Abstract
Purpose
We evaluated the impact of myopia on corneal biomechanical properties in primary open-angle glaucoma (POAG) and nonglaucoma patients, and the effect of modification of glaucoma on myopic eyes.
Methods
This cross-sectional study included 66 POAG eyes (33 myopia, 33 nonmyopia) and 66 normal eyes (33 myopia, 33 nonmyopia). Seven corneal biomechanical parameters were measured by ultra-high-speed Scheimpflug imaging, including corneal deformation amplitude (CDA), inward/outward corneal applanation length (ICA, OCA), inward/outward corneal velocity (ICV, OCV), radius, and peak distance (PD).
Results
Mean age (SD) of the 65 male (49%) and 67 female (51%) patients was 59 (9.82) years. Myopia was associated with significantly higher CDA (adjusted effect = 0.104, P = 0.001) and lower OCV (adjusted effect = −0.105, P < 0.001) in the POAG group. Within the nonglaucoma group, myopic eyes had a significantly lower OCV (adjusted effect = −0.086, P < 0.001) and higher CDA (adjusted effect = 0.079, P = 0.001). All parameters except PD suggested that glaucoma modified the effect of myopia on corneal biomechanics. Percentage differences in the adjusted myopic effect between POAG and nonglaucoma patients was 31.65, 27.27, 31.65, 50.00, 22.09, and 60.49 for CDA, ICA, OCA, ICV, OCV, and radius, respectively.
Conclusions
Myopia had a significant impact on corneal biomechanical properties in the POAG and nonglaucoma groups. The differences in corneal biomechanical parameters suggest that myopia is correlated with significantly lower ocular rigidity. POAG may enhance the effects of myopia on most of these parameters.
Keywords: corneal biomechanics, myopia, glaucoma
Glaucoma is one of the leading causes of irreversible blindness worldwide, and primary open-angle glaucoma (POAG) is the most common type of glaucoma.1,2 Many studies have found that myopia has been associated with POAG.1–3 This association is notably higher in moderate to high myopia with axial length more than 26 mm.1,2 However, the pathogenesis for why myopia increases the susceptibility for and progression of glaucoma remains controversial.4–7 Many proposed theories focus on the deformity of the lamina cribrosa. Jonas et al.4–6 found that stretching of the globe in the long-axial-length myopic eye made the optic nerve head enlarged, and the lamina cribrosa stretched and thinned. All of these factors may contribute to the increase in nerve fiber susceptibility to higher IOP.5–7
In myopia, the globe usually is elongated and scleral thickness and scleral rigidity are reduced. The viscoelastic properties of the cornea also are altered. Since the cornea, sclera, peripapillary ring, and lamina cribrosa are formed primarily by the same extracellular matrix constituents, corneal biomechanical properties can represent the elasticity of collagen fibers in the eyeball as a whole.8,9
Corneal biomechanical properties can be measured by many techniques. The Ocular Response Analyzer (ORA) was the first instrument launched and is extensively used to measure corneal biomechanical properties in terms of corneal hysteresis (CH) and corneal resistance factor (CRF).10–14 The ultra-high speed Scheimpflug camera (Corvis ST) is a new device with precise, repeatable, and reproducible measurements of corneal biomechanical properties. The device provides quantitative information, including the magnitude and direction of the displacement of the corneal apex. Published data have shown that the device has excellent reproducibility.15,16
Most of the available studies on corneal biomechanics have been conducted using the ORA.10,11 High myopes were reported to have significantly lower CH.10,12 However, the data obtained from the ORA denote only the rate-dependent viscoelastic properties of the cornea, which only represent a portion of the currently available measures of cornea biomechanical properties. The Corvis ST provides the advantage of dynamic cross-sectional imaging during the deformation, which may give additional information about the biomechanical status of the cornea. Currently, only a few studies have been done using the Corvis ST to evaluate the corneal biomechanical properties.15,16
Since myopia is one of the significant risk factors for glaucoma and could alter the biomechanical properties of the eye, the understanding of these properties in relation to myopia and glaucoma can be useful in detection and understanding of the pathophysiology. We evaluated the impact of myopia on corneal biomechanical properties in POAG and nonglaucoma patients and the effect modification of glaucoma on these myopic effects.
Methods
This study followed the tenets of the Declaration of Helsinki and was approved by the institutional review ethics committee of the Faculty of Medicine, Chulalongkorn University. This cross-sectional study was conducted at the general ophthalmology clinic and the glaucoma clinic, King Chulalongkorn Memorial Hospital, Bangkok, Thailand, between September 2015 and July 2016. Written informed consent was obtained from each subject.
Participants
Subjects were classified into 4 groups (myopia with POAG, nonmyopia with POAG, myopia without glaucoma, nonmyopia without glaucoma). Inclusion criteria were age over 40 years and willingness to participate in the study. Exclusion criteria were any of the following: (1) presence of corneal and other ocular pathology (except for nonvisually significant cataract and normal age-related posterior vitreous detachment), (2) history of corneal surgery, (3) pregnancy, (4) history of cataract surgery, (5) presence or history of underlying connective tissue disease, (6) inability to communicate and give consent, or (7) inability to perform the test, which required maintaining posture in the upright position for a few minutes.
