxide dismutase assay kit (WST-1 method) respectively. *p<0.05, versus control group. #p<0.05, versus PQ group.
Abbreviations: GSH, glutathione; MDA, malondialdehyde; PQ, paraquat; SOD, superoxide dismutase; TBA, thiobarbituric acid; WST-1, 2-(4-Iodophenyl)-3-(4-nitropheny)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt.
Porous Se@SiO2 nanospheres could decrease the degree of PQ-induced ALI
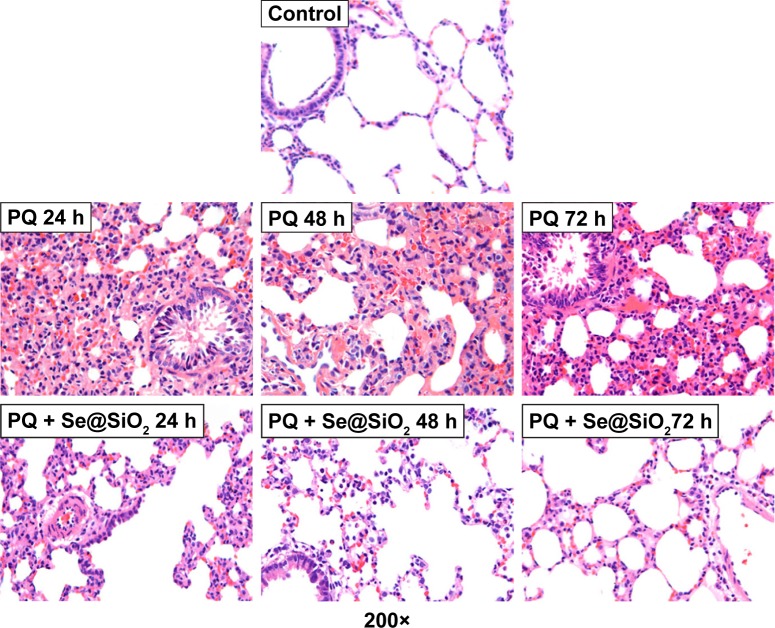
The wet-to-dry weight ratio was significantly increased at each time point after PQ treatment. The wet-to-dry weight ratio was reduced after treating with the porous Se@SiO2 nanospheres compared with the PQ group (Figure 4). In Figure 5, the pathology of the rat lung tissues shows that the normal structure of the lung tissues was damaged, and edema was present in the PQ group. These manifestations were markedly alleviated after porous Se@SiO2 nanosphere treatment.
Figure 4.
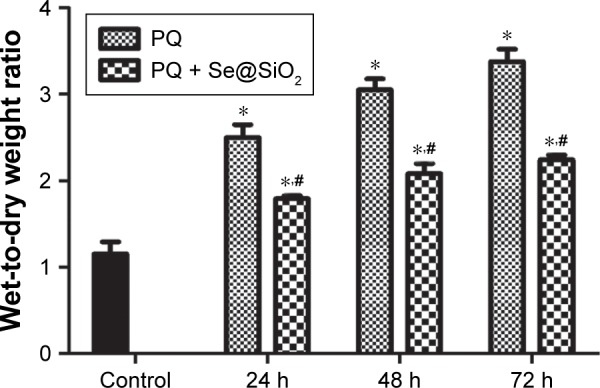
Wet-to-dry weight ratio of rat lung tissues.
Notes: The wet-to-dry weight ratio was calculated by dividing the wet weight by the dry weight. *p<0.05, versus control group. #p<0.05, significant change relative to PQ group.
Abbreviation: PQ, paraquat.
Figure 5.
Histological analysis of rat lung tissues.
Note: The rat lung tissues were stained with hematoxylin and eosin.
Abbreviation: PQ, paraquat.
