Supplemental Digital Content is available in the text
Keywords: cytomegalovirus, ELISPOT, Pneumocystis pneumonia
Abstract
The clinical importance of pulmonary cytomegalovirus (CMV) co-infection in patients with Pneumocystis jirovecii pneumonia (PCP) is uncertain. We therefore determined the association of CMV infection with outcomes in non-HIV-infected patients with PCP by assessing CMV viral load and CMV-specific T-cell response.
We prospectively enrolled all non-HIV-infected patients with confirmed PCP, over a 2-year period. Real-time polymerase chain reaction from bronchoalveolar lavage was performed to measure CMV viral load, and CMV enzyme-linked immunospot assays of peripheral blood were used to measure CMV-specific T-cell responses. The primary outcome was 30-day mortality.
A total of 76 patients were finally analyzed. The mortality in patients with high BAL CMV viral load (>2.52 log copies/mL, 6/32 [18%]) showed a nonsignificant trend to be higher than in those with low CMV viral load (2/44 [5%], P = .13). However, the mortality in patients with low CMV-specific T-cell responses (<5 spots/2.0 × 105 PBMC, 6/29 [21%]) was significantly higher than in patients with high CMV-specific T-cell response (2/47 [4%], P = .048). Moreover, the 2 strata with high CMV viral load and low CMV-specific T-cell responses (4/14 [29%]) and low CMV viral load and low CMV-specific T-cell responses (2/15 [13%]) had poorer outcomes than the 2 strata with high CMV viral load and high CMV-specific T-cell responses (2/18 [11%]) and low CMV viral load and high CMV-specific T-cell responses (0/29 [0%]).
These data suggest that the CMV replication and impaired CMV-specific T-cell responses adversely affect the outcomes in non-HIV-infected patients with PCP.
1. Introduction
Pneumocystis jirovecii pneumonia (PCP) is considered an important opportunistic infection in immunocompromised hosts.[1,2] Cytomegalovirus (CMV) is also a major cause of morbidity and a preventable cause of mortality in immunocompromised patients.[3] It is frequently reactivated in patients infected with other pathogens, especially in respiratory specimens of patients with PCP.[4] CMV viral replication has both direct and indirect effects in immunocompromised hosts.[5,6] However, previous studies found no significant influence of CMV co-infection on the outcome of PCP,[7–9] although some studies have suggested that the clinical impact of CMV co-infection PCP might be affected by adjunctive corticosteroid therapy.[10,11] Thus, the clinical significance of concomitant CMV pulmonary replication in PCP is uncertain.
In the past 20 years, it has become clear that CMV-specific immunity plays a crucial role in controlling CMV infection.[12,13] Analysis of the CMV-specific T-cell response can allow direct quantification of host immunity to CMV, and combined knowledge of host and viral factors could help to assess the role of CMV co-infection in PCP. We therefore prospectively evaluated the clinical significance of CMV pulmonary infection in non-HIV-infected patients with PCP by assessing CMV viral load in bronchoalveolar lavage (BAL) fluid and CMV-specific T-cell responses in peripheral blood.
2. Methods
2.1. Study design
All non-HIV-infected adult patients (aged ≥16 years) who were diagnosed with PCP were prospectively enrolled at Asan Medical Center, a 2700-bed tertiary hospital in Seoul, South Korea, between January 2014 and December 2015. We enrolled adult patients with confirmed PCP (see below) who agreed to additional sampling for peripheral blood mononuclear cells (PBMCs). We excluded HIV-infected patients with confirmed PCP. We used the fourth generation HIV-1/2 antigen/antibody combination immunoassay for the diagnosis of HIV infection. In addition, in the case of a negative immunoassay result in which acute HIV-1 infection was still suspected, we performed plasma HIV-1 RNA testing to diagnose acute HIV-1 infection and minimize the window period. Decisions regarding antiviral therapy for CMV, such as ganciclovir, were made by the attending physicians based on each patient's initial clinical features, blood tests, microbiological results, and image findings. The results for CMV-specific cell-mediated immunity (CMV CMI) were concealed from the attending physicians because they might have affected decisions on antiviral therapy. The study was approved by the Institutional Review Board of the Asan Medical Center (IRB No. 2014-0198).
2.2. Definitions
Pulmonary CMV co-infection was defined as positive BAL quantitative CMV polymerase chain reaction (qPCR) with or without positive BAL CMV culture. Based on PaO2 while breathing room air or on the alveolar–arterial oxygen difference ([A–a] DO2), patients were classified into those with mild (PaO2 >70 mm Hg or[A–a] DO2<35), moderate (PCP: PaO2 ≤70 mm Hg or[A–a] DO2 ≥35), or severe (PaO2 ≤60 mm Hg or[A–a] DO2 ≥45) PCP before bronchoscopy.[2] Antiviral therapy was defined as intravenous ganciclovir treatment for at least 1 week, and failure of the initial treatment regimen was defined as clinical deterioration during the first 5 days of treatment or lack of improvement after 7 or more days of treatment.[14] The primary outcome was 30-day mortality from the time of BAL.