POAG was defined as patients those who had gonioscopically open anterior chamber angles and met the glaucoma criteria based on the International Society of Geographical and Epidemiological Ophthalmology (ISGEO) guidelines:17 Category 1, a visual field (VF) defect that is consistent with glaucomatous optic neuropathy, and either a vertical cup-to-disc ratio (C/D) ≥ 0.7 (97.5th percentile) or C/D asymmetry between both eyes ≥ 0.2 (97.5th percentile); Category 2, VF results are not definitive or are unattainable due to patient inability to perform an adequate quality test, and optic disc has a C/D ≥ 0.9 (99.5th percentile) or C/D asymmetry between both eyes ≥ 0.3 (99.5th percentile); or Category 3, VF testing and optic disc examination are not possible in the subject, and visual acuity (VA) is less than 20/400 (for any ophthalmic pathology) and IOP > 21 mm Hg (99.5th percentile).
In conjunction with ISGEO criteria, subjects in whom C/D assessment was difficult and all subjects belonging to categories 2 (4 in the nonmyopia group) and 3 (1 in the myopia and 2 in the nonmyopia groups) were further evaluated with optical coherence tomography (OCT) of the retinal nerve fiber layer, optic nerve head, and ganglion cell layer, and optic disc photography. Clinical assessment and interpretation of all tests were individually performed for each patient by one of the glaucoma specialists (SC, AM, VT) to confirm the diagnosis for all subjects.
Myopia was defined as refractive error (spherical equivalence) more than −4.00 diopters (D) and axial length ≥ 26 mm. Nonmyopia was defined as refractive error (spherical equivalence) between −0.50 and +0.50 D and axial length < 26 mm.
Ocular Examination
All subjects received an ocular examination, including Snellen VA measurement, slit-lamp examination, and Goldmann applanation tonometry. Dynamic gonioscopy and disc assessment under stereoscopic biomicroscopy were performed by glaucoma specialists (SC, AM, VT). Diagnosis was confirmed in conjunction with the results of automated perimetry (24–2 Swedish Interactive Threshold Algorithm Standard on Humphrey Field Analyzer-2; Carl Zeiss Meditec, Dublin, CA), OCT (Cirrus HD-OCT 4000, Carl Zeiss Meditec), and optic disc photography (KOWA nonmyd α–D fundus camera, KOWA, Nagoya, Japan) as aforementioned.
Refraction (spherical equivalent) was measured with an autorefractor (Nidek AR530-A; Nidek, Gamagori, Japan). Axial length was measured by IOLMaster (Carl Zeiss Meditec). All ocular examinations including Corvis ST imaging were performed within the same day. Only one of the eyes that met the criteria was included to reduce intraindividual confounding. If both eyes were eligible, only right eyes were selected for analysis.
Image Acquisition
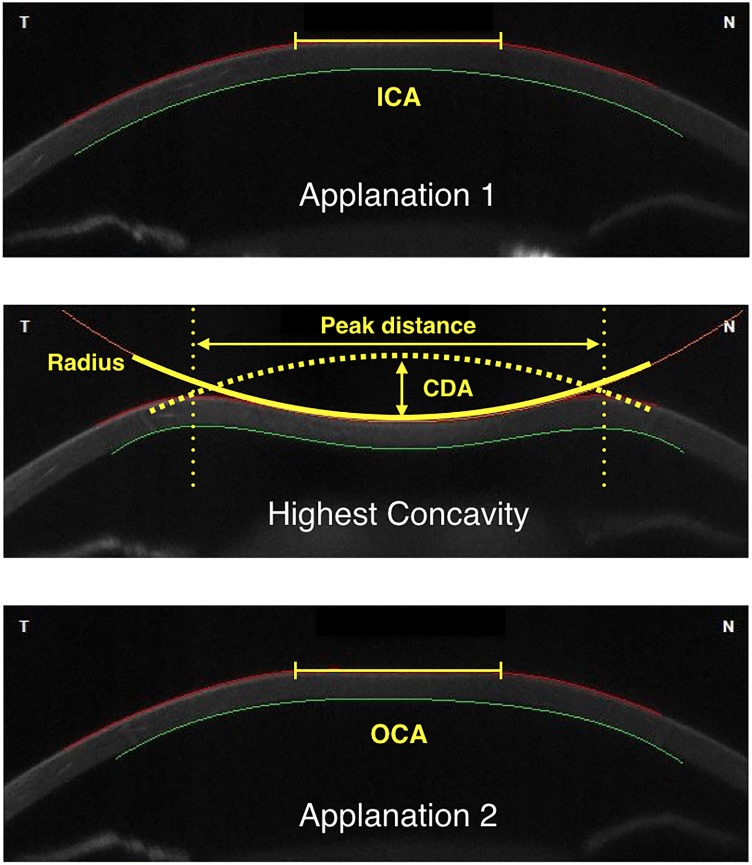
An ultra-high-speed Scheimpflug camera (Corvis ST; OCULUS Optikferäte GmbH, Wetzlar, Germany) was used in the study. It records the corneal dynamic response to a symmetrically metered air puff (size 3.06 mm, pressure 60 mm Hg) at the rate of 4330 image frames per second covering a horizontal distance of 8 mm. The machine automatically captures the corneal reaction when the cornea is aligned centrally. From the resting state, the cornea bends inward to the first flattened (applanation) state and continues to move inward until it reaches the maximum deformation state (highest concavity). After reaching this state, the cornea moves outward and passes the second applanation point before going back to its resting state. The corneal deformation response parameters were calculated automatically after automated tracing of the anterior and posterior corneal surfaces at each image frame. The parameters used in this study were: (1) corneal deformation amplitude (CDA): the distance of the maximum corneal deformation amplitude measured from the resting state to highest concavity at the corneal apex; (2) inward corneal applanation (ICA) length: the length of the flattened cornea at the first applanation; (3) outward corneal applanation (OCA) length: length of the flattened cornea at the second applanation; (4) inward corneal velocity (ICV): corneal velocity during the first applanation moment; (5) outward corneal velocity (OCV): corneal velocity during the second applanation moment; (6) peak distance (PD): distance between two bending points of the cornea at the highest concavity; and (7) radius: the radius of the circle that best fits the corneal curve at the highest concavity. A diagram illustrating these parameters is shown in the Figure. The Corvis ST also can measure optical pachymetry and IOP. We used pachymetry data of the Corvis ST for the central corneal thickness (CCT). However, we used standard Goldman applanation tonometry (GAT) IOP for analysis in this study.
