Abstract
Progressive cerebral infarction (PCI) is associated with high rates of mortality and disability. Many studies have shown that Dl-3n-butylphthalide (NBP) is effective against acute ischemic stroke. The administration of NBP can result in an increased number of capillaries in the ischemic region, promote the establishment of collateral circulation, protect the mitochondria, and narrow the infarction area, among other effects. In the present study, we evaluated the efficacy and safety of NBP for the treatment of PCI.
Between March 2008 and May 2012, we performed a randomized, double-blind placebo-controlled study including 304 inpatients with PCI. These patients were randomly assigned to the test (152 cases) and control groups (152 cases). The test group received 200 mg of NBP soft capsules orally, 15 minutes before each meal, 3 times daily. The control group received 200 mg of placebo soft capsules orally, 15 minutes before each meal, 3 times daily. Treatment was administered during 21 days. The National Institute of Health Stroke Scale (NIHSS) score was assessed before the treatment and on days 7, 14, 21, and 30 after treatment. The Barthel index (BI) was assessed on the same days and on day 90.
In the test group, the NIHSS scores on days 7, 14, 21, and 30 were 14.75 ± 4.85, 11.62 ± 3.49, 8.87 ± 5.17, and 6.38 ± 4.93, respectively. In the control group, they were 16.08 ± 3.76, 13.28 ± 5.02, 11.05 ± 4.25, and 8.43 ± 5.41 (P < .05), respectively. The BI on days 7, 14, 21, 30, and 90 were 51.57 ± 15.11, 61.21 ± 16.39, 70.48 ± 18.21, 76.41 ± 19.02, and 81.10 ± 15.52 for the test group and 46.79 ± 18.42, 55.93 ± 19.12, 64.84 ± 17.67, 70.65 ± 18.54, and 76.54 ± 17.05 for the control group (P < .05), respectively. Adverse events were elevation of alanine aminotransferase and aspartate aminotransferase (P > .05).
NBP was useful to improve the outcome of patients with PCI and decreased their disability for activities of daily living. NBP was an efficacious and safe treatment for PCI.
Keywords: Dl-3n-butylphthalide, progressive cerebral infarction, therapeutic effect
1. Introduction
Progressive cerebral infarction (PCI) accounts for 26% to 43%[1] of cerebral infarction cases, and it is associated with high rates of mortality and disability. PCI has a complex pathogenesis.[2–5] It is associated with the expansion and regeneration of the thrombus, decreased cerebral perfusion, and the inability to rapidly establish sufficient collateral circulation in the ischemic region. Additionally, there is encephaledema along with reperfusion injuries, among other processes. The current treatment of PCI includes subcutaneous injection of low-molecular heparin calcium, intravenous treatments including constant injections of low heparin doses, low doses of urokinase, recombinant tissue plasminogen activator, ozagrel, and edaravone, among others. However, these agents are associated with poor efficacy, and PCI survivors may not be capable of self-maintenance regarding activities of daily living.
Apium graveolens (Celery) is a plant in the family Apiaceae that has been cultivated as a vegetable since antiquity; its extracts are used in medicines. Dl-3n-butylphthalide (NBP) is derived from the seeds of Apium graveolens and was shown to improve the outcomes of cerebral infarction by increasing the number of capillaries in the ischemic region, promoting the establishment of collateral circulation, enhancing cerebral blood flow, protecting the mitochondria, improving the cerebral energy metabolism and narrowing the infarction area, among other effects.[6–10] Several multicenter, open-label clinical studies on NBP for the treatment of acute ischemic stroke showed that NBP was both effective and safe.[11–13] Although many studies have evaluated the effects of NBP for acute cerebral infarction, as far as we know, no previous clinical studies have evaluated NBP for the treatment of PCI. Therefore, we performed a clinical study to evaluate the efficacy and safety of NBP for the treatment of PCI.
2. Materials and methods
2.1. Ethical approval, informed consent
This research was approved by the Medical Ethics Committee of the Second Hospital of Baoding City. Written informed consent was formally obtained from all participates.
2.2. Subjects
Between March 2008 and May 2012, 304 patients with PCI were included in the study. Patients were randomly assigned to the test (n = 152) and control (n = 152) groups.