2.3. Microbiological methods
PCP was diagnosed as a positive test result in an immunohistochemical (IHC) antibody assay (Dako, Santa Babara, CA) for P jirovecii using BAL fluid in patients with respiratory symptoms and radiological findings compatible with PCP. All the IHC tests for PCP were read by 1 experienced clinical microbiologist (H.S.). Additional microbiological investigations performed on BAL included Gram stain, acid-fast stain, and cultures for conventional bacteria, mycobacteria, fungi, and viruses. CMV DNA load (CMV qPCR) in BAL was measured using a Cobas Amplicor CMV Monitor-Test Kit on a COBAS Amplicor Analyzer (Roche Molecular Systems, Branchburg). For respiratory virus screening and identification of viruses other than CMV, including respiratory syncytial virus, influenza virus, parainfluenza virus, and adenovirus were detected by real-time PCR.
2.4. ELISPOT
A peripheral venous blood sample (∼8 mL) was collected from each patient for the CMV enzyme-linked immunospot (ELISPOT) assay for T cells producing interferone gamma (IFN-γ) (i.e., T-track CMV, Regensburg, Germany). Briefly, PBMCs were immediately (within 30 minutes) separated and collected. The collected cells were suspended at 2.0 × 106 cells/mL and plated in 4 wells (2.0 × 105 cells/well) precoated with the antihuman IFN-γ antibody. The samples were stimulated with phytohemagglutinin (PHA, positive control), pp65, IE1, and medium only (negative control) and incubated for 18 hours. The resulting spots were counted with an automated microscope (ELISPOT 04 HR; Autoimmune Diagnostika GmbH, Strassberg, Germany). Background values, obtained from the negative control wells, were subtracted from all values.
2.5. Statistical analysis
Patient characteristics were summarized using percentages or median and interquartile range (IQR). Categorical variables were compared using the χ2 test or Fisher exact test. Continuous variables were analyzed by the Mann–Whitney U-test. The time-to-event analysis was performed using Kaplan–Meier estimates and the log-rank test. Cox proportional hazard regression models for multivariate analyses were conducted using backward elimination. Factors with P <.10 in the univariate analyses were considered for entry into a multivariate analysis, which was limited to 2 factors because of the number of events. Correlations between the variables (multicollinearity) were also considered. All tests were 2-tailed, and differences were considered significant at P <.05. Diagnostic performance was expressed in terms of sensitivity, specificity, positive predictive value, negative predictive value, positive likelihood ratio, and negative likelihood ratio. We assessed the optimal cut-off point for each of the tests used for predicting mortality by constructing a receiver operating characteristic (ROC) curve that plotted sensitivity against the rate of false-positive results over a range of cut-off values.[15] We selected the optimal cut-off value as the point on the ROC curve farthest from the diagonal line that maximized the sum of the sensitivity and the specificity.[16] All statistical calculations were carried out with SPSS version 21.0 (SPSS, Armonk, NY), and figures were created with GraphPad Prism 5.01 for Windows (GraphPad Software, San Diego, CA).
3. Results
3.1. Patients characteristics
During the study period, a total of 82 patients with confirmed PCP were screened for possible enrollment in the study. Of these 82 patients, 6 (7%) were excluded because they refused informed consent. Therefore, 76 patients with confirmed PCP were included in the final analysis. Hematological diseases, including 28 (37%) lymphomas and 4 (5%) hematopoietic stem cell transplants, were the most common underlying diseases; solid organ transplants, including 19 (25%) kidney, 4 (5%) pancreas, 4 (5%) liver, and 2 (3%) heart transplants, were the second most common. The initial PCP was mild in 24 (32%) patients, moderate in 19 (25%), and severe in 33 (43%). All patients were treated with trimethoprim–sulfamethoxazole as initial therapy for PCP.
