Abstract
To evaluate the visual outcomes of cataract surgery in patients with uveitis, and to determine risk factors for the recurrence of uveitis and postoperative complications.
Eighty patients with uveitis who underwent phacoemulsification with intraocular lens (IOL) implantation were included in this retrospective study. We analyzed the following data: patient characteristics, medications used, visual acuity, and complications of cataract surgery.
The mean ± standard deviation time from cataract surgery to the last visit was 20.8 ± 10.4 months. Best-corrected visual acuity improved significantly after surgery (P < .001). The visual outcome was worse in patients with Behçet disease than in patients with other etiologies of uveitis. Gender (P = .018) and IOL type (P = .020) were significantly associated with recurrent uveitis after surgery. The incidence of recurrent inflammation was not significantly different between patients who did or did not receive systemic therapy (P = .43). Perioperative systemic therapies (P = .011) and recurrent uveitis within 3 months of surgery (P = .043) were associated with posterior capsular opacification. Perioperative systemic therapies (P = .026) and recurrent uveitis after surgery (P = .006) were also significantly associated with cystoid macular edema.
Patients with uveitis could benefit from cataract surgery. Patients with Behçet disease had worse postoperative prognosis than patients with other etiologies of uveitis. A heparin-surface-modified IOL may reduce the incidence of recurrent inflammation.
Keywords: cataract surgery, postoperative complications, uveitis, visual outcome
1. Introduction
Cataract is a common complication in patients with uveitis and is the main cause of reversible blindness in these patients. Cataract occurs in up to 50% to 70% of patients with uveitis.[1,2] Preoperative complications, including anterior synechiae, posterior synechiae, and pupillary membrane formation, may pose surgical challenges. In addition, recurrent inflammation increases the incidence of postoperative complications and often affects the visual prognosis. In recent years, phacoemulsification with intraocular lens (IOL) implantation has become the main surgical method for treating uveitis (complicated cataract), and the visual prognosis of patients who undergo this procedure is favorable.[3–5]
However, there is no consensus on the optimal surgical procedures and perioperative therapeutic regimens for different etiologies of uveitis. Recently, Lin et al[6] reported that preoperative visual acuity (P = .020) and disease etiology (P = .011) were likely to influence visual prognosis, postoperatively. However, risk factors were not established for recurrent inflammation and postoperative complications, both of which were strongly associated with the visual outcomes. In addition, the distribution of etiologies of uveitis and the statistical data varied widely among different races. Several studies have evaluated cataract surgery outcomes in Asian patients with uveitis. However, it may hold true for a specific region in Asia.
Therefore, the aims of this study were to evaluate the efficacy of phacoemulsification with posterior chamber IOL (PC-IOL) implantation in patients with various causes of uveitis and to identify risk factors for postoperative complications.
2. Methods
2.1. Data collection
The patients’ medical records were reviewed and the following information was retrieved: age at surgery, gender, cause of uveitis, medications prescribed, ocular complications, systemic diseases, cataract classification, ocular findings, surgical method, IOL type, best-corrected visual acuity (BCVA). Preoperative uveitis-associated fundal disease, macular thickness, and optic atrophy were measured with ultrasonography and optical coherence tomography (OCT). Postoperatively, patients were followed up and the following data were recorded: BCVA, intraocular pressure, ocular findings (anterior segment inflammation, iris and capsular features, and IOL position), and postoperative complications. Postoperative posterior segment was also evaluated with OCT and ultrasonography. All the charts were reviewed by a single observer.
2.2. Patients
The Ethics Committee of the Eye and Ear, Nose, and Throat Hospital, Fudan University, approved our study. Written informed consent was obtained from all patients during the visit after being informed of the nature and possible consequences of the study. The study was registered at www.clinicaltrials.gov (Registration Number: NCT02182921).