2.3. PCI diagnostic criteria
The diagnostic criteria for PCI were as follows: (a) patients diagnosed with first-onset cerebral infarction; (b) the disease course extended from 6 hours to 7 days; (c) after regular treatment for cerebral infarction, the primary nervous symptoms and signs of the cerebral infarction progressively worsened and the National Institute of Health Stroke Scale (NIHSS) score increased no less than 2 points; (d) cerebral hemorrhage and hemorrhage after infarction were excluded by head computed tomography and magnetic resonance imaging (MRI); (e) there were several sites of cerebral infarction.[1]
2.4. Inclusion criteria
Patients eligible for this study were (a) those diagnosed with PCI; (b) aged between 40 and 80 years; (c) without serious disturbance of consciousness, could cooperate with the medical examination and had normal swallowing function; and (d) those who agreed and gave written informed consent to participate in the study.
2.5. Exclusion criteria
Patients were excluded if (a) they were younger than 40 years and older than 80 years; (b) had a hospitalization shorter than 14 days or suffered from serious consciousness disturbance; (c) dementia or swallowing difficulties; (d) brain lesion was shown by MRI of the head and was associated with worsening of nervous symptoms and signs and a congenital vascular abnormality; (e) serious heart, liver, and kidney function abnormalities or any other serious diseases; (f) serious mental impairment and could not cooperate with the medical examination; (g) presented allergies to the study drug or celery; and (h) patients who were pregnant or breast-feeding.
2.6. Medicines and medication
(1) The test group received 200 mg of NBP soft capsules (100 mg/capsule) orally, 15 minutes before each meal, 3 times daily for 21 days. NBP capsules were produced by Shijiazhuang Pharmaceutical Group Pharmaceutical Co. Ltd (approval number: H20050299). (2) The control group received 200 mg of placebo which consisted of soft capsules identical in appearance, quantity, packaging, formulation, batch number, and dosage as the NBP capsules. These were orally administered 15 minutes before each meal, 3 times daily for 21 days. (3) Enteric-coated aspirin, 100 mg/tablet, was taken 30 minutes after supper, once daily; rosuvastatin, 10 mg/tablet, was taken 30 minutes after supper, once daily; ginkgo biloba extracts 20 ml (specification: 5 mL/ampoule; Beijing Double Crane Pharmacy Co., Ltd. Approval number: 080801) in 250 mL of 0.9% sodium chloride injection were administered by intravenous drip once daily for 14 days; and citicoline sodium chloride injection (specification: 250 mL (0.5 g)/bottle) (Beijing Double Crane Pharmacy Co., Ltd. Approval number: 030801) was administered by intravenous drip once daily for 14 days. Patients with cerebral edema were administered mannitol or glycerin fructose as well as other symptomatic treatments (e.g., blood pressure and glycemic control) as needed. The study drugs were coded according to the randomization form and labeled with following information: medicine code, quantity of capsules, dosage, storage requirements, and package inserts, among other information.
2.7. Experimental methods and operation
The subjects were included in each specific group according to a random number. The basic information and disease history of the subjects were recorded and they underwent a physical examination before commencing treatment. Complications, NIHSS scores, and functional disability of activities of daily living were recorded before commencing treatment. Subjects underwent head computed tomography and MRI, electrocardiogram, chest radiograph, and routine laboratory tests, including complete blood counts, hemagglutination test, liver and renal function tests, blood biochemistry tests, urinalysis, and stool microbiology examinations.
2.8. Observation indicators
(1) The curative effect was evaluated according to the NIHSS scores on days 7, 14, 21, and 30 after treatment. (2) Functional outcomes were evaluated according to the Barthel index (BI) score on days 7, 14, 21, 30, and 90 after treatment. (3) Safety indicators were the detection of alterations in blood coagulation, platelet count (PLT), and liver and kidney function parameters, before the treatment and on days 7 and 21 of treatment. Adverse events were recorded and timely treatment (such as protecting the liver function) was administered if needed. Patients with adverse events were followed up until these were resolved.
2.9. Statistical analysis
(1) All statistical tests were 2-sided, and a P < .05 was considered statistically significant. (2) Descriptive data are presented as mean ± standard deviation (SD) and frequencies (constituent ratio). (3) Multivariate analysis of variance and repeated measures was performed, and a least square difference test was used simultaneously to compare between groups at each time point. The Bonferroni test was used at different time points for within group differences. (4) The χ2 test was used for between group comparisons.