3.2. Comparison of PCP patients with and without pulmonary CMV infection
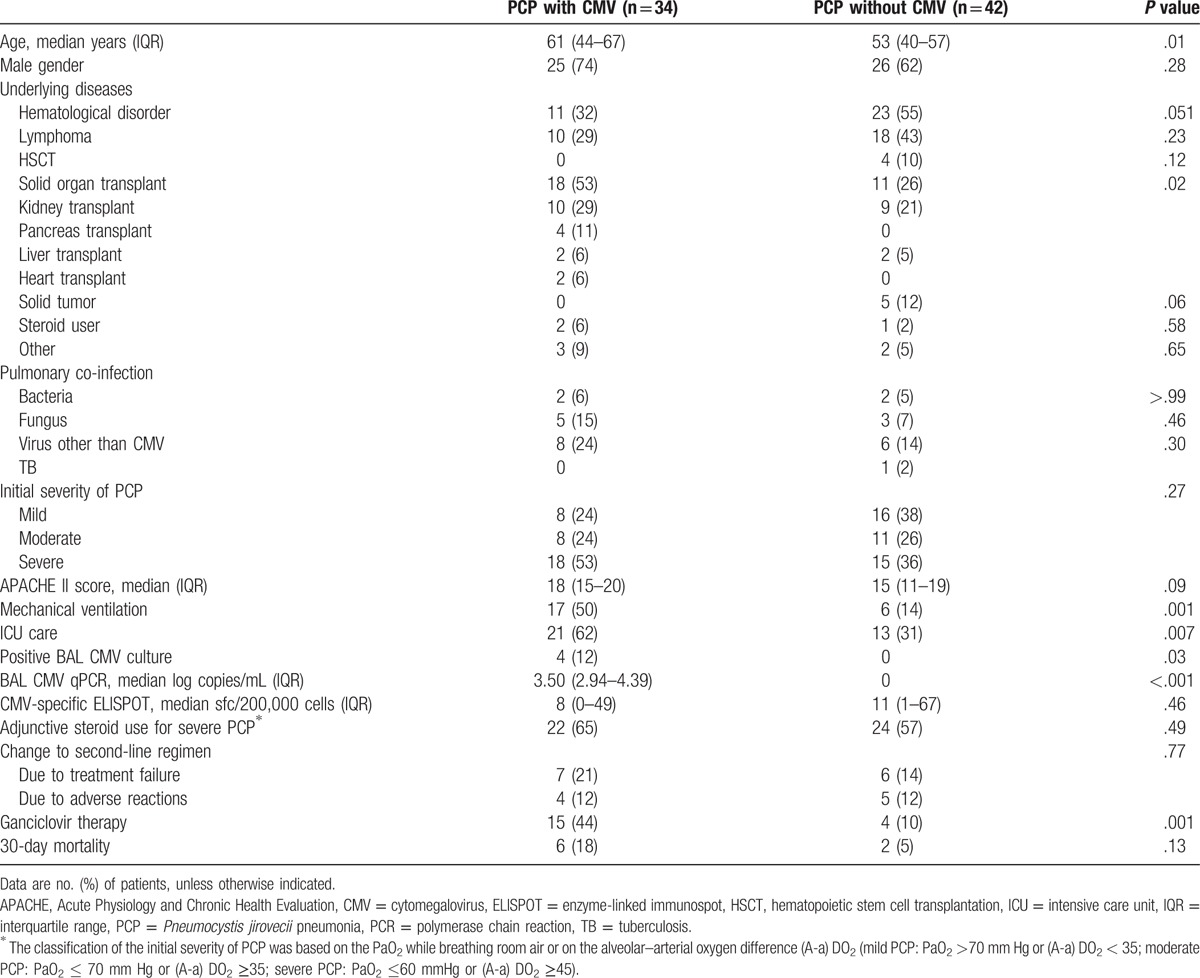
Of the 76 patients with PCP, 34 (45%) with positive CMV qPCR and/or CMV culture (4 [5%]) from BAL fluid were classified as patients with pulmonary CMV co-infection. The demographic data, clinical characteristics, and mortality of patients with and without pulmonary CMV co-infection are shown in Table 1. The 34 patients with pulmonary CMV co-infection were older (P = .01) and included more solid organ transplant recipients than the 43 patients without pulmonary CMV co-infection (53% vs. 26%, P = .02). Conversely, hematological disorders (32% vs. 55%, P = .051) and solid tumors receiving chemotherapy (0% vs. 12%, P = .06) tended to be more common in those without pulmonary CMV co-infection. More of the patients with pulmonary CMV co-infection received mechanical ventilation (50% vs. 14%, P = .001), ICU care (62% vs. 31%, P = .007), and ganciclovir therapy (44% vs. 10%, P = .001). However, 30-day mortality was not significantly different between the PCP patients with and without CMV (18% vs 5%, P = .13)
Table 1.
Comparison the characteristics of Pneumocystis jirovecii pneumonia with and without pulmonary cytomegalovirus infection.