Porous Se@SiO2 nanospheres inhibited PQ-induced inflammation
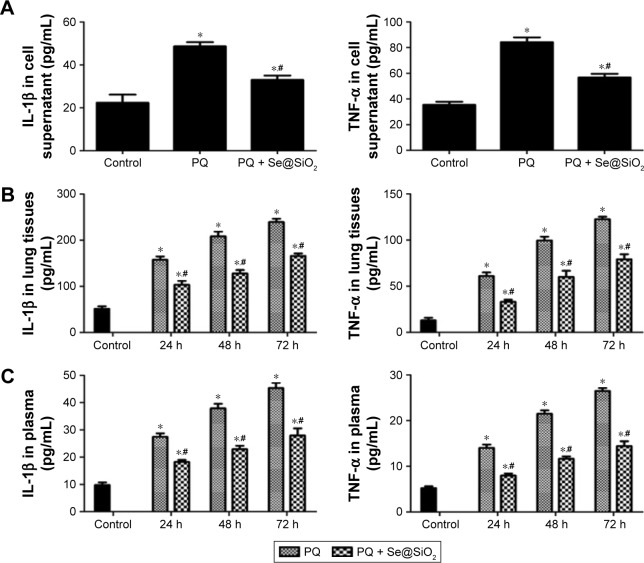
Next, we detected the levels of IL-1β and TNF-α in the cell supernatants, the rat lung tissues and the plasma samples. IL-1β and TNF-α levels were increased in the cell supernatants of the PQ group compared with those of the control group (Figure 6A). Additionally, the expression levels of IL-1β and TNF-α were reduced in the PQ + Se@SiO2 group compared with the PQ group. In the rat lung tissues and plasma, IL-1β and TNF-α levels also increased after PQ treatment at each time point. Additionally, the porous Se@ SiO2 nanospheres decreased the expression of IL-1β and TNF-α in the PQ + Se@SiO2 group compared with the PQ group (Figure 6B and C).
Figure 6.
Expression of IL-1β and TNF-α after porous Se@SiO2 nanosphere treatment.
Notes: (A) The RLE-6TN cells were treated with Se@SiO2, PQ and PQ + Se@SiO2. The expression levels of IL-1β and TNF-α in the supernatants of RLE-6TN cells were measured by ELISA. *p<0.05, versus control group. #p<0.05, versus PQ group. (B and C) The levels of IL-1β and TNF-α in the rat lung tissues and plasma were detected by ELISA. *p<0.05, versus control group. #p<0.05, versus PQ group.
Abbreviations: ELISA, enzyme-linked immunosorbent assay; IL-1β, interleukin-1β; PQ, paraquat; RLE-6TN, rat type II alveolar cells; TNF-α, tumor necrosis factor-α.
NF-κB and p-NF-κB levels were reduced after porous Se@SiO2 nanosphere treatment
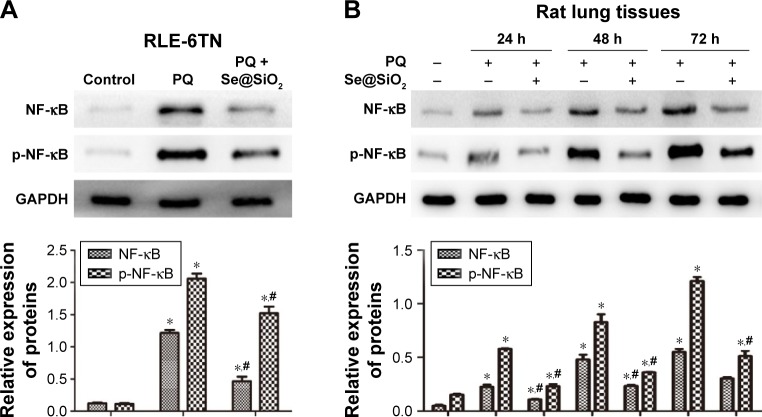
As the Western blotting results show, the expression levels of NF-κB and p-NF-κB were significantly increased in the PQ group in vitro (Figure 7A) and in vivo (Figure 7B). The levels of NF-κB and p-NF-κB were reduced in the cells and rat lung tissues at each time point after porous Se@SiO2 nanosphere treatment.
Figure 7.
Level of NF-κB and p-NF-κB after porous Se@SiO2 nanospheres treatment.
