Abstract
Preclinical evidence has established that the host commensal microbiota can contribute to therapeutic response in cancer models, a finding supported by early clinical data. This connection between the microbiome and clinical outcome in oncology is cause for new consideration in the administration of antibiotics and microbiota-modulating interventions to improve outcomes.
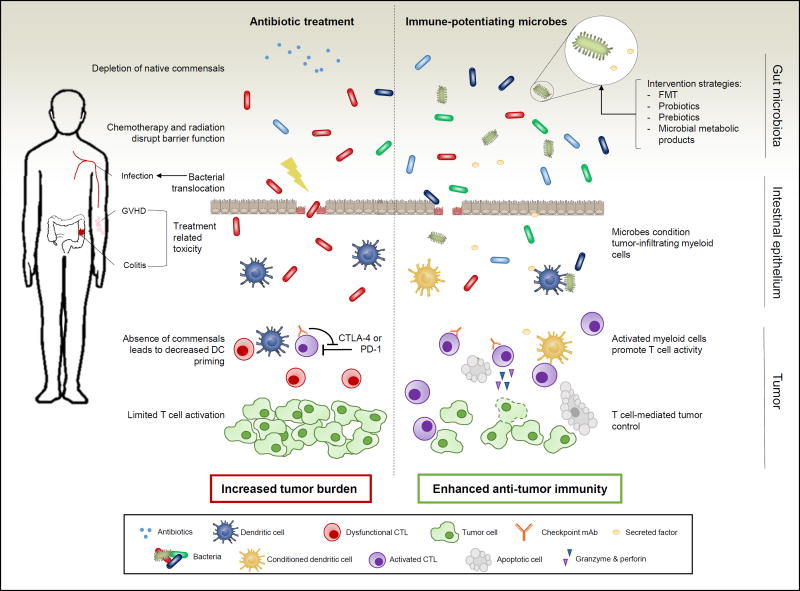
In this issue of Clinical Cancer Research, Galloway-Peña and colleagues discuss the implications of antibiotic use in cancer care. The authors highlight the role that the microbiota plays in treatment efficacy and the overall health of the cancer patient (see Figure 1) (1).
Figure 1. The impact of the microbiota in cancer treatment outcomes.
The potentially harmful role of the microbiota post-antibiotics treatment (left) or the beneficial contribution of commensal bacteria (right) are indicated. Antibiotics deplete native commensals that can lead to the outgrowth of pathogenic bacteria and more severe treatment-related toxicity. Chemotherapy and radiation disrupt barrier function allowing for bacterial translocation and subsequent infection. The reduction in specific commensals leads to decreased DC priming and limited T cell activation in the tumor microenvironment. Alternatively, the presence of immune-potentiating microbes or their byproducts can ultimately condition tumor-infiltrating myeloid cells. These activated myeloid cells promote T cell activation and ultimately enhancing immune-mediated tumor control.
When an oncologist treats a patient, the overall goal is to eradicate malignancy while minimally affecting healthy tissue. Immunotherapy can harness the immune system’s already carefully calibrated ability to preferentially mount responses against foreign antigens (including tumor antigens) over self-antigens, and has the potential to provide remarkably durable clinical benefit. However, a key host variable that impacts this process are the trillions of foreign organisms that have co-evolved with us—our commensal microbiota.
The microbiota represents a complex community of bacteria, archaea, protists, fungi, and viruses that exist in symbiosis within human hosts. The gut harbors 1013 bacteria alone and it is here, at this critical interface between the immune system and the outside world, that the microbiota plays a key role fine-tuning immunity. It is well established that the microbiota can influence human health in a vast number of ways (2). More recently its importance in cancer, beyond inflammation-driven tumors, has become increasingly evident. Immune modulating drugs are revolutionizing treatment in many cancers. In order to continue to capitalize on the promise of immunotherapy, it is imperative to understand how gut microbiota affects host immunity in the context of cancer. Chemotherapy and radiation can lead to disruption of the intestinal barrier, potentially allowing bacteria to translocate across the epithelium, leading to a more pronounced effect of the microbiota on health for cancer patients.