Phacoemulsification with PC-IOL implantation was performed at the Eye, Ear, Nose, and Throat Hospital between May 2012 and May 2014. Originally, 151 patients were enrolled, and finally 74 subjects had a follow-up at least 6 months. Patients were excluded from this study if they had histories of ocular surgery, trauma, infection, lens subluxation, and diabetes. Patients were eligible if uveitis had been in a quiescent state for ≥3 months. Quiescence was defined as the presence of ≤ 1+ cells (5–10 cells per high-power field) in the anterior chamber and no active vitritis, as observed using a narrow slit beam.[7]
The classification of uveitis was based on the definitions proposed by the Standardization of Uveitis Nomenclature (SUN) working group.[8] Clinical manifestation, a history of uveitis-associated systemic disease, routine, and immunological examinations were recorded to comment on the etiology of uveitis.
2.3. Surgical technique
Tropicamide was administered 30 minutes before surgery to fully dilate the pupil. A 2.6-mm main temporal corneal incision was made. If pupil dilation was insufficient with other methods, it was managed by synechiolysis. Hydrodissection, chopping, nucleus rotation, and phacoemulsification were performed after making a 5.5-mm continuous curvilinear capsulorhexis. A HQ201-HEP (HexaVision, Paris, France), ZCB00 (Abbott Medical Optics, Anasco, Puerto Rico), SN60WF (Alcon Laboratories, Fort Worth, TX), or PC 525Y ErgomaX (HumanOptics, Erlangen, Germany) IOL was implanted using a dedicated injector. All the surgeries were performed by the same surgeon, YL.
2.4. Perioperative protocol
Routine postoperative ophthalmic management included topical levofloxacin, prednisolone acetate, and pranoprofen, which were administered 4 times daily for 1 month. Patients with a history of frequent inflammatory attacks and patients who developed significant postoperative inflammation were also treated with tobramycin-dexamethasone eye drops or a 4-week course of a systemic corticosteroid (since 3 days before the cataract surgery). Oral prednisolone (5 mg/tablet) was prescribed at 20 to 30 mg/d with a gradual dose tapering. If severe inflammation occurred, a higher steroid dose or intravenous methylprednisolone were given. Immunosuppressants (e.g., cyclosporine and methotrexate) were prescribed to refractory patients.
2.5. Statistical analysis
Statistical analyses were performed using SPSS statistical software version 19.0 (SPSS-IBM Inc, Chicago, IL). For patients who underwent binocular cataract surgery, the data were collected for 1 randomly selected eye. Quantitative data are presented as the mean ± standard deviation (SD). Paired t tests were used to compare the preoperative, postoperative, and final visual acuities. Unpaired t tests and one-way analysis of variance (ANOVA) were used to determine significant differences between 2 groups or more than 2 groups, respectively. Survival analysis was performed to compare the incidence of relapsing uveitis after surgery in patients treated with or without systemic medications. χ2 tests and univariate logistic regression, followed by multivariable backwards regression, were used to identify independent risk factors for outcomes of interest. P values of < .05 were considered statistically significant.
3. Results
3.1. Patient characteristics at baseline
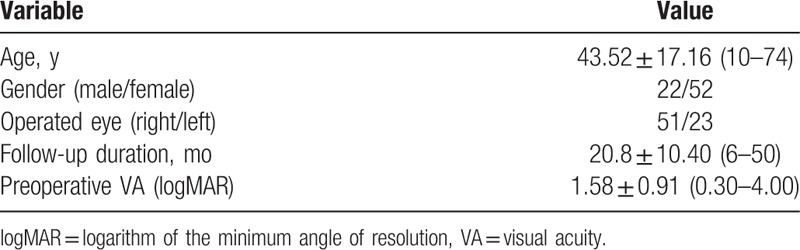
Our study included 74 patients (22/52 males/females) with different types of uveitis. Table 1 shows the baseline characteristics of the 74 eyes analyzed in this study. The mean ± SD (range) age of patients at cataract surgery was 43.52 ± 17.16 years (10–74 years) and the time from cataract surgery to the final visit was 20.8 ± 10.40 months (6–50 months). The main indication for phacoemulsification with IOL implantation was poor preoperative visual acuity and the logarithm of the minimum angle of resolution (logMAR) was 1.58 ± 0.91.