Notes: (A and B) The expression levels of NF-κB and p-NF-κB protein in the RLE-6TN cells and rat lung tissues were determined by Western blotting. *p<0.05, versus control group. #p<0.05, significant change relative to PQ group.
Abbreviations: GAPDH, glyceraldehyde 3-phosphate dehydrogenase; NF-κB, nuclear factor-κB; p-NF-κB, phosphorylated NF-κB; PQ, paraquat; RLE-6TN, rat type II alveolar cells.
Discussion
At present, the mechanism of PQ-induced ALI is still not conclusively known. Most scholars believe that oxidative stress has an important function in this process. Traditional antioxidants, such as vitamin C, N-acetylcysteine, and others for the treatment of PQ poisoning have been used clinically and have shown an effect.23,24 In this study, we found that porous Se@SiO2 nanospheres could alleviate oxidative stress and reduce inflammatory cytokine production in PQ-induced ALI.
As a trace element, Se participates in regulating the activity of GSH.25 It has been demonstrated that selenium supplementation leads to higher GSH peroxidase activity and less oxidative damage in ALI.26,27 Significant advantages of porous Se@SiO2 nanospheres in both economic terms and stability (room temperature storage) are due to the SiO2-coated structure. The porous structure gives Se@SiO2 nanospheres the potential to be multifunctional and makes them slow-releasing. Controlled-release systems play special roles in disease treatment.28,29 Compared with normal Se nanoparticles, Se in the Se@SiO2 nanospheres is limited by SiO2. Accompanying the entrance of pyrrolidinovalerophe-none into an aqueous solution, trace amounts of Se can be released into the solution.20 Due to their controlled-release, porous Se@SiO2 nanospheres may have advantages in bio-safety and in vivo stability. Thus, SiO2-coated ultra-small Se particles may help to alleviate PQ-induced ALI.
Studies have shown that Se may reduce the expression of ROS and help combat oxidative stress.30–32 ROS and MDA levels are known to reflect the degree of peroxidation. GSH and SOD are main endogenous antioxidants and can effectively remove the O2– and reduce oxidation reactions and the subsequent acute inflammatory response. When these antioxidants are imbalanced, there will be airway inflammation, airway hyperresponsiveness and tissue damage, neutrophil infiltration and ultimately the formation of lung injury.2,6,33 In our study, the results showed that the levels of ROS were markedly increased in the PQ group. In the rat model of PQ poisoning, MDA levels increased and GSH and SOD levels decreased at each time point. However, the porous Se@SiO2 nanospheres reduced the expression of ROS in vitro. MDA levels decreased, and GSH and SOD levels increased after porous Se@SiO2 nanosphere treatment compared with the PQ group. These data indicated that the porous Se@SiO2 nanospheres could alleviate PQ-induced oxidative stress in ALI.
NF-κB is a primary factor that regulates the cytokine network, and its activation could cause imbalances in the regulation of this network. NF-κB must be phosphorylated to be trafficked into the nucleus to activate the expression of inflammatory factors. p-NF-κB can initiate transcription and expression of many inflammatory cytokines (IL-1β, TNF-α, IL-6) and then form an “inflammatory cascade” to initiate pulmonary inflammation.34 In this study, we observed that the expression levels of NF-κB, p-NF-κB, IL-1β and TNF-α were significantly increased in the PQ-poisoned rats and in RLE-6TN cells. Additionally, these indicators were reduced upon treatment with porous Se@SiO2 nanospheres compared with the PQ group. NF-κB is an oxidative stress-sensitive transcription factor that can be activated by oxygen free radicals as a second messenger. TNF-α and IL-1β are thought of as important inflammatory mediators in the early stages of acute lung injury. TNF-α can trigger the synthesis of leukotriene and prostaglandin E2, stimulate the infiltration of granulocytes into the lungs, and cause lung injury.35 IL-1β regulates the activity of helper T lymphocytes, the accumulation of chemotactic granulocytes, macrophages and lymphocytes, and mediates alveolar inflammation.36 TNF-α can also activate neutrophils to mediate the alveolar inflammatory response by synergizing with IL-1β. Our research showed that the wet-to-dry weight ratio of the rat lung tissues was reduced and lung tissue damage was significantly alleviated after porous Se@SiO2 nanosphere treatment. Thus, these results indicated that porous Se@SiO2 nanospheres may reduce PQ-induced inflammation by suppressing ROS.