3. Results
3.1. Compliance analysis
Of the 152 subjects in the test group, 6 subjects discontinued the study: 5 subjects were transferred to other hospitals, and 1 subject discontinued treatment. The rate of discontinuation in the test group was 3.95%. Of the 152 subjects in the control group, 11 subjects discontinued the study: 7 subjects were transferred to other hospitals, and 4 subjects discontinued treatment. The rate of discontinuation in the control group was 7.24%. All the discontinuations were attributed to lack of disease improvement. There was no significant difference in the discontinuation rates between groups (χ2 = 1.558, P > .05).
3.2. Patient disposition and comparison of baseline data between test and control groups
A total of 146 subjects in the test group were evaluated, including 77 males and 69 females, aged between 42 and 78 years. In the control group, there were 141 subjects, including 75 males and 66 females, aged between 43 and 79 years. There were no significant differences between groups in terms of baseline characteristics, including age, sex, previous medical history, complications, time of progression, and NIHSS scores, P > .05 (Table 1).
Table 1.
Comparison of baseline data between the test and control groups.

3.3. Treatment effect estimation
3.3.1. Comparison of the test and control groups before and after treatment by NIHSS scores
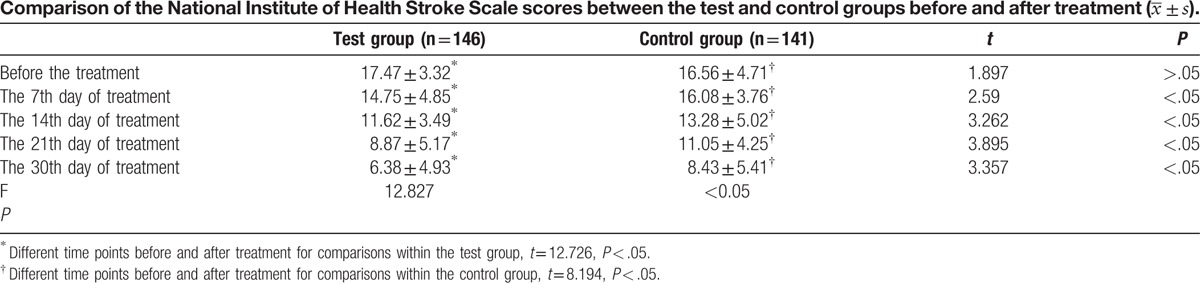
When comparing the groups according to the NIHSS scores, there was a statistically significant difference (P < .05) between groups on days 7, 14, 21, and 30 after treatment. In the test group, the effect of treatment was obvious from day 7 (Table 2).
Table 2.
3.3.2. Comparison of the test and control groups after the treatment by the BI score
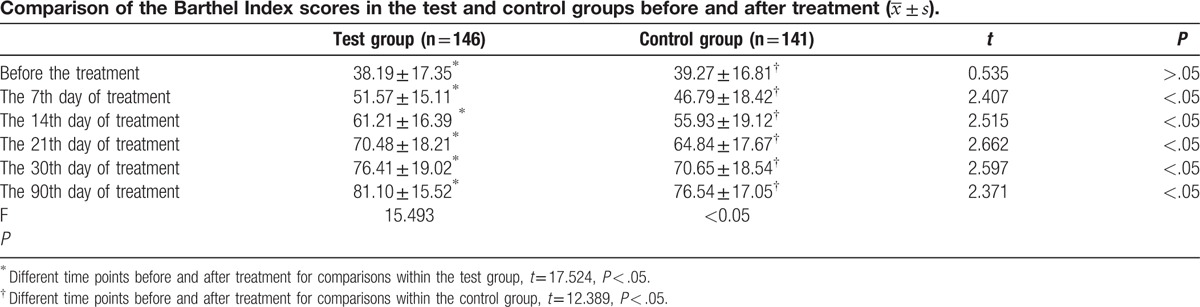
There was a significant difference (P < .05) between groups according to the BI score on days 7, 14, 21, 30, and 90 after the treatment (Table 3).
Table 3.