Figure.
Diagram illustrates each state of corneal deformation during Corvis ST measurement, and the parameters: Top: First flattened state or the first applanation point. Middle: Maximum deformation state or point of highest concavity. Bottom: Second flattened state or second applanation point.
All images were taken by a single operator (RP). After each scan, the video output was checked for any artifacts and the corneal boundary lines were reviewed for their accuracy. The scan was repeated if the quality score (QS) bar did not show “OK” the video revealed the presence of artifact, or boundary lines were in wrong positions. If a qualified image could not be obtained by the third attempted scan, the subject was not included in the study.
Interobserver reproducibility of studied parameters was calculated from 10 eyes of 10 normal subjects with 2 sets of scans. Each scan was performed separately by 2 operators—an investigator (RP) and a trained technician—with 5-minute intervals between each scan. The sequence of operators was randomized.
Statistical Analysis
Sample size was calculated based on 2-tailed testing, an effect size of d = 0.05, 80% power and α error probability of 0.05. This calculation suggested a total sample size of at least 31 participants per group. Data were analyzed as means and standard deviations for continuous variables and counts, and percentages for categorical variables. Inferential analysis was conducted using general linear modeling to obtain unadjusted (bivariate) and adjusted (multivariate) effects for each of the seven outcomes. Along with the study predictor (myopia) the effects of age, sex, VA, and CCT were assessed for the glaucoma and nonglaucoma groups to gauge whether they were either confounders or independent risk factors. Additional clinical parameters, including C/D ratio and mean deviation (MD) on the VF, were considered for the glaucoma group only. Covariate selection in the adjusted models was based on statistical significance and/or a confounding effect, with covariates resulting in a more than a 20% change in the unadjusted myopia effect included in the multivariate model as confounders.18 Once the final models were determined, adjusted effects and model-based means of each group (estimated marginal means) were generated. Estimated marginal mean was the calculated mean that corrected for the baseline differences and adjusted for other potential confounding variables in the model. As we considered seven different outcomes, there was a potential multiplicity problem (inflation of type I error). Consequently, we used Bonferroni correction to control family-wise type 1 error (αFW = 0.05/7 = 0.007). Finally, given the potential effect modification phenomenon of POAG and myopia, a stratified analysis was conducted. Impact of myopia on outcomes was analyzed separately in each group; then, percentage difference in adjusted myopic effect between POAG and nonglaucoma patients was calculated for evaluating effect modification. In this way, we could gauge whether a preexisting condition of myopia enhances (or even diminishes) the difference in corneal biomechanics between patients with and without glaucoma. Intraclass correlations (ICC) were calculated to assess the interobserver reproducibility. All analyses were performed using the R statistical package version 3.2.1 (R Project for Statistical Computing, Vienna, Austria).
Results
We recruited 66 POAG eyes (33 myopia, 33 nonmyopia) and 66 normal eyes (33 myopia, 33 nonmyopia). Among the POAG subjects, 2 in the myopia group and 3 in the nonmyopia group had normal-tension glaucoma. Table 1 shows the demographics and clinical characteristics for all groups in the study population. The mean age (SD) of the 65 male (49%) and 67 female (51%) patients studied was 59 (9.8) years. Mean (SD) spherical equivalent was −7.78 (3.37) D in the myopia and +0.05 (0.96) D in the nonmyopia groups. Mean (SD, range) IOP was 14.56 (3.22, 8–23) in POAG and 13.78 (2.88, 8–20) in the nonglaucoma groups.
Table 1.