As Se has multiple functions in addition to its role as an antioxidant, we speculated that the porous Se@SiO2 nanospheres may also prevent PQ-induced ALI by modulating cell apoptosis, proliferation, or by regulating immune cells.37,38 In our research, we found that the porous Se@SiO2 nanospheres could alleviate PQ-induced ALI by suppressing oxidative stress. However, the appropriate dose of porous Se@SiO2 nanospheres, the optimal method of drug-delivery, the distribution of porous Se@SiO2 nanospheres and the efficacy of this treatment compared with other traditional antioxidative drugs need to be further researched.
Conclusions
In this study, we confirmed that porous Se@SiO2 nanospheres could suppress oxidative stress in PQ-induced ALI. Additionally, this treatment could also reduce NF-κB and inflammatory cytokines (IL-1β and TNF-α), possibly by inhibiting ROS. This study demonstrates that porous Se@SiO2 nanospheres may be a therapeutic method in future treatments for PQ poisoning.
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (No 71432007-2, 81272002).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Novaes RD, Goncalves RV, Marques DC, et al. Effect of bark extract of Bathysa cuspidata on hepatic oxidative damage and blood glucose kinetics in rats exposed to paraquat. Toxicol pathol. 2012;40(1):62–70. doi: 10.1177/0192623311425059. [DOI] [PubMed] [Google Scholar]
- 2.Dinis-Oliveira RJ, Duarte JA, Sanchez-Navarro A, Remiao F, Bastos ML, Carvalho F. Paraquat poisonings: mechanisms of lung toxicity, clinical features, and treatment. Crit Rev Toxicol. 2008;38(1):13–71. doi: 10.1080/10408440701669959. [DOI] [PubMed] [Google Scholar]
- 3.Chen JG, Eldridge DL, Lodeserto FJ, et al. Paraquat ingestion: a challenging diagnosis. Pediatrics. 2010;125(6):e1505–e1509. doi: 10.1542/peds.2009-2601. [DOI] [PubMed] [Google Scholar]
- 4.Yamashita M, Yamashita M, Ando Y. A long-term follow-up of lung function in survivors of paraquat poisoning. Hum Exp Toxicol. 2000;19(2):99–103. doi: 10.1191/096032700678815729. [DOI] [PubMed] [Google Scholar]
- 5.Lin JL, Lin-Tan DT, Chen KH, Huang WH. Repeated pulse of methyl-prednisolone and cyclophosphamide with continuous dexamethasone therapy for patients with severe paraquat poisoning. Crit Care Med. 2006;34(2):368–373. doi: 10.1097/01.ccm.0000195013.47004.a8. [DOI] [PubMed] [Google Scholar]
- 6.Kim H, Lee SW, Baek KM, Park JS, Min JH. Continuous hypoxia attenuates paraquat-induced cytotoxicity in the human A549 lung carcinoma cell line. Exp Mol Med. 2011;43(9):494–500. doi: 10.3858/emm.2011.43.9.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dinis-Oliveira RJ, Duarte JA, Remiao F, Sanchez-Navarro A, Bastos ML, Carvalho F. Single high dose dexamethasone treatment decreases the pathological score and increases the survival rate of paraquat-intoxicated rats. Toxicology. 2006;227(1–2):73–85. doi: 10.1016/j.tox.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 8.Amirshahrokhi K, Bohlooli S. Effect of methylsulfonylmethane on paraquat-induced acute lung and liver injury in mice. Inflammation. 2013;36(5):1111–1121. doi: 10.1007/s10753-013-9645-8. [DOI] [PubMed] [Google Scholar]