3.4. Safety analysis
3.4.1. Analysis of adverse events
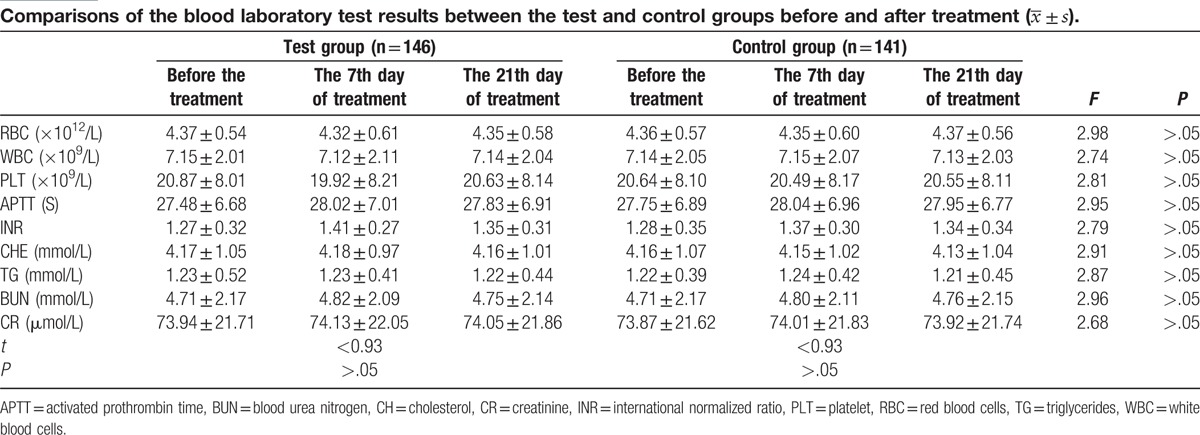
There were no significant differences (P > .05) between groups in the results of red blood cells (RBC), white blood cells (WBC), Platelet (PLT), cholesterol (CH), triglycerides (TG), blood urea nitrogen (BUN), creatinine (CR), activated prothrombin time (APTT), and international normalized ratio (INR) before and after treatment (Table 4).
Table 4.
In the test group, alanine aminotransferase (ALT) elevation was detected in 17 subjects and aspartate aminotransferase (AST) elevation was detected in 9 subjects. In the control group, ALT elevation was detected in 13 subjects and AST elevation in 5 subjects. However, there were no significant differences (χ2 = 1.405, P > .05) in the incidence of these AEs between groups (Table 5).
Table 5.
Comparison of alterations in ALT and AST between the test and control groups before and after treatment.

4. Discussion
A total of 304 subjects were enrolled in the study. Of the 152 subjects in the test group, 6 subjects discontinued, resulting in a dropout rate of 3.95% and a total of 146 evaluated subjects. In the control group, 11 subjects discontinued, resulting in a dropout rate of 7.24% and 141 evaluated subjects. There was no significant difference (χ2 = 1.558, P > .05) between groups in terms of discontinuations. This finding indicates that there was no obvious selection or design bias in this study.
PCI generally presents as cerebral infarction in which the primary neurological symptoms and signs continue to worsen within 7 days of routine treatment. Further, the current PCI treatment has been associated with poor outcomes.[1–5] Therefore, we conducted this study to evaluate whether NBP treatment could safely result in definite curative effects in subjects with PCI.
The physiopathological mechanisms of PCI are associated with the expansion of thrombus, collateral circulation angiemphraxis, release of excitatory amino acids, decrease in brain tissue perfusion, damaged mitochondria, generation of free radicals, intracellular calcium overload, brain cell edema, apoptosis, and other effects.[14,15]
Table 2 shows that the NIHSS scores in the test group gradually decreased starting on day 7 of treatment and were lower than those in the control group. The degree of improvement of the neurological impairment was more pronounced in the test group compared with the control group. This indicates that NBP could be useful for improving the neurological impairment and disability rate of patients with PCI. This result was consistent with those of previous research studies that showed that NBP treatment was efficacious in improving the outcomes of patient with acute ischemic stroke.[11–13] Several studies[16–21] have shown that NBP is a lipid-soluble drug that can directly pass through the blood brain barrier. By triggering various pathways, NBP seems to interrupt the development of physiopathological processes that lead to cerebral ischemia. Additionally, it promotes an increase in the number of capillaries, helps rebuild microcirculation, and protects the mitochondria, which improves the brain energy metabolism after cerebral infarction. Other NBP effects are improvement of cerebral perfusion in the ischemic area and reduction of the infarction area. Moreover, it limits the release of glutamic acid, decreases the calcium concentration in the cell, decreases the free radical generation, and enhances the antioxidase activity, which contributes to decreased cell apoptosis after cerebral ischemia. Thus, NBP mitigates the degree of neurological impairment in patients with PCI.
