Abstract
To evaluate the refractive outcomes of balanced salt solution infiltration during small-incision lenticule extraction (SMILE).
This randomized prospective study enrolled 52 patients (104 eyes) with myopic astigmatism. Patients underwent SMILE to correct the myopic astigmatism in Daping Hospital of the Third Military Medical University between January and July 2013. One eye of each patient received traditional SMILE (control group) and the other received a modified SMILE procedure (liquid infiltration group). The corrected distance visual acuity (CDVA), postoperative uncorrected distance visual acuity (UDVA), refraction, wavefront aberration, intraocular pressure (IOP), modulation transfer function (MTF) cut-off frequency, and objective scattering index (OSI) were evaluated.
UDVA in the liquid infiltration group was significantly higher than that in the control group at 1 day postoperatively, but not at 1 month after surgery. Moreover, OSI and MTF cut-off frequency in the liquid infiltration group were higher than those in the control group at early follow-up. However, no significant intergroup difference was observed in the OSI and MTF cut-off frequency at 3 months after surgery. In addition, the predictability was better in the liquid infiltration group than in the control group. The changes of horizontal coma in the liquid infiltration group were lesser than those in the control group. However, no intergroup difference was observed in the reduction of IOP at 1 month after surgery.
The modified SMILE procedure results in better visual outcomes than did the traditional SMILE procedure when used for treating myopic astigmatism.
Keywords: balanced salt solution infiltration, myopic astigmatism, small-incision lenticule extraction
1. Introduction
Refractive surgery is an effective option for patients seeking independence from glasses or contact lenses to correct refractive error.[1] A newly developed surgical method, small-incision lenticule extraction (SMILE), has gained widespread acceptance in correcting myopia and myopic astigmatism via an all-in-one VisuMax femtosecond laser system (Carl Zeiss Meditec AG, Berlin, Germany).[2,3] SMILE is a flapless surgery, and compared with laser-assisted in situ keratomileusis (LASIK), it could reduce corneal nerve damage and dry eye.[4,5] Moreover, SMILE has better predictability than dose LASIK.[5,6]
However, lenticule dissection is a limitation in SMILE surgery,[3,6] because of the probability of corneal epithelial and stromal damage during separation in SMILE,[7–9] which could delay the speed of visual recovery after surgery. Several studies have reported that immersing the cornea in water could significantly reduce the thermal damage to the cornea.[8–10] A recent modified surgical technique with balanced salt solution infiltration dissection was investigated in this study. With this technique, the epithelium and stroma should be less disrupted. Therefore, a prospective study was conducted to evaluate and compare the clinical outcomes between the modified and SMILE procedures.
2. Patients and methods
This prospective study was performed in accordance with the tenets of the Declaration of Helsinki and approved by the ethical committee of Daping Hospital of the Third Military Medical University. All the patients provided signed informed consent.
2.1. Patients
In this randomized study, we enrolled 104 eyes of 52 patients, including 17 males and 35 females, with the mean age of 24.04 ± 5.47 years (ranged from 18 to 36 years). All the patients had undergone SMILE to correct myopic astigmatism in Daping Hospital of the Third Military Medical University between January and July 2013. The patients included in this study did not have any surgical contraindication and the alterations of refraction state for both eyes were less than 0.5 D per year in 2 to 3 years. Moreover, patients with abnormal ocular motor parameters were excluded. The refraction state was assessed by subjective optometry and no cycloplegia was utilized.
One eye of each patient was randomized into the control group, in which the eyes received the traditional SMILE procedure. The contralateral eye received liquid infiltration dissection during SMILE surgery (liquid infiltration group).
2.2. Surgery
The corrected surgery in this study was performed by an experienced surgeon. SMILE was performed using a VisuMax femtosecond laser as previously described by Shah et al.[11] All patients received topical an aesthesia with 3 drops of 0.4% oxybuprocaine hydrochloride (Santen Pharmaceutical Co., Osaka, Japan) before surgery. The parameters of VisuMax were set as follows: work power was 500 kHz, dot spacing and line spacing both were 3 μm, pulse energy ranged from 150 to 170 nJ, designed cap thickness ranged from 120 to 130 μm, optical zone diameter ranged from 6.2 to 6.5 mm, base thick ranged from 10 to 15 μm and incision was performed at a perpendicular direction. After scanning by femtosecond laser, 0.2 mL mild balanced buffer solution (BBS) was utilized to wash cornea surface by anterior chamber irrigator and segregator with a trace of BBS accessed to the above lenticule with “S” path. By using the same method, surgery below lenticule was also conducted. The surgery was performed using a single hand, without any other surgical equipment was applied. No accident or complications occurred during any of the surgeries.
In the control group, the lenticules were dissected using a thin blunt spatula (Seibel, Rhein Medical, Inc., Tampa, FL) and extracted using a coaxial Tan DSAEK forceps (Asico, Westmont, IL) through a small incision. In the liquid infiltration group, the balanced salt solution was injected into the small-incision after identifying the anterior and posterior layers of the lenticule. Thereafter, the lenticules were separated and extracted through the small incision.