Demographics and Clinical Characteristics of the Study Groups
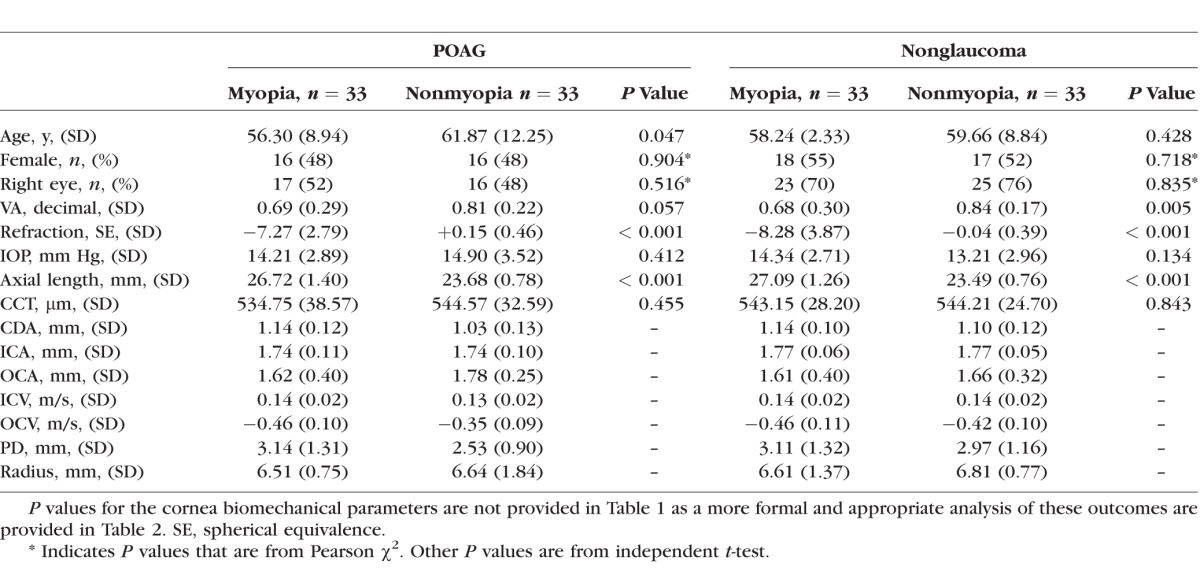
Of the patients with POAG, the mean (SD) C/D ratio was 0.73 (0.10) in the myopia and 0.71 (0.29) in the nonmyopia groups (P = 0.10). Mean (SD) visual field mean deviation was −6.09 (6.14) and −7.57 (8.68) dB, respectively (P = 0.60). Mean (SD) number of medications was 1.73 (0.84) and 1.70 (0.64), respectively (P = 0.87)
The estimated marginal means along with the effect size are shown in Table 2. The clinical parameters that resulted in changing the unadjusted effect of myopia more than 20% were used for calculating the adjusted effect.
Table 2.
Estimated Marginal Means Along With Effect Size (and 95% CI) for Myopia and Nonmyopia Patients by Glaucoma Status
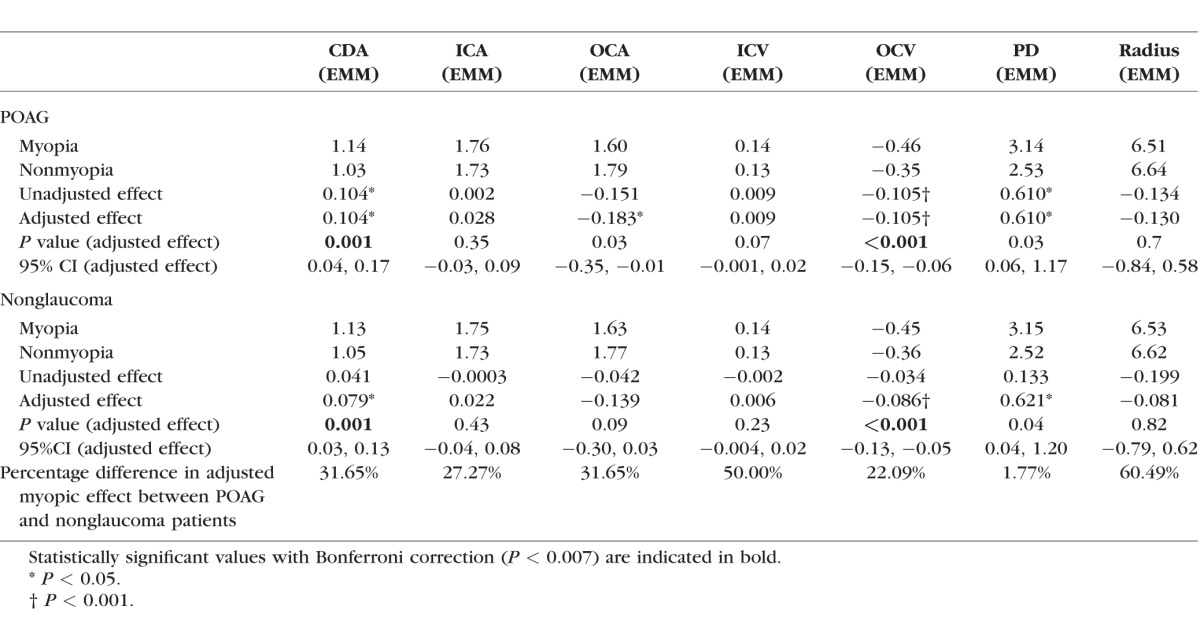
In the POAG group, CDA did not need to be adjusted (there was no confounder change in myopia effect >20% in our confounder adjusting model). No adjustments were needed for ICV, OCV, and PD. However, ICA needed adjustment for age, VA, C/D, CCT, and MD; OCA required adjustment for MD; and Radius required adjustment for age, IOP, and CCT.