- 9.Djukic MM, Jovanovic MD, Ninkovic M, et al. Protective role of glutathione reductase in paraquat induced neurotoxicity. Chem Biol Interact. 2012;199(2):74–86. doi: 10.1016/j.cbi.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 10.Li SP, Han JY, Sun P, Wu GY, Bai XY. Effect of SP-A/B in lipoic acid on acute paraquat poisoning. World J Emerg Med. 2014;5(1):57–62. doi: 10.5847/wjem.j.issn.1920-8642.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park MH, Hong JT. Roles of NF-kappaB in cancer and inflammatory diseases and their therapeutic approaches. Cells. 2016;5(2):E15. doi: 10.3390/cells5020015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu Y, Liu JP, Zhu Y, Lu NH. The importance of toll-like receptors in NF-kappaB signaling pathway activation by helicobacter pylori infection and the regulators of this response. Helicobacter. 2016;21(5):428–440. doi: 10.1111/hel.12292. [DOI] [PubMed] [Google Scholar]
- 13.Wullaert A, Bonnet MC, Pasparakis M. NF-kappaB in the regulation of epithelial homeostasis and inflammation. Cell Res. 2011;21(1):146–158. doi: 10.1038/cr.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El Assar M, Angulo J, Rodriguez-Manas L. Oxidative stress and vascular inflammation in aging. Free Radic Biol Med. 2013;65:380–401. doi: 10.1016/j.freeradbiomed.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Ali S, Mann DA. Signal transduction via the NF-kappaB pathway: a targeted treatment modality for infection, inflammation and repair. Cell Biochem Funct. 2004;22(2):67–79. doi: 10.1002/cbf.1082. [DOI] [PubMed] [Google Scholar]
- 16.Rayman MP. The importance of selenium to human health. Lancet. 2000;356(9225):233–241. doi: 10.1016/S0140-6736(00)02490-9. [DOI] [PubMed] [Google Scholar]
- 17.Spallholz JE. On the nature of selenium toxicity and carcinostatic activity. Free Radic Biol Med. 1994;17(1):45–64. doi: 10.1016/0891-5849(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 18.Prathna TC, Chandrasekaran N, Raichur AM, Mukherjee A. Biomimetic synthesis of silver nanoparticles by Citrus limon (lemon) aqueous extract and theoretical prediction of particle size. Colloids Surf B Biointerfaces. 2011;82(1):152–159. doi: 10.1016/j.colsurfb.2010.08.036. [DOI] [PubMed] [Google Scholar]
- 19.He Y, Chen S, Liu Z, Cheng C, Li H, Wang M. Toxicity of selenium nanoparticles in male Sprague-Dawley rats at supranutritional and nonlethal levels. Life Sci. 2014;115(1–2):44–51. doi: 10.1016/j.lfs.2014.08.023. [DOI] [PubMed] [Google Scholar]
- 20.Liu X, Deng G, Wang Y, et al. A novel and facile synthesis of porous SiO2-coated ultrasmall Se particles as a drug delivery nanoplatform for efficient synergistic treatment of cancer cells. Nanoscale. 2016;8(16):8536–8541. doi: 10.1039/c6nr02298g. [DOI] [PubMed] [Google Scholar]
- 21.Deng G, Niu K, Zhou F, et al. Treatment of steroid-induced osteonecrosis of the femoral head using porous Se@SiO2 nanocomposites to suppress reactive oxygen species. Sci Rep. 2017;(7):43914. doi: 10.1038/srep43914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu Y, Tan J, Xie H, Wang J, Meng X, Wang R. HIF-1alpha regulates EMT via the Snail and beta-catenin pathways in paraquat poisoning-induced early pulmonary fibrosis. J Cell Mol Med. 2016;20(4):688–697. doi: 10.1111/jcmm.12769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Awadalla EA. Efficacy of vitamin C against liver and kidney damage induced by paraquat toxicity. Exp Toxicol Pathol. 2012;64(5):431–434. doi: 10.1016/j.etp.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 24.Ahmad I, Shukla S, Kumar A, et al. Biochemical and molecular mechanisms of N-acetyl cysteine and silymarin-mediated protection against maneb- and paraquat-induced hepatotoxicity in rats. Chem Biol Interact. 2013;201(1–3):9–18. doi: 10.1016/j.cbi.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 25.Birringer M, Pilawa S, Flohe L. Trends in selenium biochemistry. Nat Prod Rep. 2002;19(6):693–718. doi: 10.1039/b205802m. [DOI] [PubMed] [Google Scholar]