Table 1.
Patient characteristics.
3.2. Anatomical classification and etiology of uveitis
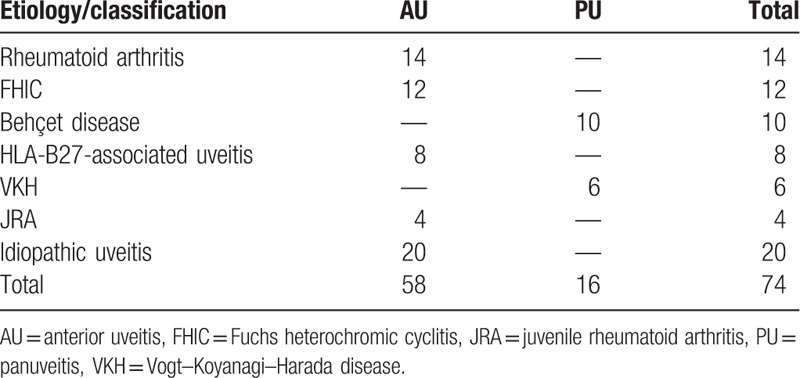
As shown in Table 2, of the 74 eyes evaluated, 58 (78.4%) were diagnosed with anterior uveitis and 16 eyes (21.6%) were diagnosed with panuveitis. Twenty eyes (27.0%) were diagnosed with idiopathic anterior uveitis. The higher prevalence was found in rheumatoid arthritis (14 eyes, 18.9%), Fuchs heterochromic cyclitis (FHIC; 12 eyes, 16.2%), and Behçet disease (10 eyes, 13.5%).
Table 2.
Etiology and anatomical classification of uveitis.
3.3. Visual acuity outcomes according to the etiology of uveitis
Among the 74 eyes evaluated, the Snellen value before surgery was <20/200 in 44 eyes (59.5%) eyes and was ≥ 20/80 in just 7 eyes (9.5%) eyes. At the final visit, the Snellen value was ≥ 20/80 in 63 eyes (85.1%). The BCVA was significantly different between the preoperative visit and first postoperative visit (within 48 hours after surgery) (P < .001), and between the first postoperative visit and the final visit (P < .001). However, the BCVA was the same at the preoperative visit and the last visit in 2 eyes (2.7%), and deteriorated in 1 eye (1.4%).
In Figure 1, all Snellen values were converted to the equivalent logMAR to calculate the median visual acuity. This figure shows the visual acuity outcomes according to the etiology of uveitis. Preoperatively, the mean logMAR was 2.56 in eyes with Behçet disease, and it was significantly different to the logMAR of uveitis with other etiologies (P < .05) except for FHIC (P = .094). The BCVA of eyes with FHIC was worse than that of eyes with idiopathic anterior uveitis (P = .012) and rheumatoid arthritis (P = .031). The BCVA of eyes with Behçet disease at the first and last visits after surgery was significantly worse than that of eyes with other types of uveitis. Eyes with FHIC showed marked improvements in visual outcomes after surgery.
Figure 1.
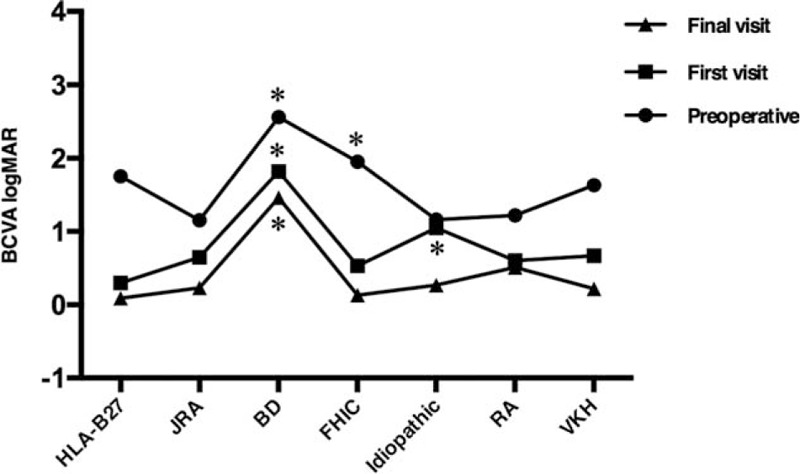
Visual outcomes of patients according to the etiology of uveitis. ∗P < .05 versus other etiologies of uveitis. BCVA = best-corrected visual acuity, BD = Behçet disease, CME = cystoid macular edema, FHIC = Fuchs heterochromic cyclitis, JRA = juvenile rheumatoid arthritis, logMAR = logarithm of the minimum angle of resolution, PCO = posterior capsular opacification, RA = rheumatoid arthritis, VKH = Vogt–Koyanagi–Harada disease.