In the nonglaucoma group, CDA required adjustments for VA and IOP; ICA required adjustments for sex, age, VA, IOP, and CCT; OCA required adjustments for IOP; ICV required adjustment for age, VA, and IOP; OCV required adjustment for VA and IOP; PD required adjustments for VA and IOP, and Radius required adjustment for VA and IOP to achieve an appropriate comparison between myopia and nonmyopia.
For POAG patients, there were significantly higher values for CDA (adjusted effect = 0.104, P = 0.001) and significantly lower values for OCV (adjusted effect = −0.105, P < 0.001) associated with myopia. For the nonglaucoma group, myopia also had significantly higher CDA (adjusted effect = 0.079, P = 0.001) and significantly lower OCV (adjusted effect = -0.086, P < 0.001). It should be noted that the minus symbol of OCV indicates the outward direction of the velocity; thus, the greater magnitude of the absolute value in eyes with myopia is associated with a greater amount of velocity for the OCV.
Further analysis was performed to gauge whether POAG was an effect modifier of myopia. POAG substantially modified the effect of myopia on all corneal biomechanical parameters with the exception of PD. Among POAG patients, the impact of myopia was 31.65%, 27.27%, 31.65%, 50.00%, 22.09%, and 60.49% higher on CDA, ICA, OCA, ICV, OCV, and radius, respectively.
General linear models were created to evaluate the effects of sex, age, VA, IOP, CCT, and severity of glaucoma on each parameter in the POAG and nonglaucoma groups. The data are shown in Supplementary Tables 1 and 2.
For interobserver analysis, the ICCs (95% confidence interval [CI]) of CDA, ICA, OCA, ICV, OCV, PD, and radius were 0.82 (0.30–0.95), 0.77 (0.13–0.94), 0.71 (0.28–0.93), 0.75 (0.07–0.94), 0.91 (0.63–0.98), 0.77 (0.03–0.94), and 0.87 (0.45–0.97), respectively.
Discussion
We evaluated corneal biomechanical properties between myopia and nonmyopia in POAG patients and controls using Corvis ST imaging. The results showed that myopia was associated with significantly increased CDA and decreased OCV among POAG subjects. Increased CDA and decreased OCV were correlated with myopia in the nonglaucoma group. Furthermore, our data suggested that POAG modifies the effect of myopia in all parameters except PD. There was a moderate to excellent reproducibility of the Corvis ST parameters.19
Results from population-based studies have indicated a strong link between myopia and POAG.2,3,20 In the Blue Mountains Eye Study, compared to emmetropic eyes, high and low myopia had odds ratios of 3.3 and 2.3, respectively, in developing POAG.3 Likewise, the Beaver Dam Eye Study showed that the risk of glaucoma was increased by 60% in patients compared with those without myopia.20 The data suggested that myopia was an independent risk factor for POAG. In the Singapore Malay Eye Study, the investigators found an association between longer axial length and POAG.2 This finding led to the presumption that axial myopia might be the main biometric constituent that underlies the risk for POAG.
Several theories have been proposed to explain the link between myopia and glaucoma. Most have focused on the scleral change in myopia. Myopic eyes are associated with longer axial length, which is associated with changes in the sclera and contiguous lamina cribrosa. Fong et al.21 proposed that the elongated myopic eye causes deformability of the lamina cribrosa. The stretching and thinning of this nerve-supporting structure may increase the susceptibility to IOP.4–6,22,23
There is increasing evidence of changes in the biomechanical properties of myopic eyes.13,24–27 Data suggest that there is scleral remodeling, which leads to scleral thinning in myopia.13 Studies in animal models have demonstrated a significant reduction of scleral collagen fibrils and decrease in the rate of proteoglycan synthesis in the sclera of myopic eyes.24,25 This change could lead to weakening of scleral mechanical properties.26 Similar changes appear to occur in the cornea with the development of myopia.27 According to Kotecha et al. corneal biomechanical properties, which are represented partly by elasticity and viscoelasticity of the cornea, may represent overall globe biomechanics.13,28
Myopic eyes had significantly higher CDA than emmetropic eyes in POAG and nonglaucoma subjects. CDA represents the maximum deformation amplitude, which is measured at the corneal apex from the start to the highest concavity. It denotes the flexibility of the eye in response to a certain amount of pressure. Our study showed that myopic eyes could deform farther and deeper than emmetropic eyes. Low ocular rigidity in myopia may be responsible for this finding. Several studies have investigated the association of myopia and corneal rigidity as representative of overall global rigidity. They used the ORA to measure the biomechanics of the cornea and found that myopia had significantly lower corneal hysteresis, indicating possibly softer and more flexible eyes.11–14 With the Corvis ST technology, we had the opportunity to study the rigidity of the eye with more quantitative parameters, including the magnitude of the displacement. Our findings affirmed the relative “flaccidity” of the eye in myopia.