In preclinical studies, multiple groups have found that the composition of the intestinal microbiota is a key component of anti-tumor immunity and therapeutic efficacy (3–6). It is now appreciated that the same genetically identical mouse strain from two different vendors can harbor unique commensal organisms and that this diversity can impact systemic immune responses. This includes communities of commensal bacteria associated with potent or weak therapeutic efficacy with checkpoint blockade antibodies such as anti-PD-L1 (3). The disparity between mice can be eliminated with transfer of fecal material by gavage, and in one model the administration of a single microbe associated with improved response, Bifidobacterium breve, improved efficacy of anti-PD-L1, highlighting the potential for clinical intervention strategies. In parallel studies, CTLA-4 blockade was found to rely on the gut microbiota and anti-tumor effects were reported to be dependent upon distinct Bacteroides species (4). Similarly, CpG-oligonucleotide immunotherapy and platinum-based chemotherapy required the presence of intestinal microbiota for maximal potency (5). Clinical data also point to the microbiota accounting for variability in treatment-related toxicity. In melanoma patients treated with ipilimumab, a positive association was identified between resistance to immune-mediated colitis and bacteria belonging to the Bacteroidetes phylum (7).
Given the evidence pointing to commensals as an important determinant of response to treatment, it is paramount to critically evaluate the use of a common microbiota-modulating agent in cancer—antibiotics. Galloway-Peña and colleagues describe the dual properties of antibiotics in oncology. Historically, antimicrobial treatment has been crucial for maintaining the overall health of cancer patients through treatment and prevention of infectious complications (8). Yet this precedent has led to injudicious administration, which can have negative consequences as well. In addition to their intended role impeding pathogenic bacteria, antibiotics can also deplete native microflora, which is a key regulator of anti-tumor immunity. Oncologists caring for cancer patients are therefore challenged with finding a balance in antimicrobial administration -- preventing and treating infection without entering the vicious cycle of recurring infection, increasing multi-drug resistance, and subsequent broad spectrum antibiotic treatment leading to intestinal dysbiosis. Mucosal barrier injury from cytotoxic chemotherapy in conjunction with antibiotic treatment can lead to the proliferation of opportunistic pathogenic bacteria that can translocate across the compromised epithelium and lead to infection (9).
Antibiotic use has also been shown to increase the severity of graft-versus-host disease (GVHD) and mortality in mice and humans receiving allogenic hematopoietic stem-cell transplantation (HSCT) (10). Furthermore, antibiotics specific for gram-positive bacteria were reported to lead to cyclophosphamide-resistant tumors in animal models via dampened induction of pathogenic T helper 17 (pTH17) cells and memory TH1 responses (6). Follow-up clinical studies in chronic lymphocytic leukemia (CLL) patients treated with cyclophosphamide and cisplatin confirmed that antibiotic treatment targeting Gram-positive bacteria was independently associated with shorter progression-free survival and overall survival (11). These data underscore the potential repercussions from compromising commensal communities with antibiotic use and the need to refine antimicrobial treatment strategies.
Given preclinical evidence that the commensal population in the gut is both influential and pliable, several strategies are possible to utilize the microbiota to improve patient outcomes. The composition of the intestinal microbiome can be analyzed by 16S and shotgun sequencing to develop prognostic or diagnostic tools. Retrospective analysis of pre-treatment stool samples can establish correlations between the microbiota and clinical outcomes, including response to therapy and toxicity. Such data might also be used to predict response to therapy and to devise more personalized regimens to minimize the risk of treatment-related toxicity and also define the impact of antibiotics. Biomarkers may also identify patient candidates for microbiota intervention strategies such as fecal transplantation, prebiotics, and probiotics.