3.4. Postoperative uveitis recurrence and associated risk factors
Among the 74 eyes evaluated, inflammation recurred in 34 eyes (45.9%) in the follow-up period after surgery and uveitis recurred within 3 months after surgery in 14 eyes (18.9%). The mean ± SD (range) time from cataract surgery to the recurrence of inflammation was 6.23 ± 5.62 months (0–19 months).
In the perioperative period, 24 patients were given a 4-week course of systemic therapy, which included a corticosteroid and/or immunosuppressant, while the other 50 patients were prescribed topical eye drops alone. The decision to administer systemic therapies was made by the experienced attending doctor. The incidence of recurrent uveitis was not significantly different between patients who did or did not receive systemic therapy (P = .43).
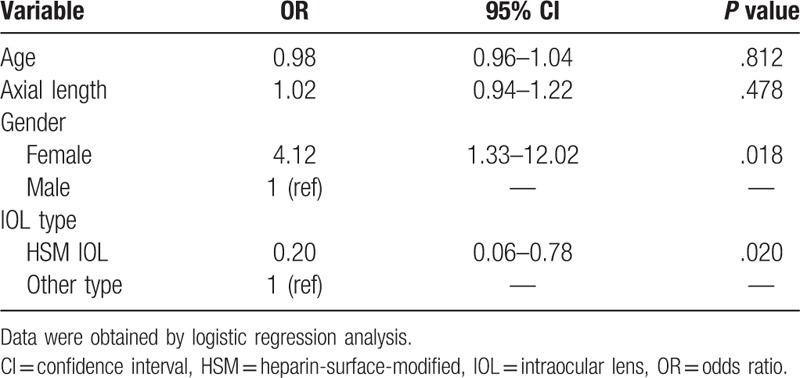
We used χ2 tests to determine which factors were associated with the development of postoperative uveitis, including age, axial length, gender, and IOL type. Logistic regression analysis revealed that females were more likely to experience recurrent uveitis (P = .018). Implantation of a heparin-surface-modified (HSM) IOL seemed to reduce the incidence of relapse inflammation (P = .020) (Table 3).
Table 3.
Risk factors for postoperative recurrent uveitis.