OCV or the velocity at the second applanation represents how fast the deformed cornea goes back to its neutral position. We found significantly faster OCV in myopic eyes in POAG and nonglaucoma subjects. Myopic eyes tend to recoil backward more rapidly. We speculated that the emmetropic eye has the rigidity to dampen the force applied to the eye, while the myopic eye has a poorer ability to dampen this force.
We found that CDA and OCV differ between myopia and nonmyopia. The characteristics of these differences are similar to what has been described as the differences between glaucoma and nonglaucomatous eyes in previous studies. Lee et al.29 compared Corvis ST parameters between POAG and normal subjects. The study recruited only emmetropic eyes (refractive error less than −3.0 D), and found significantly higher CDA and faster OCV in glaucomatous eyes compared to normal eyes. There was no significant difference in ICA, ICV, and radius.29 These outcomes are similar to what we found in myopic eyes compared to emmetropic eyes, suggesting common ocular biomechanical changes in glaucoma and myopia.
The percentage difference in adjusted myopic effect between POAG and nonglaucoma patients shows the impact of POAG in modifying the myopia effect on each parameter. This percentage difference should be interpreted based on clinical judgement. From our results, the relative change in CDA, ICA, OCA, ICV, OCV, and Radius suggested that POAG had a major impact in modifying the effect of myopia. However, given that myopia did not show a statistically significant effect for ICA, ICV, and Radius in the POAG and nonglaucoma groups, the effect of glaucoma on these parameters was likely to be independent of the myopic condition. Interestingly, the myopic effect on PD was not modified by glaucoma (adjusted effect was 0.61 and was 0.62 in the POAG and nonglaucoma groups), although it was significantly increased in myopic eyes in both groups. This may imply that higher PD is consistent with myopia but not necessarily glaucoma per se.
Experiments in animal models revealed that a stiff sclera in monkey eyes was less susceptible to biomechanical change in response to chronic IOP elevation; thus, being protective against glaucoma development.30,31 In previous corneal biomechanical studies of glaucoma using the ORA, lower CH was associated with risk for glaucoma development.32–34 Moreover, lower CH eyes had faster rates of VF loss than higher CH eyes.35 It was hypothesized that lower CH in glaucomatous eyes reflects the loss of capacity to absorb energy from an air puff.29 Lower CH means that eyes deform deeper and reform faster than normal eyes. We speculated that the loss of energy absorption also may be found in myopic eyes and contribute to the increased risk for glaucoma in these eyes. Our results supported this hypothesis by demonstrating that there is greater deformation and velocity during reformation of the myopic eye in response to pressure change. Moreover, the difference in the deformation response was even greater in glaucomatous eyes.
Our study intentionally included only patients with high myopia with confirmed long axial length, in other words, only those with axial myopia, avoiding potential error when using only a subject's refraction as seen in prior myopia studies. The reason was that we wanted to focus on the effect of elongation of the globe on biomechanical changes in eyes. Refraction could be affected by several factors, especially lens-induced refractive errors. With the stricter criteria, our data likely represent results seen in myopia that are due to a long globe. Furthermore, we also restricted our inclusion only to those eyes without any laser or ocular surgery to avoid their possible effects on corneal biomechanics.
Several factors can potentially affect the Corvis ST parameters. Leung et al.15 and Tian et al.36 found a positive correlation between CDA and age. Lee et al.29 reported the significant effect of CCT on OCV. IOP also was found to affect CDA, OCV, and PD.29,36 To decrease these potential confounding effects, our study statistically adjusted for age, sex, Goldmann IOP, and CCT in our Corvis ST parameters.
We chose to examine and compare the myopia effect on POAG and nonglaucoma populations. Given the lack of knowledge of myopia's and POAG's impact on corneal biomechanics, it also would be valid to compare the impact of glaucoma on myopic and nonmyopic patients. We chose to focus on the first comparison mainly because there are existing proposed effects of myopia on ocular rigidity and tissue remodeling, which have been suggested to affect the risk for glaucoma development.
There are some limitations to our study. As a cross-sectional study, we cannot prove a causal relationship for myopia and glaucoma to the changes of the biomechanical properties measured by the Corvis ST machine. Also, we obtained only single measurements in subjects to prevent discomfort and inconvenience to the patients. To avoid potential measurement error, the image quality was checked immediately and only the measurements that passed the quality of scan measure were included for analysis. Moreover, it often was difficult to diagnose glaucoma in high myopia patients due to their glaucomatous-like disc appearance. Hence, our diagnosis was made by glaucoma specialists and based on ISGEO criteria. However, there remains the possibility of an incorrect diagnosis in this group. Lastly, all of the POAG patients were on medications for this condition and it is uncertain what effect this might have had on differences between POAG and nonglaucoma patients. However, it would have been unethical to withdraw medication use from the patients just for the purposes of studying disease severity.