3.3. Comparison of PCP patients with high CMV-specific T-cell responses and low CMV-specific T-cell responses
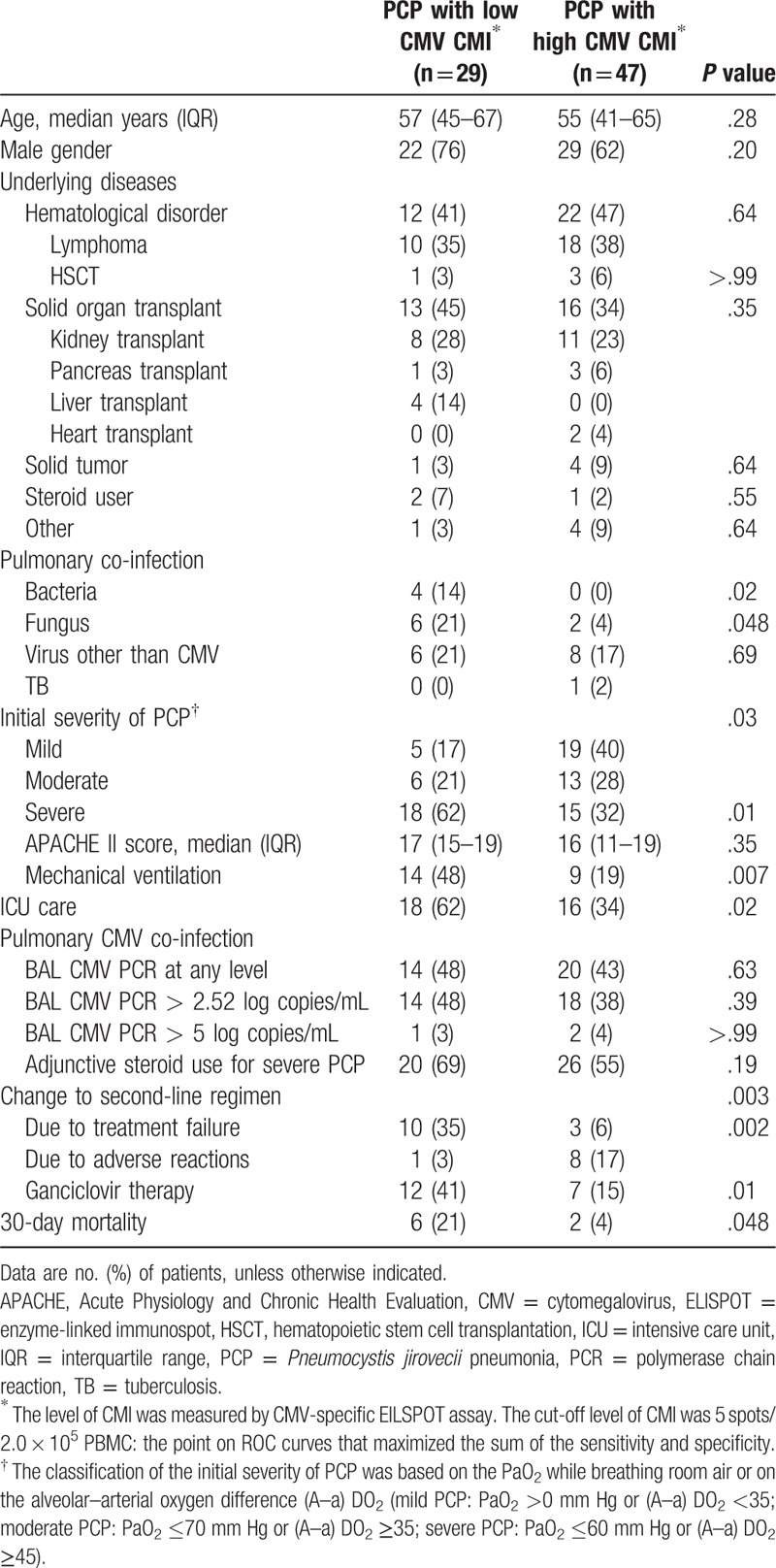
The patients were divided into 2 groups based on the CMV ELISPOT results with a cut-off value (≥5 sfc/200,000 cells) selected from the ROC curves. Of the 76 patients, 29 (38%) patients were classified into the low CMV-specific CMI group and 47 (62%) into the high CMV-specific CMI group. The characteristics and mortality of the patients stratified by low and high CMV ELISPOT results are shown in Table 2. Age, male gender, and underlying diseases were comparable in the 2 groups. Pulmonary co-infection due to bacterial (14% vs. 0%, P = .02) and fungal organisms (21% vs 4%, P = .048) was more common in the low CMV CMI group, as was severe PCP (62% vs 32%, P = .01). In addition, mechanical ventilation (48% vs 19%, P = .007), ICU care (62% vs. 34%, P = .02), salvage treatment for PCP due to initial treatment failure (35% vs 6%, P = .002), as well as ganciclovir therapy (41% vs 15%, P = .01) were significantly more common among the PCP patients with low CMV CMI. Thirty-day mortality was also higher in this group (21% vs 4%, P = .048).