3.5. Postoperative complications according to the type of uveitis
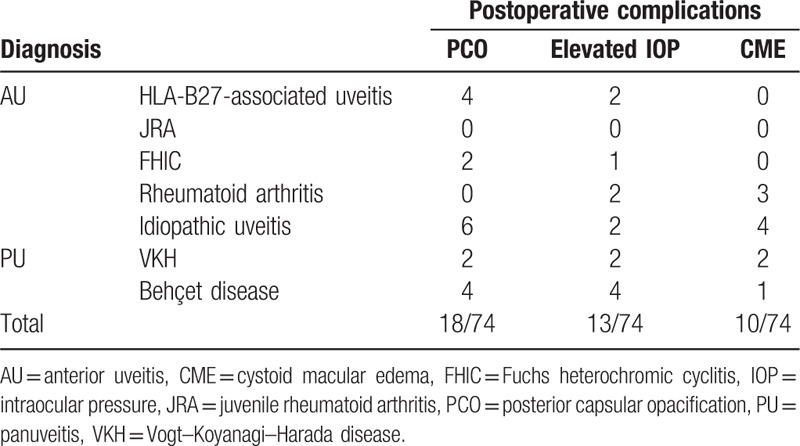
Preoperatively, no cystoid macular edema (CME) was found in the included patients. A history of glaucoma and antiglaucoma therapy was found in 26 patients. Table 4 shows the rates of common postoperative complications including posterior capsular opacification (PCO), elevated intraocular pressure (IOP), and CME according to the etiological and anatomical types of uveitis. Elevated IOP occurred postoperatively in 13 patients (17.6%). Of these, 11 had transient elevated IOP, which could be controlled by topical treatment and 2 patients had uncontrolled IOP requiring trabeculectomy. In addition, 4 of these patients with elevated IOP had antiglaucoma therapy preoperatively. CME occurred in 10 patients (13.5%) and corticosteroids were administered to these patients. PCO occurred in 18 patients (24.3%) who underwent posterior capsulotomy with a neodymium-doped yttrium aluminum garnet (Nd:YAG) laser owing to obvious decreases in visual acuity.
Table 4.
Postoperative complications according to the etiology of uveitis.
3.6. Risk factors for postoperative complications
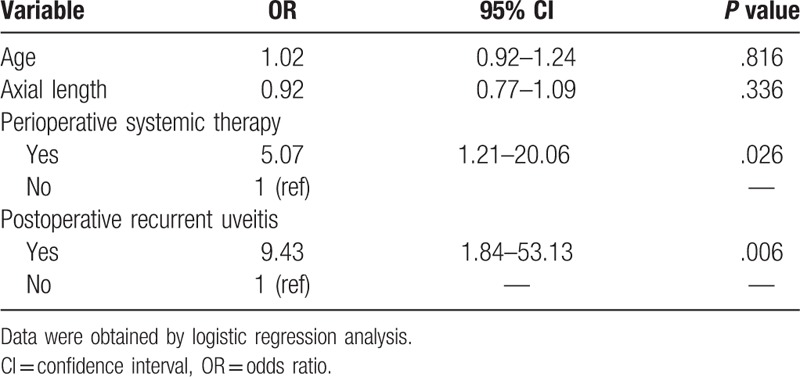
We used χ2 tests to determine which factors were associated with the development of the postoperative complications PCO and CME. As shown in Table 5, perioperative systemic therapies (P = .011) and the recurrence of uveitis within 3 months of surgery (P = .043) were associated with PCO. Perioperative systemic therapies (P = .026) and the recurrence of uveitis (P = .006) were also associated with CME (Table 6).
Table 5.
Risk factors for postoperative posterior capsular opacification.
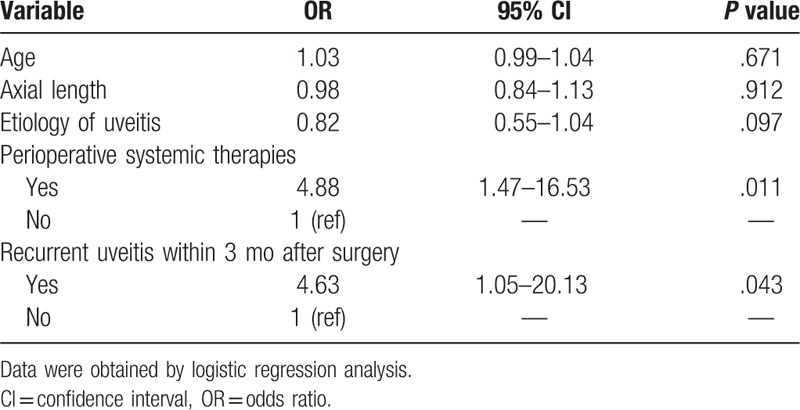
Table 6.
Risk factors for postoperative cystoid macular edema.