In conclusion, to our knowledge this is the first study to demonstrate the impact of myopia and glaucoma on corneal biomechanical properties measured by the new Corvis ST. Myopic eyes had significantly lower ocular rigidity than nonmyopic eyes. Furthermore, POAG seemed to enhance the effects of myopia in relationship to most of the corneal biomechanical parameters. Our findings may have some pathophysiologic implications for the development of glaucoma among those with myopia.
Supplementary Material
Acknowledgements
This article was presented as oral presentation at 32nd Asia-Pacific Academy of Ophthalmology Congress, Singapore, March 2017.
The authors thank Tanate Chira-Adisai, MD, for his kind help in providing important clinical data.
Supported by the Ratchadaphiseksomphot Endowment Fund, and the Ratchadaphiseksomphot Fund, Faculty of Medicine, Chulalongkorn University, and by Research to Prevent Blindness Unrestricted Grant and the NIH-NEI EY002162 Core Grant for Vision Research.
Disclosure: S. Chansangpetch, None; R. Panpruk, None; A. Manassakorn, None; V. Tantisevi, None; P. Rojanapongpun, None; C.P. Hurst, None; S.C. Lin, None
References
- 1. Chen SJ, Lu P, Zhang WF, Lu JH. . High myopia as a risk factor in primary open angle glaucoma. Int J Ophthalmol. 2012; 5: 750– 753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Perera SA, Wong TY, Tay WT, Foster PJ, Saw SM, Aung T. . Refractive error, axial dimensions, and primary open-angle glaucoma: the Singapore Malay Eye Study. Arch Ophthalmol. 2010; 128: 900– 905. [DOI] [PubMed] [Google Scholar]
- 3. Mitchell P, Hourihan F, Sandbach J, Wang JJ. . The relationship between glaucoma and myopia: the Blue Mountains Eye Study. Ophthalmology. 1999; 106: 2010– 2015. [DOI] [PubMed] [Google Scholar]
- 4. Jonas JB, Berenshtein E, Holbach L. . Anatomic relationship between lamina cribrosa, intraocular space, and cerebrospinal fluid space. Invest Ophthalmol Vis Sci. 2003; 44: 5189– 5195. [DOI] [PubMed] [Google Scholar]
- 5. Jonas JB, Berenshtein E, Holbach L. . Lamina cribrosa thickness and spatial relationships between intraocular space and cerebrospinal fluid space in highly myopic eyes. Invest Ophthalmol Vis Sci. 2004; 45: 2660– 2665. [DOI] [PubMed] [Google Scholar]
- 6. Jonas JB, Budde WM. . Optic nerve damage in highly myopic eyes with chronic open-angle glaucoma. Eur J Ophthalmol. 2005; 15: 41– 47. [DOI] [PubMed] [Google Scholar]
- 7. Kim HS, Park KH, Jeoung JW, Park J. . Comparison of myopic and nonmyopic disc hemorrhage in primary open-angle glaucoma. Jpn J Ophthalmol. 2013; 57: 166– 171. [DOI] [PubMed] [Google Scholar]
- 8. Morita T, Shoji N, Kamiya K, Fujimura F, Shimizu K. . Corneal biomechanical properties in normal-tension glaucoma. Acta Ophthalmol. 2012; 90: e48– e53. [DOI] [PubMed] [Google Scholar]
- 9. Radhakrishnan H, Miranda MA, O'Donnell C. . Corneal biomechanical properties and their correlates with refractive error. Clin Exp Optom. 2012; 95: 12– 18. [DOI] [PubMed] [Google Scholar]
- 10. Wong YZ, Lam AK. . The roles of cornea and axial length in corneal hysteresis among emmetropes and high myopes: a pilot study. Curr Eye Res. 2015; 40: 282– 289. [DOI] [PubMed] [Google Scholar]
- 11. Del Buey MA, Lavilla L, Ascaso FJ, Lanchares E, Huerva V, Cristobal JA. . Assessment of corneal biomechanical properties and intraocular pressure in myopic spanish healthy population. J Ophthalmol. 2014; 2014: 905129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Plakitsi A, O'Donnell C, Miranda MA, Charman WN, Radhakrishnan H. . Corneal biomechanical properties measured with the Ocular Response Analyser in a myopic population. Ophthalmic Physiol Opt. 2011; 31: 404– 412. [DOI] [PubMed] [Google Scholar]
- 13. Jiang Z, Shen M, Mao G,et al. . Association between corneal biomechanical properties and myopia in Chinese subjects. Eye (Lond). 2011; 25: 1083– 1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shen M, Fan F, Xue A, Wang J, Zhou X, Lu F. . Biomechanical properties of the cornea in high myopia. Vision Res. 2008; 48: 2167– 2171. [DOI] [PubMed] [Google Scholar]
- 15. Leung CK, Ye C, Weinreb RN. . An ultra-high-speed Scheimpflug camera for evaluation of corneal deformation response and its impact on IOP measurement. Invest Ophthalmol Vis Sci. 2013; 54: 2885– 2892. [DOI] [PubMed] [Google Scholar]