Table 2.
Comparison the characteristics of Pneumocystis jirovecii pneumonia with low CMV-specific cell-mediated immunity and high CMV-specific cell-mediated immunity.
3.4. Risk factors for 30-day mortality
Univariate and multivariate analyses of factors associated with 30-day mortality are shown in Table 3. In the univariate analysis, age, steroid use, pulmonary fungal co-infection, mechanical ventilation, ICU care, low CMV ELISPOT, and ganciclovir therapy were associated with an increased risk of 30-day mortality. In the multivariate analysis, age (hazard ratio [HR] 1.1, 95% confidence interval [CI] 1.0–1.2, P = .01) and low CMV CMI (low CMV ELISPOT [< 5 sfc/200,000 cells], HR 5.3, 95% CI 1.0–26.3, P = .04) were independently associated with 30-day mortality. Supplementary Fig. 1, shows Kaplan–Meier survival curves comparing patients with high BAL CMV viral load to those with low CMV viral load (P = .051), and patients with high CMV CMI to those with low CMV CMI (P = .02), respectively. On the basis of these results, we stratified the study patients into 4 groups by BAL CMV viral load and CMV CMI, and compared the 30-day mortalities (Fig. 1). Supplementary Table 1, shows the characteristics of patients with PCP with respect to the strata. Each stratum contains 14 patients for high viral replication with low CMI, 15 for low viral replication with low CMI, 18 for high viral replication with high CMI, and 29 for low viral replication with high CMI. The strata with high CMV viral loads and low CMV-specific T-cell responses (4/14 [29%]) and low CMV viral load and low CMV-specific T-cell responses (2/15 [13%]) had worse outcomes than the strata with high CMV viral load and high CMV-specific T-cell response (2/18 [11%]) and low CMV viral load and high CMV-specific T-cell responses (0/29 [0%]).
Table 3.
Risk factors for 30-day mortality in patients with Pneumocystis jirovecii pneumonia.
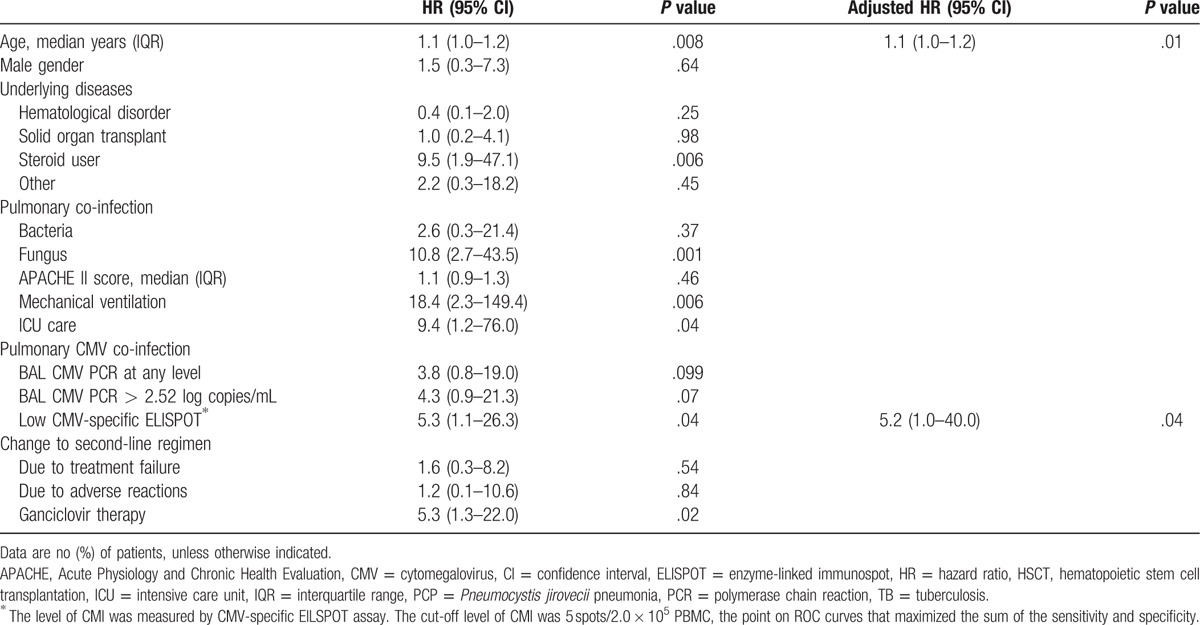
Figure 1.