4. Discussion
Cataract is the main complication of patients with uveitis and can lead to vision loss. For many years, intraocular inflammation was considered a major risk factor for cataract surgery and was regarded as a contraindication for cataract surgery. With the development of new techniques, phacoemulsification with PC-IOL implantation has become the main surgical method for treating cataract. However, there are 2 questions that need to be addressed. The first question is whether the surgical outcomes differ between patients with different etiologies of uveitis. The second question is what risk factors are associated with recurrent uveitis and postoperative complications.
In our study, the BCVA increased significantly between the first and last visits (P < .001). Previous studies have demonstrated that strict control of preoperative inflammation significantly improved the visual outcomes of phacoemulsification with IOL implantation.[9–11] In terms of the etiologies of uveitis, patients with Behçet disease experienced worse postoperative outcomes than patients with other etiologies. The main factors associated with worse outcomes of Behçet disease were reported to include pre-existing chorioretinopathy or glaucoma-related optic neuropathy.[12–14] Recently, Oray et al[15] reviewed the clinical photographs of 259 patients with Behçet-related uveitis, and found that 62 patients (24%) had localized retinal nerve fiber layer defects. The presence of localized retinal nerve fiber layer defects was reported to be a risk factor for poor visual prognosis and may be caused by microvascular ischemia. In our study, the visual acuity of patients with FHIC was worse before surgery, but then markedly improved postoperatively. These findings suggested that the worse preoperative visual acuity in patients with FHIC was mainly due to severe lens opacity and that the better outcomes were probably due to less-severe pre-existing vision-limiting pathology and less postoperative inflammation.[4,16]
Relapse inflammation after surgery increases the risk of complications and may affect the visual outcomes. However, few studies have focused on the risk factors for recurrent uveitis. Implantation of an HSM-IOL seemed to reduce the incidence of recurrent uveitis in our study. A previous study suggested that postoperative inflammation was associated with the biological compatibility of the IOL.[17] A prospective, randomized study of 60 patients showed no obvious differences at 6 months between the hydrophobic AcrySof and the hydrophilic Akreos adapt in terms of uveal and capsular biocompatibility, and visual outcomes.[18] Krall et al[19] subsequently reported that the mean flare value was significantly lower in patients implanted with an HSM-IOL than in patients implanted with an uncoated IOL (14.92 ± 7.47 ph/ms vs 16.73 ± 7.81 ph/ms, respectively; P = .04). Patients implanted with an HSM-IOL had significantly weaker inflammatory reactions in the early postoperative stage compared with patients implanted with an uncoated IOL. In addition, we found that the incidence of recurrent uveitis was not significantly different between patients with and without perioperative systemic therapy (P = .43). Similarly, Mora et al compared the intensive topical steroids alone and the same topical regimen combined with oral steroids before cataract surgery in uveitic patients. It was suggested that preoperative intensive topical steroids alone could comparably prevent uveitis relapse.[20] However, previous studies revealed that administration of oral prednisolone achieved better recovery of the blood–aqueous barrier after cataract surgery, and reduced the incidence of recurrent uveitis.[21,22] The lack of association in the present study was probably due to the fact that patients considered to have a higher risk of relapse inflammation or persistent chronic inflammation attacks were more likely to receive systemic corticosteroids.
Patients with complicated cataract are thought to experience more postoperative complications than patients with age-related cataract. In our study, the most common postoperative complications were PCO (24.3% of patients), elevated IOP (17.6%), and CME (13.5%). A study of 61 patients (72 eyes) followed up for ≥ 5 years (mean 91 months) showed that macular edema or scarring occurred in 17 eyes (24%), PCO occurred in 69 eyes (96%), and glaucoma drainage surgery was required up to the latest follow-up examination in 11 eyes (15%).[23] Recently, Takayama et al[24] reviewed 17 patients who underwent coaxial microincision cataract surgery and that postoperative complications were rare; elevated IOP and exacerbated uveitis occurred in 1 eye each.