- 16. Hon Y, Lam AK. . Corneal deformation measurement using Scheimpflug noncontact tonometry. Optom Vis Sci. 2013; 90: e1– e8. [DOI] [PubMed] [Google Scholar]
- 17. Foster PJ, Buhrmann R, Quigley HA, Johnson GJ. . The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol. 2002; 86: 238– 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hosmer DW, Lemeshow S. . Applied Logistic Regression. New York: Wiley; 2000. [Google Scholar]
- 19. Koo TK, Li MY. . A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016; 15: 155– 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wong TY, Klein BE, Klein R, Knudtson M, Lee KE. . Refractive errors, intraocular pressure, and glaucoma in a white population. Ophthalmology. 2003; 110: 211– 217. [DOI] [PubMed] [Google Scholar]
- 21. Fong DS, Epstein DL, Allingham RR. . Glaucoma and myopia: are they related? Int Ophthalmol Clin. 1990; 30: 215– 218. [DOI] [PubMed] [Google Scholar]
- 22. Kim TW, Kim M, Weinreb RN, Woo SJ, Park KH, Hwang JM. . Optic disc change with incipient myopia of childhood. Ophthalmology. 2012; 119: 21– 26.e3. [DOI] [PubMed] [Google Scholar]
- 23. Cahane M, Bartov E. . Axial length and scleral thickness effect on susceptibility to glaucomatous damage: a theoretical model implementing Laplace's law. Ophthalmic Res. 1992; 24: 280– 284. [DOI] [PubMed] [Google Scholar]
- 24. Rada JA, Nickla DL, Troilo D. . Decreased proteoglycan synthesis associated with form deprivation myopia in mature primate eyes. Invest Ophthalmol Vis Sci. 2000; 41: 2050– 2058. [PubMed] [Google Scholar]
- 25. McBrien NA, Cornell LM, Gentle A. . Structural and ultrastructural changes to the sclera in a mammalian model of high myopia. Invest Ophthalmol Vis Sci. 2001; 42: 2179– 2187. [PubMed] [Google Scholar]
- 26. McBrien NA, Gentle A. . Role of the sclera in the development and pathological complications of myopia. Prog Retin Eye Res. 2003; 22: 307– 338. [DOI] [PubMed] [Google Scholar]
- 27. Muscat S, McKay N, Parks S, Kemp E, Keating D. . Repeatability and reproducibility of corneal thickness measurements by optical coherence tomography. Invest Ophthalmol Vis Sci. 2002; 43: 1791– 1795. [PubMed] [Google Scholar]
- 28. Kotecha A. . What biomechanical properties of the cornea are relevant for the clinician? Surv Ophthalmol. 2007; 52 suppl 2: S109– S114. [DOI] [PubMed] [Google Scholar]
- 29. Lee R, Chang RT, Wong IY, Lai JS, Lee JW, Singh K. . Novel Parameter of Corneal Biomechanics That Differentiate Normals From Glaucoma. J Glaucoma. 2016; 25: e603– 609. [DOI] [PubMed] [Google Scholar]
- 30. Girard MJ, Suh JK, Bottlang M, Burgoyne CF, Downs JC. . Biomechanical changes in the sclera of monkey eyes exposed to chronic IOP elevations. Invest Ophthalmol Vis Sci. 2011; 52: 5656– 5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Steinhart MR, Cone FE, Nguyen C,et al. . Mice with an induced mutation in collagen 8A2 develop larger eyes and are resistant to retinal ganglion cell damage in an experimental glaucoma model. Mol Vis. 2012; 18: 1093– 1106. [PMC free article] [PubMed] [Google Scholar]
- 32. Congdon NG, Broman AT, Bandeen-Roche K, Grover D, Quigley HA. . Central corneal thickness and corneal hysteresis associated with glaucoma damage. Am J Ophthalmol. 2006; 141: 868– 875. [DOI] [PubMed] [Google Scholar]
- 33. Abitbol O, Bouden J, Doan S, Hoang-Xuan T, Gatinel D. . Corneal hysteresis measured with the Ocular Response Analyzer in normal and glaucomatous eyes. Acta Ophthalmol. 2010; 88: 116– 119. [DOI] [PubMed] [Google Scholar]
- 34. Anand A, De Moraes CG, Teng CC, Tello C, Liebmann JM, Ritch R. . Corneal hysteresis and visual field asymmetry in open angle glaucoma. Invest Ophthalmol Vis Sci. 2010; 512: 6514– 6518. [DOI] [PubMed] [Google Scholar]
- 35. Medeiros FA, Meira-Freitas D, Lisboa R, Kuang TM, Zangwill LM, Weinreb RN. . Corneal hysteresis as a risk factor for glaucoma progression: a prospective longitudinal study. Ophthalmology. 2013; 120: 1533– 1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tian L, Wang D, Wu Y,et al. . Corneal biomechanical characteristics measured by the CorVis Scheimpflug technology in eyes with primary open-angle glaucoma and normal eyes. Acta Ophthalmol. 2016; 94: e317– 324. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.