- 26.Kim KS, Suh GJ, Kwon WY, et al. Antioxidant effects of selenium on lung injury in paraquat intoxicated rats. Clin Toxicol (Phila) 2012;50(8):749–753. doi: 10.3109/15563650.2012.708418. [DOI] [PubMed] [Google Scholar]
- 27.Bhattacharjee A, Basu A, Biswas J, Bhattacharya S. Nano-Se attenuates cyclophosphamide-induced pulmonary injury through modulation of oxidative stress and DNA damage in Swiss albino mice. Mol Cell Biochem. 2015;405(1–2):243–256. doi: 10.1007/s11010-015-2415-1. [DOI] [PubMed] [Google Scholar]
- 28.Robinson E, Kaushal S, Alaboson J, et al. Combinatorial release of dexamethasone and amiodarone from a nano-structured parylene-C film to reduce perioperative inflammation and atrial fibrillation. Nanoscale. 2016;8(7):4267–4275. doi: 10.1039/c5nr07456h. [DOI] [PubMed] [Google Scholar]
- 29.Kim K, Jo MC, Jeong S, et al. Externally controlled drug release using a gold nanorod contained composite membrane. Nanoscale. 2016;8(23):11949–11955. doi: 10.1039/c6nr00362a. [DOI] [PubMed] [Google Scholar]
- 30.Gong G, Meplan C, Gautrey H, Hall J, Hesketh JE. Differential effects of selenium and knock-down of glutathione peroxidases on TNFalpha and flagellin inflammatory responses in gut epithelial cells. Genes Nutr. 2012;7(2):167–178. doi: 10.1007/s12263-011-0256-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu ZL, Yin XB, Lin ZQ, et al. Inhibitory effect of selenium against Penicillium expansum and its possible mechanisms of action. Curr Microbiol. 2014;69(2):192–201. doi: 10.1007/s00284-014-0573-0. [DOI] [PubMed] [Google Scholar]
- 32.Hassanin KM, Abd El-Kawi SH, Hashem KS. The prospective protective effect of selenium nanoparticles against chromium-induced oxidative and cellular damage in rat thyroid. Int J Nanomedicine. 2013;8:1713–1720. doi: 10.2147/IJN.S42736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qian J, Ye Y, Lv L, Zhu C, Ye S. FTY720 attenuates paraquat-induced lung injury in mice. Int Immunopharmacol. 2014;21(2):426–431. doi: 10.1016/j.intimp.2014.05.025. [DOI] [PubMed] [Google Scholar]
- 34.Aksentijevich I, Zhou Q. NF-kappaB pathway in autoinflammatory diseases: dysregulation of protein modifications by ubiquitin defines a new category of autoinflammatory diseases. Front Immunol. 2017;8:399. doi: 10.3389/fimmu.2017.00399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dinis-Oliveira RJ, Remiao F, Duarte JA, et al. P-glycoprotein induction: an antidotal pathway for paraquat-induced lung toxicity. Free Radic Biol Med. 2006;41(8):1213–1224. doi: 10.1016/j.freeradbiomed.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 36.Butt Y, Kurdowska A, Allen TC. Acute lung injury: a clinical and molecular review. Arch Pathol Lab Med. 2016;140(4):345–350. doi: 10.5858/arpa.2015-0519-RA. [DOI] [PubMed] [Google Scholar]
- 37.Liu H, Xu H, Huang K. Selenium in the prevention of atherosclerosis and its underlying mechanisms. Metallomics. 2017;9(1):21–37. doi: 10.1039/c6mt00195e. [DOI] [PubMed] [Google Scholar]
- 38.Wrobel JK, Power R, Toborek M. Biological activity of selenium: revisited. IUBMB Life. 2016;68(2):97–105. doi: 10.1002/iub.1466. [DOI] [PubMed] [Google Scholar]