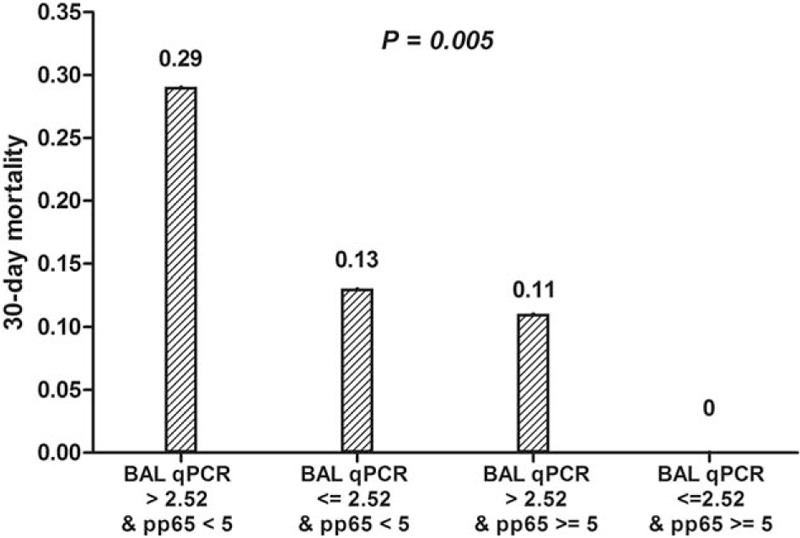
The 4-group model using quantitative CMV PCR for BAL fluid and CMV ELISPOT results for blood to predict outcomes in Pneumocystis jirovecii pneumonia. BAL = bronchoalveolar lavage, CMV = cytomegalovirus, ELISPOT = enzyme-linked immunospot, PCR = polymerase chain reaction.
3.5. Diagnostic performances of BAL CMV viral load and CMV-specific ELISPOT for predicting mortality
We estimated the diagnostic performances of BAL CMV qPCR and the CMV-specific ELISPOT assay for predicting 30-day mortality. The detailed data for each cut-off value for predicting 30-day mortality in patients with confirmed PCP are shown in Supplementary Table 2.
4. Discussion
In this study, we prospectively assessed the association of pulmonary CMV co-infection with clinical outcomes in non-HIV-infected PCP patients, using BAL CMV viral load and CMV-specific T-cell response. As expected, patients with pulmonary CMV co-infection had worse prognostic factors, such as solid organ transplantation, mechanical ventilation, and ICU care. However, the mortality of patients with and without pulmonary CMV co-infection was not significantly different (18% vs 5%, P = .13). In contrast, low CMV-specific T-cell response was significantly associated with mortality, even though the patients with low CMV-specific T-cell response had similar poor prognostic factors (mechanical ventilation and ICU care) as those with pulmonary CMV co-infection. These findings provide further insight into the complex host–virus interactions in critically ill patients with PCP.
In previous studies, reactivation of CMV was observed in about 30% of critically ill patients in the ICU and was independently associated with prolonged ICU stay and increased mortality.[17–21] Whether CMV reactivation is the cause of adverse outcomes or a simple marker of immunocompromised and debilitated status has not been established. In addition, the effectiveness of anti-CMV agents, such as ganciclovir in patients with CMV infections, has not been tested. We hypothesized that if the CMV-specific T-cell response was intact when CMV was reactivated, the host might eventually control viral replication and the immunomodulatory or immunopathological effect of CMV would be minimized. However, if CMV was reactivated but the CMV-specific T-cell response was not intact, the host would eventually be unable to control viral replication and CMV would have a strong immunomodulatory or immunopathological effect. In this context, we assumed that stratification by viral load and CMV-specific T-cell response would help us evaluate whether the beneficial effect of ganciclovir use outweighed its toxic effect. To test this hypothesis, we selected non-HIV-infected patients with confirmed PCP because PCP is one of the most important infections in immunocompromised hosts, and it is frequently accompanied by CMV co-infection. We clearly showed that CMV replication and impaired CMV-specific T-cell responses adversely affected the outcomes in these patients. Further studies are needed to generalize these findings to other critically ill patients, and to investigate whether antiviral therapy is beneficial in particular subgroups of patients with CMV co-infection.