2.3. Postoperative medication
After surgery, patients were prescribed tobramycin and dexamethasone eye drops (Alcon Laboratories, Inc., Fort Worth, TX) twice on the surgical day. Following the instillation of tobramycin and dexamethasone eye drops, levofloxacin eye drops (Oftaquix; Santen Oy, Tampere, Finland) and protein-free calf blood extract eye gel (Shenyang Xing Qi Pharmaceutical Co., Ltd, Shenyang, China) were applied 4 times per day in the first week after surgery. From the second week after surgery, the patients were asked to instill a loteprednol etabonate ophthalmic suspension (Lotemax and Alrex, Bausch & Lomb Pharmaceuticals, Rochester, NY) 2 times per day and polyethylene glycol eye drops (Alcon Laboratories, Inc.) 4 times per day for 1 month.
2.4. Refractive outcomes
Visual parameters of all patients were assessed both preoperatively and postoperatively. The preoperative corrected distance visual acuity (CDVA) and postoperative uncorrected distance visual acuity (UDVA) were recorded as the logarithm of the minimum angle of resolution (logMAR). Meanwhile, the preoperative central corneal thickness was assessed using an ultrasound pachymeter (Advent; Mentor O & O, Inc., Norwell, MA). Intraocular pressure (IOP) was assessed using Schiotz indentation tonometer (Schiotz, John Weiss & Son Ltd, London, UK) both preoperatively and postoperatively. Moreover, pre- and postoperative refraction was evaluated using the Auto Kerato-Refractometer (ARK-510A; NIDEK, Hiroishi, Japan). The evaluation of total wavefront aberrations was conducted using SCHWIND Corneal and Ocular Wavefront Analyzers.
In addition, the modulation transfer function (MTF) cut-off frequency and objective scattering index (OSI) were measured by Optical Quality Analysis System (OQAS, Visiometrics, Terrassa, Spain) at 1 day, 1 week, 1 and 3 months postoperatively. All estimations were carried out under mesopic conditions with a pupil diameter of 4.0 mm. All patients wore glasses to avoid the influence of their refractive error. A 0.01 MTF value was set as the criterion for the MTF cut-off frequency of the double-pass instrument. The OSI was calculated as the ratio of the amount of light within an annular area between 12 and 20 minutes of the arc compared to that recorded at the central peak of the arc within 1 minute in the acquired double-pass image. An OSI value close to 1.0 is usually recorded in eyes with low scattering.[12,13]
2.5. Statistical analyses
All data are shown as mean ± standard deviation (SD). Intergroup and intra-group comparisons were performed using Student's t -test. P < .05 was set as the cut-off for significant differences between groups.
3. Results
3.1. Preoperative parameters
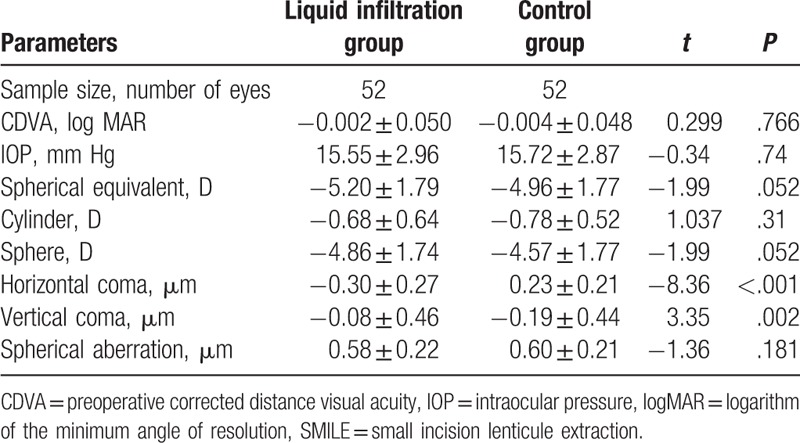
Demographics of the included patients are summarized in Table 1. The analytical results showed no significant intergroup differences in terms of logMAR CDVA (P = .766), IOP (P = .74), spherical equivalent (t = −1.99, P = .052), cylinder (P = .31), sphere (P = .052), and spherical aberration (P = .181); however, intergroup differences were detected in terms of horizontal coma (P < .001) and vertical coma (P = .002).
Table 1.
Preoperative parameters.