Despite our growing understanding of the microbiota, Galloway-Peña et al. raise several outstanding questions in the cancer-microbiome connection. As microbiome sequencing techniques become more accessible and widely adopted, reproducible collection, processing, and analysis methods must be employed to ensure robust results. The importance of longitudinal sampling in cancer patients remains unknown. While the commensal community of healthy individuals is relatively stable, cancer patients experience a barrage of assaults to their intestinal homeostasis including chemotherapy, radiation, and antibiotics. The vast majority of current microbiota studies focus only on the bacterial component, which is further limited by the inability to culture many of these microbes. Improved culture methods will be necessary to carefully study isolates and assign functional importance. To paint a more comprehensive picture of the microbiota it may be necessary to expand attention beyond bacteria to the broader ecological community and include viruses and fungi.
Deeper mechanistic interrogation into the role of the microbiota in cancer relies on animal models that faithfully recapitulate phenomena relevant to human health. This includes humanized microbiota and cancer models, and incorporating autochthonous models to more closely mirror disease progression. Due to the inherent differences between mice and humans, the ultimate test for microbiota manipulations will have to rely on interventional clinical trials. Such studies can validate correlations retrospectively identified in patients, but more importantly assess whether specific manipulations can improve therapeutic efficacy or decrease treatment-related toxicity. More systematic tracking of antibiotic use in patients in the context of immunotherapy administration will be important for understanding clinical impact. Interactions are beginning to be defined between the composition of the microbiota and germline polymorphisms of the host, diet, and exercise. As such, an integrated analysis of multiple dimensions of data in patients may reveal additional clues regarding the biology of commensal bacteria and impact on treatment outcomes.
Acknowledgments
Funding: Work underlying some aspects of this commentary were supported by R35 CA210098 from the National Cancer Institute.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest.
References
- 1.Galloway-Peña JR, Jenq RR, Shelburne SA. Can Consideration of the Microbiome Improve Antimicrobial Utilization and Treatment Outcomes in the Oncology Patient? Clin Cancer Res. doi: 10.1158/1078-0432.CCR-16-3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kamada N, Seo S-U, Chen GY, Núñez G. Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol. 2013 May;13(5):321–35. doi: 10.1038/nri3430. [DOI] [PubMed] [Google Scholar]
- 3.Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti–PD-L1 efficacy. Science. 2015 Nov 27;350(6264):1084–9. doi: 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vétizou M, Pitt JM, Daillère R, Lepage P, Waldschmitt N, Flament C, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015 Nov 27;350(6264):1079–84. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA, et al. Commensal Bacteria Control Cancer Response to Therapy by Modulating the Tumor Microenvironment. Science. 2013 Nov 22;342(6161):967–70. doi: 10.1126/science.1240527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Viaud S, Saccheri F, Mignot G, Yamazaki T, Daillère R, Hannani D, et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science. 2013 Nov 22;342(6161):971–6. doi: 10.1126/science.1240537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dubin K, Callahan MK, Ren B, Khanin R, Viale A, Ling L, et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat Commun. 2016 Feb 2;7:10391. doi: 10.1038/ncomms10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, et al. Executive Summary: Clinical Practice Guideline for the Use of Antimicrobial Agents in Neutropenic Patients with Cancer: 2010 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2011 Feb 15;52(4):427–31. doi: 10.1093/cid/ciq147. [DOI] [PubMed] [Google Scholar]
- 9.Taur Y, Pamer EG. Microbiome mediation of infections in the cancer setting. Genome Med. 2016;8:40. doi: 10.1186/s13073-016-0306-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shono Y, Docampo MD, Peled JU, Perobelli SM, Velardi E, Tsai JJ, et al. Increased GVHD-related mortality with broad-spectrum antibiotic use after allogeneic hematopoietic stem cell transplantation in human patients and mice. Sci Transl Med. 2016 May 18;8(339):339ra71–339ra71. doi: 10.1126/scitranslmed.aaf2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pflug N, Kluth S, Vehreschild JJ, Bahlo J, Tacke D, Biehl L, et al. Efficacy of antineoplastic treatment is associated with the use of antibiotics that modulate intestinal microbiota. OncoImmunology. 2016 Jun 2;5(6):e1150399. doi: 10.1080/2162402X.2016.1150399. [DOI] [PMC free article] [PubMed] [Google Scholar]