The results of the BI score in Table 3 show that in the test group, the BI score gradually increased from day 7 after treatment and reached higher levels than in the control group. In the test group, subjects showed greater improvement in functional outcomes than those in the control group. The present results showed that NBP treatment for patients with PCI was beneficial for the recovery of neurological impairment, which led to significant improvements in the self-maintenance ability and quality of life. Further, our results support the conclusions of other researchers.[11–13] NBP is a novel drug that was developed as an anticerebral ischemic agent, and its active ingredient is dl-3-n-butylphthalide. The mechanism underlying the effects of NBP for PCI may be associated with following pharmacologic actions[16–21]: (1) NBP selectively inhibits arachidonic acid and various physiopathological processes mediated by its metabolism; thus, it reverts the microvascular spasm and inhibits the platelet aggregation. NBP prevents further expansion of the thrombus, decreasing the infarct size by inhibiting the synthesis of TXA2 in the cerebral cortex cells and facilitating the synthesis of PGI2. (2) NBP acts directly on the cerebral mitochondria in the ischemic area, enhancing the mitochondria membrane fluidity and the activity of the enzyme, complex IV, in the respiratory chain of the mitochondria. This leads to a decrease of the mitochondrial membrane potential and protects the structure and function of the mitochondria, enhancing the levels of ATP and phosphocreatine in brain cells. Thus, NBP improves the ischemic tolerance, adjusts the cerebral energy metabolism in the ischemic state, reduces the generation of free radicals, enhances the cerebral blood flow in the ischemic area, reduces the infarct size, and diminishes brain edema. All these effects seem to lead to an overall improvement of the neurological impairment after ischemia. (3) NBP facilitates the collateral circulation, increases the number of microvessels, rebuilds the microcirculation in the ischemic area, it maintains the structure and form of microvessels intact, and enhances perfusion to the ischemic area.
Tables 4 and 5 show that there were few changes in the red blood cells (RBC), white blood cells (WBC), Platelet (PLT), cholesterol (CH), triglycerides (TG), blood urea nitrogen (BUN), creatinine (CR), activated prothrombin time (APTT), and international normalized ratio (INR) in the test group before treatment and on days 7 and 21 after treatment. Additionally, we found no significant differences between the test and control groups. Thus, these findings indicate that NBP has very little influence on blood cells, blood coagulation, blood glucose, blood lipids, and renal function. In this study, NBP did cause alterations of ALT and AST after treatment, but no significant differences were observed between the test and control groups. These parameters normalized with treatment. This finding indicates that NBP treatment is safe for patients with PCI, which may be attributed to the fact that NBP is extracted from celery seeds. Further, this finding is consistent with that of previous studies.[11–13]
The present study results showed that NBP treatment for PCI obviously improved the disability rate of the patients with PCI and greatly improved their functional outcomes in terms of activities of daily living. NBP was found to be an effective and safe treatment for patients with PCI.
Acknowledgments
The authors thank the patients and their families for their participation. They also thank their families. The authors wish to express their sincere thanks to Professor Jiasong Guo to polish this paper who from the Department of Histology&Embryology School of Basic Medical Sciences Southern Medical University Guangzhou, China.
Footnotes
Abbreviations: ALT = alanine aminotransferase, AST = aspartate aminotransferase, BI = the Barthel index, NBP = Dl-3n-butylphthalide, NIHSS = The National Institute of Health Stroke Scale, PCI = progressive cerebral infarction.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Philipps J, Thomalla G, Glahn J, et al. Treatment of progressive stroke with tirofiban—experience in 35 patients. Cerebrovasc Dis 2009;28:435–8. [DOI] [PubMed] [Google Scholar]