The finding of no significant association of CMV viral load with outcome in patients with PCP is consistent with previous studies in PCP patients.[9,22] However, the CMV-specific T-cell response was independently associated with 30-day mortality. A possible explanation for this observation is that PCP together with a critical illness may decrease cell-mediated immunity (immune paralysis), leading to uncontrollable CMV replication. Eventually, the direct or indirect effect of CMV replication and immunosuppression may increase the risk of bacterial or fungal co-infection[23,24] and lead to adverse outcomes. Furthermore, studies using animal models have shown that CMV infection inhibits immune responses against P jirovecii, and contributes to delayed clearance of the organism.[25,26]
This study had a few limitations. First, the sample size and the number of events were not enough to allow robust conclusions. Despite that, the study involved relatively large numbers of fairly homogenous patients with confirmed PCP infections and allowed us to demonstrate a statistically significant effect of low cell-mediated immunity on 30-day mortality. Second, although both pp65 and IE-1 are considered dominant T-cell targets for CMV infection,[27] it has not been known which platform technologies for various target antigens optimally reflect the protective T-cell response in CMV infection. As such, further studies are needed for our findings to be applicable to other target antigens, such as IE-2, pp50, or pp150, other platform technologies for producing antigens, such as overlapping peptides or CMV cell lysates, and other diagnostic methods such as ELISA-based IFN-gamma releasing assays or FACS-based intracellular cytokine staining. Third, we evaluated the CMV-specific CMI only at the time of PCP diagnosis. Thus, we could not evaluate whether the CMV-specific immune response was affected after ganciclovir therapy. The measurement of CMV-specific CMI at several time points during the disease course will help us further understand the kinetics of CMV-specific CMI in patients with CMV co-infection.
In conclusion, this study suggests that low CMV CMI with high BAL CMV viral load is associated with 30-day mortality and vice versa. This finding could be helpful in guiding further study of the use of antivirals in patients with CMV co-infection. Further large-scale epidemiological studies and application of these research findings are needed to therapeutically benefit patients with PCP and CMV co-infection.
Acknowledgment
The authors thank Ms. Vanessa Topping from the Scientific Publications Team at Asan Medical Center for her editorial assistance in preparing this manuscript.
Supplementary Material
Footnotes
Abbreviations: BAL = bronchoalveolar lavage, CMI = cell-mediated immunity, CMV = cytomegalovirus, ELISPOT = enzyme-linked immunospot, FACS = fluorescence-activated cell sorting, HIV = human immunodeficiency virus, IHC = immunohistochemical, IQR = interquartile range, PBMC = peripheral blood mononuclear cells, PCP = Pneumocystis jirovecii pneumonia, PCR = polymerase chain reaction, PHA = phytohemagglutinin.
Funding: This study was supported by grants of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant no. HI15C1763) and from the National Research Foundation of Korea funded by the Ministry of Education (NRF-2015R1D1A1A01059315).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Sepkowitz KA. Opportunistic infections in patients with and patients without acquired immunodeficiency syndrome. Clin Infect Dis 2002;34:1098–107. [DOI] [PubMed] [Google Scholar]
- [2].Pfaller MA, Anaissie EJ. Pneumocystis. Clinical Mycology. 2nd ed.2009;Edinburgh: Churchill Livingstone, 385–401; Chapter 7. [Google Scholar]
- [3].Ramanan P, Razonable RR. Cytomegalovirus infections in solid organ transplantation: a review. Infect Chemother 2013;45:260–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Salomon N, Perlman DC. Cytomegalovirus pneumonia. Semin Respir Infect 1999;14:353–8. [PubMed] [Google Scholar]
- [5].George MJ, Snydman DR, Werner BG, et al. The independent role of cytomegalovirus as a risk factor for invasive fungal disease in orthotopic liver transplant recipients. Boston Center for Liver Transplantation. Am J Med 1997;103:106–13. [DOI] [PubMed] [Google Scholar]
- [6].Hodson EM, Jones CA, Webster AC, et al. Antiviral medications to prevent cytomegalovirus disease and early death in recipients of solid-organ transplants: a systematic review of randomised controlled trials. Lancet 2005;365:2105–15. [DOI] [PubMed] [Google Scholar]