Until now, few studies have examined the risk factors for postoperative complications.[6,25] Our results suggest that perioperative systemic therapies and recurrent uveitis were significant risk factors for PCO and CME. In clinical practice, the application of systemic therapies and recurrent uveitis indicate a diagnosis of complicated uveitis, which is associated with the development of postoperative complications. By contrast, previous studies indicated that the administration of prophylactic corticosteroids was associated with decreased incidences of Nd:YAG capsulotomy and CME.[5,26]
In conclusion, our retrospective study showed that most patients experienced significant improvements in visual acuity after cataract surgery. Patients with Behçet disease appeared to have a worse prognosis than patients with other etiologies of uveitis. The HSM-IOL might help to reduce postoperative recurrent uveitis. Patients with relapse inflammation were at increased risk of postoperative complications, including PCO and CME. Further, we have to point out that larger randomized double-blind prospective studies are needed to demonstrate the affecting factors of complications.
Footnotes
Abbreviations: ANOVA = analysis of variance, AU = anterior uveitis, BCVA = best-corrected visual acuity, CI = confidence interval, CME = cystoid macular edema, FHIC = Fuchs heterochromic cyclitis, HSM = heparin-surface-modified, IOL = intraocular lens, IOP = intraocular pressure, JRA = juvenile rheumatoid arthritis, logMAR = logarithm of the minimum angle of resolution, Nd:YAG = neodymium-doped yttrium aluminum garnet, OCT = optical coherence tomography, OR = odds ratio, PC-IOL = posterior chamber IOL, PCO = posterior capsular opacification, PU = panuveitis, SD = standard deviation, SUN = standardization of uveitis nomenclature, VA = visual acuity, VKH = Vogt–Koyanagi–Harada disease.
Trial Registration Number: NCT02182921.
YZ and XZ contributed equally to this work.
Publication of this article was supported by the National Science Foundation of China (81100653, 81270989, and 81470613), and the National Health and Family Planning Commission of the People's Republic of China (201302015).
The authors have no conflicts of interest to disclose.
References
- [1].Okhravi N, Lightman SL, Towler HM. Assessment of visual outcome after cataract surgery in patients with uveitis. Ophthalmology 1999;106:710–22. [DOI] [PubMed] [Google Scholar]
- [2].Kump LI, Cervantes-Castaneda RA, Androudi SN, et al. Analysis of pediatric uveitis cases at a tertiary referral center. Ophthalmology 2005;112:1287–92. [DOI] [PubMed] [Google Scholar]
- [3].Estafanous MF, Lowder CY, Meisler DM, et al. Phacoemulsification cataract extraction and posterior chamber lens implantation in patients with uveitis. Am J Ophthalmol 2001;131:620–5. [DOI] [PubMed] [Google Scholar]
- [4].Javadi MA, Jafarinasab MR, Araghi AA, et al. Outcomes of phacoemulsification and in-the-bag intraocular lens implantation in Fuchs’ heterochromic iridocyclitis. J Cataract Refract Surg 2005;31:997–1001. [DOI] [PubMed] [Google Scholar]
- [5].Elgohary MA, Mccluskey PJ, Towler HM, et al. Outcome of phacoemulsification in patients with uveitis. Br J Ophthalmol 2007;91:916–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lin CP, Yeh PT, Chen PF, et al. Cataract extraction surgery in patients with uveitis in Taiwan: risk factors and outcomes. J Formos Med Assoc 2014;113:377–84. [DOI] [PubMed] [Google Scholar]
- [7].Hogan MJ, Kimura SJ, Thygeson P. Signs and symptoms of uveitis. I. Anterior uveitis. Am J Ophthalmol 1959;47(5 pt 2):155–70. [DOI] [PubMed] [Google Scholar]