- [2].Eriksson M, Stecksén A, Glader EL, et al. Riks-Stroke Collaboration. Discarding heparins as treatment for progressive stroke in Sweden 2001 to 2008. Stroke 2010;41:2552–8. [DOI] [PubMed] [Google Scholar]
- [3].Siegler JE, Boehme AK, Albright KC, et al. A proposal for the classification of etiologies of neurologic deterioration after acute ischemic stroke. J Stroke Cerebrovasc Dis 2013;22:e549–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tada Y, Uno M, Matsubara S, et al. Reversibility of ischemic findings on 3-T T2∗-weighted imaging after emergency superficial temporal artery-middle cerebral artery anastomosis in patients with progressive ischemic stroke. Neurol Med Chir (Tokyo) 2010;50:1006–11. [DOI] [PubMed] [Google Scholar]
- [5].Derflinger S, Fiebach JB, Böttger S, et al. The progressive course of neurological symptoms in anterior choroidal artery infarcts. Int J Stroke 2015;10:134–7. [DOI] [PubMed] [Google Scholar]
- [6].Zhao Q, Zhang C, Wang X, et al. (S)-ZJM-289, a nitric oxide-releasing derivative of 3-n-butylphthalide, protects against ischemic neuronal injury by attenuating mitochondrial dysfunction and associated cell death. Neurochem Int 2012;60:134–44. [DOI] [PubMed] [Google Scholar]
- [7].Kapoor S. Dl-3-n-butylphthalide and its emerging beneficial effects in neurology. Chin Med J (Engl) 2012;125:3360. [PubMed] [Google Scholar]
- [8].Huang JZ, Chen YZ, Su M, et al. dl-3-n-Butylphthalide prevents oxidative damage and reduces mitochondrial dysfunction in an MPP(+)-induced cellular model of Parkinson's disease. Neurosci Lett 2010;475:89–94. [DOI] [PubMed] [Google Scholar]
- [9].Lu XL, Luo D, Yao XL, et al. Dl-3n-butylphthalide promotes angiogenesis via the extracellular signal-regulated kinase 1/2 and phosphatidylinositol 3-kinase/Akt-endothelial nitric oxide synthase signaling pathways. J Cardiovasc Pharmacol 2012;59:352–62. [DOI] [PubMed] [Google Scholar]
- [10].Li J, Li Y, Ogle M, et al. DL-3-n-butylphthalide prevents neuronal cell death after focal cerebral ischemia in mice via the JNK pathway. Brain Res 2010;1359:216–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cui LY, Liu XQ, Zhu YC, et al. Effects of dl-3-butylphthalide on treatment of acute ischemic stroke with moderate symptoms: a multi-center, randomized, double-blind, placebo-control trial. Zhonghua Shenjingke Zazhi 2005;38:251–4. [Google Scholar]
- [12].Cui LY, Li SW. D1-3-n-butylphthalinde curing acute ischemic stroke: random and double-blind control study. Zhongguo Xiandai Shenjing Jibing Zazhi 2006;6:263–5. [Google Scholar]
- [13].Cui LY, Li SW, Zhang WW, et al. Effects of dl-3-butylphthalide soft capsules on treatment of acute ischemic stroke: multi-center, randomized, double-blind, double-dummy and aspirin-control study. Zhonghua Shenjingke Zazhi 2008;41:727–30. [Google Scholar]
- [14].Li HY, Ji XJ, Han D, et al. Analysis of risk factors for progressive ischemic stroke. Guoji Haoxueguanbing Zazhi 2005;13:817–9. [Google Scholar]
- [15].Del Bene A, Palumbo V, Lamassa M, et al. Progressive lacunar stroke: review of mechanisms, prognostic features, and putative treatments. Int J Stroke 2012;7:321–9. [DOI] [PubMed] [Google Scholar]
- [16].Liu CL, Liao SJ, Zeng JS, et al. Dl-3n-butylphthalide prevents stroke via improvement of cerebral microvessels in RHRSP. J Neurol Sci 2007;260:106–13. [DOI] [PubMed] [Google Scholar]
- [17].Liao SJ, Lin JW, Pei Z, et al. Enhanced angiogenesis with dl-3n-butylphthalide treatment after focal cerebral ischemia in RHRSP. Brain Res 2009;1289:69–78. [DOI] [PubMed] [Google Scholar]
- [18].Cao WY, Deji QZ, Li QF, et al. Effects of dl-3n-butylphthalide on the expression of VEGF and bFGF in transient middle cerebral artery occlusion rats. Sichuan Da Xue Xue Bao Yi Xue Ban 2009;40:403–7. [PubMed] [Google Scholar]
- [19].Zhang L, Lü L, Chan WM, et al. Effects of DL-3-n-butylphthalide on vascular dementia and angiogenesis. Neurochem Res 2012;37:911–9. [DOI] [PubMed] [Google Scholar]
- [20].Wang X, Li Y, Zhao Q, et al. Design, synthesis and evaluation of nitric oxide releasing derivatives of 3-n-butylphthalide as antiplatelet and antithrombotic agents. Org Biomol Chem 2011;9:5670–81. [DOI] [PubMed] [Google Scholar]
- [21].Cui YH, Zhang CD, Guo W, et al. Effects of butylphthalide on the apoptosis of PC-12 cells under the induction of β-amyloid peptide. Zhonghua Yi Xue Za Zhi 2010;90:3235–7. [PubMed] [Google Scholar]