- [7].Bozzette SA, Arcia J, Bartok AE, et al. Impact of Pneumocystis carinii and cytomegalovirus on the course and outcome of atypical pneumonia in advanced human immunodeficiency virus disease. J Infect Dis 1992;165:93–8. [DOI] [PubMed] [Google Scholar]
- [8].Jacobson MA, Mills J, Rush J, et al. Morbidity and mortality of patients with AIDS and first-episode Pneumocystis carinii pneumonia unaffected by concomitant pulmonary cytomegalovirus infection. Am Rreview Resp Dis 1991;144:6–9. [DOI] [PubMed] [Google Scholar]
- [9].Kim T, Moon SM, Sung H, et al. Outcomes of non-HIV-infected patients with Pneumocystis pneumonia and concomitant pulmonary cytomegalovirus infection. Scand J Infect Dis 2012;44:670–7. [DOI] [PubMed] [Google Scholar]
- [10].Jensen A-MB, Lundgren JD, Benfield T, et al. Does cytomegalovirus predict a poor prognosis in Pneumocystis carinii pneumonia treated with corticosteroids? Chest 1995;108:411–4. [DOI] [PubMed] [Google Scholar]
- [11].Benfield TL, Helweg-Larsen J, Bang D, et al. Prognostic markers of short-term mortality in AIDS-associated Pneumocystis carinii pneumonia. Chest 2001;119:844–51. [DOI] [PubMed] [Google Scholar]
- [12].Egli A, Humar A, Kumar D. State-of-the-art monitoring of cytomegalovirus-specific cell-mediated immunity after organ transplant: a primer for the clinician. Clin Infect Dis 2012;55:1678–89. [DOI] [PubMed] [Google Scholar]
- [13].Barron MA, Gao D, Springer KL, et al. Relationship of reconstituted adaptive and innate cytomegalovirus (CMV)-specific immune responses with CMV viremia in hematopoietic stem cell transplant recipients. Clin Infect Dis 2009;49:1777–83. [DOI] [PubMed] [Google Scholar]
- [14].Smego RA, Jr, Nagar S, Maloba B, et al. A meta-analysis of salvage therapy for Pneumocystis carinii pneumonia. Arch Intern Med 2001;161:1529–33. [DOI] [PubMed] [Google Scholar]
- [15].Kim SH, Bang JW, Park KH, et al. Prediction of residual immunity to smallpox, by means of an intradermal skin test with inactivated vaccinia virus. J Infect Dis 2006;194:377–84. [DOI] [PubMed] [Google Scholar]
- [16].Greiner M, Pfeiffer D, Smith RD. Principles and practical application of the receiver-operating characteristic analysis for diagnostic tests. Prev Vet Med 2000;45:23–41. [DOI] [PubMed] [Google Scholar]
- [17].Limaye AP, Kirby KA, Rubenfeld GD, et al. Cytomegalovirus reactivation in critically ill immunocompetent patients. JAMA 2008;300:413–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jain M, Duggal S, Chugh TD. Cytomegalovirus infection in non-immunosuppressed critically ill patients. J Infect Dev Ctries 2011;5:571–9. [DOI] [PubMed] [Google Scholar]
- [19].Lopez Roa P, Perez-Granda MJ, Munoz P, et al. A prospective monitoring study of cytomegalovirus infection in non-immunosuppressed critical heart surgery patients. PloS One 2015;10:e0129447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Limaye AP, Boeckh M. CMV in critically ill patients: pathogen or bystander? Rev Med Virol 2010;20:372–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Heininger A, Haeberle H, Fischer I, et al. Cytomegalovirus reactivation and associated outcome of critically ill patients with severe sepsis. Critical Care 2011;15:R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bower M, Barton SE, Nelson MR, et al. The significance of the detection of cytomegalovirus in the bronchoalveolar lavage fluid in AIDS patients with pneumonia. AIDS 1990;4:317–20. [DOI] [PubMed] [Google Scholar]
- [23].Hodson EM, Jones CA, Webster AC, et al. Antiviral medications to prevent cytomegalovirus disease and early death in recipients of solid-organ transplants: a systematic review of randomised controlled trials. Lancet 2005;365:2105–15. [DOI] [PubMed] [Google Scholar]
- [24].Kalil AC, Levitsky J, Lyden E, et al. Meta-analysis: the efficacy of strategies to prevent organ disease by cytomegalovirus in solid organ transplant recipients. Ann Intern Med 2005;143:870–80. [DOI] [PubMed] [Google Scholar]
- [25].Qureshi MH, Garvy BA, Pomeroy C, et al. A murine model of dual infection with cytomegalovirus and Pneumocystis carinii: effects of virus-induced immunomodulation on disease progression. Virus Res 2005;114:35–44. [DOI] [PubMed] [Google Scholar]
- [26].Laursen AL, Mogensen SC, Andersen HM, et al. The impact of CMV on the respiratory burst of macrophages in response to Pneumocystis carinii. Clin Exp Immunol 2001;123:239–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kim S-H, Lee H-J, Kim S-M, et al. Diagnostic usefulness of cytomegalovirus (CMV)- specific T cell immunity in predicting CMV infection after kidney transplantation: a pilot proof-of-concept study. Infect Chemother 2015;47:105–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