- [8].Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol 2005;140:509–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kawaguchi T, Mochizuki M, Miyata K, et al. Phacoemulsification cataract extraction and intraocular lens implantation in patients with uveitis. J Cataract Refract Surg 2007;33:305–9. [DOI] [PubMed] [Google Scholar]
- [10].Kosker M, Sungur G, Celik T, et al. Phacoemulsification with intraocular lens implantation in patients with anterior uveitis. J Cataract Refract Surg 2013;39:1002–7. [DOI] [PubMed] [Google Scholar]
- [11].Krishna R, Meisler DM, Lowder CY, et al. Long-term follow-up of extracapsular cataract extraction and posterior chamber intraocular lens implantation in patients with uveitis. Ophthalmology 1998;105:1765–9. [DOI] [PubMed] [Google Scholar]
- [12].Kadayifcilar S, Gedik S, Eldem B, et al. Cataract surgery in patients with Behcet's disease. J Cataract Refract Surg 2002;28:316–20. [DOI] [PubMed] [Google Scholar]
- [13].Saygili O, Szurman P, Gieselmann S, et al. [Clinical results after cataract surgery in patients with Behcet's disease]. Klin Monbl Augenheilkd 2011;228:900–4. [DOI] [PubMed] [Google Scholar]
- [14].Sullu Y, Oge I, Erkan D. The results of cataract extraction and intraocular lens implantation in patients with Behcet's disease. Acta Ophthalmol Scand 2000;78:680–3. [DOI] [PubMed] [Google Scholar]
- [15].Oray M, Onal S, Bayraktar S, et al. Nonglaucomatous localized retinal nerve fiber layer defects in Behcet uveitis. Am J Ophthalmol 2015;159:475–81. [DOI] [PubMed] [Google Scholar]
- [16].Tejwani S, Murthy S, Sangwan VS. Cataract extraction outcomes in patients with Fuchs’ heterochromic cyclitis. J Cataract Refract Surg 2006;32:1678–82. [DOI] [PubMed] [Google Scholar]
- [17].Rauz S, Stavrou P, Murray PI. Evaluation of foldable intraocular lenses in patients with uveitis. Ophthalmology 2000;107:909–19. [DOI] [PubMed] [Google Scholar]
- [18].Roesel M, Heinz C, Heimes B, et al. Uveal and capsular biocompatibility of two foldable acrylic intraocular lenses in patients with endogenous uveitis—a prospective randomized study. Graefes Arch Clin Exp Ophthalmol 2008;246:1609–15. [DOI] [PubMed] [Google Scholar]
- [19].Krall EM, Arlt EM, Jell G, et al. Intraindividual aqueous flare comparison after implantation of hydrophobic intraocular lenses with or without a heparin-coated surface. J Cataract Refract Surg 2014;40:1363–70. [DOI] [PubMed] [Google Scholar]
- [20].Mora P, Gonzales S, Ghirardini S, et al. Perioperative prophylaxis to prevent recurrence following cataract surgery in uveitic patients: a two-centre, prospective, randomized trial. Acta Ophthalmol 2016;94:e390–4. [DOI] [PubMed] [Google Scholar]
- [21].Roesel M, Heinz C, Koch JM, et al. Cataract surgery in uveitis. Ophthalmology 2008;115:1431. [DOI] [PubMed] [Google Scholar]
- [22].Meacock WR, Spalton DJ, Bender L, et al. Steroid prophylaxis in eyes with uveitis undergoing phacoemulsification. Br J Ophthalmol 2004;88:1122–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Rahman I, Jones NP. Long-term results of cataract extraction with intraocular lens implantation in patients with uveitis. Eye (Lond) 2005;19:191–7. [DOI] [PubMed] [Google Scholar]
- [24].Takayama K, Fujii S, Ishikawa S, et al. Short-term outcomes of coaxial microincision cataract surgery for uveitis-associated cataract without postoperative systemic steroid therapy. Ophthalmologica 2014;231:111–6. [DOI] [PubMed] [Google Scholar]
- [25].Yoeruek E, Deuter C, Gieselmann S, et al. Long-term visual acuity and its predictors after cataract surgery in patients with uveitis. Eur J Ophthalmol 2010;20:694–701. [DOI] [PubMed] [Google Scholar]
- [26].Belair ML, Kim SJ, Thorne JE, et al. Incidence of cystoid macular edema after cataract surgery in patients with and without uveitis using optical coherence tomography. Am J Ophthalmol 2009;148:128–35. [DOI] [PMC free article] [PubMed] [Google Scholar]