3.2. Efficacy and safety
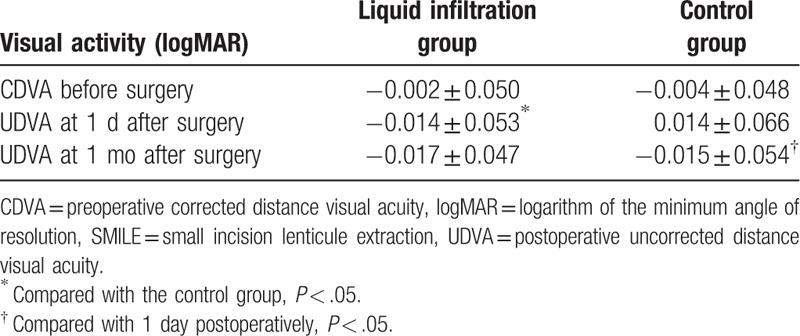
As shown in Table 2, no significant intergroup differences were identified in preoperative CDVA (P = .843) and 1-month postoperative UDVA (P = .847), while a significant decrease was detected in the 1 day postoperative UDVA in liquid infiltration group than in the control group (P = .023). However, when compared with preoperative CDVA, both 1-day and 1-month postoperative UDVA showed no remarkable alterations in both the liquid infiltration (P = .278, P = .103; respectively) and control group (P = .162, P = .261, respectively). Meanwhile, compared with 1-day postoperative UDVA, 1 month postoperative UDVA showed no obvious change in the liquid infiltration group (P = .532), whereas a significant reduction was identified in the 1-month postoperative UDVA in the control group (P = .008).
Table 2.
The logMAR CDVA and UDVA after SMILE.
3.3. Predictability
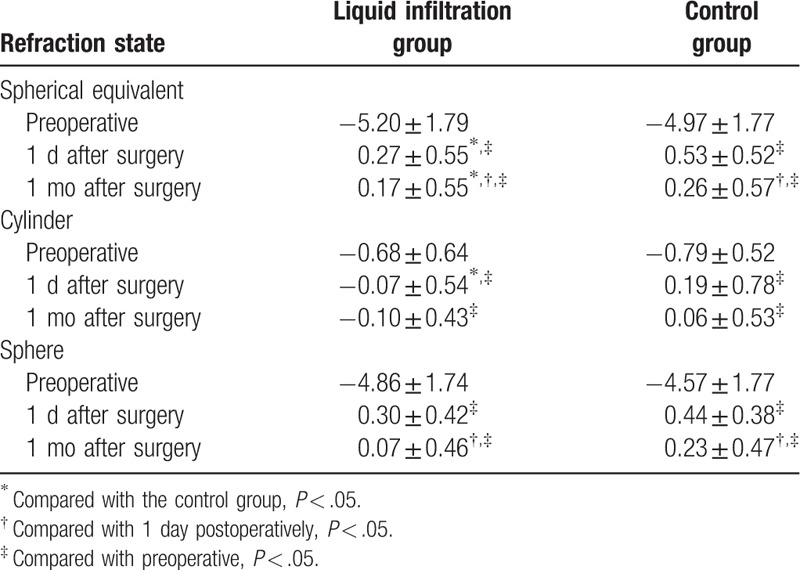
The changes in spherical equivalent, cylinder and sphere are tabulated in Table 3. Spherical equivalent showed no remarkable intergroup difference preoperatively (P = .498), but significant increases were identified 1 day (P = .013) and 1 month (P = −.031) after surgery in the liquid infiltration group than in the control group at the same time points. Meanwhile, notable increases in spherical equivalent were also obtained in both the groups at 1 day and 1 month after surgery than before surgery (all P < .001). However, spherical equivalents at 1 month after surgery in both the groups were significantly lower than the values at 1 day after surgery (both P < .001). Similar to the changes in spherical equivalent, the cylinder in the liquid infiltration group was not obviously different from the control group before surgery (P = .38) and 1 month after surgery (P = .10), but it was lower than that in the control group at 1 day after surgery (P = .048). Meanwhile, notable increases were observed 1 day and 1 month after surgery rather than before surgery in both the groups (all P < .001). However, no marked differences identified in the comparisons between 1-day and 1-month postoperative values in both the liquid infiltration (P = .63) and control groups (P = .17). Moreover, the sphere showed no remarkable intergroup differences before surgery (P = .77), as well as 1 day (P = .32) and 1 month (P = .79) after surgery. Nevertheless, remarkable changes were identified in the sphere at 1 day and 1 month after surgery rather than before surgery in both the groups (all P < .004). Similar changes were also observed in the comparisons between 1-day and 1-month postoperative values in the liquid infiltration and control group (both P < .001).
Table 3.
The changes of spherical equivalent and cylinder after SMILE.
The cumulative percentage of eyes with different refractions was calculated (Fig. 1). No significant intergroup differences were identified in the spherical equivalent and sphere before surgery (P > .05), while a marked discrepancy was detected in the preoperative cylinder between the groups (P = .02). The cumulative percentage of eyes with different refractions showed a markedly changed after surgery. For the spherical equivalent (Fig. 1A), the percentage of eyes in the range of −0.5 to 0.5 D in the liquid infiltration group was significantly higher than that in the control group at 1 day postoperation (P = .003) and at 1 month postoperation (P = .046). Meanwhile, in terms of the cylinder (Fig. 1B), the percentages of eyes was significantly higher in the range of −0.5 to 0.5 D (P = .022) and −0.25 to 0.25 D (P = .030) than in the control group. Moreover, in terms of the sphere, the percentage of eyes was also higher in the range of −0.25 to 0.25 D in the liquid infiltration group than in the control group at 1 month after surgery (P = .018, Fig. 1C).
Figure 1.
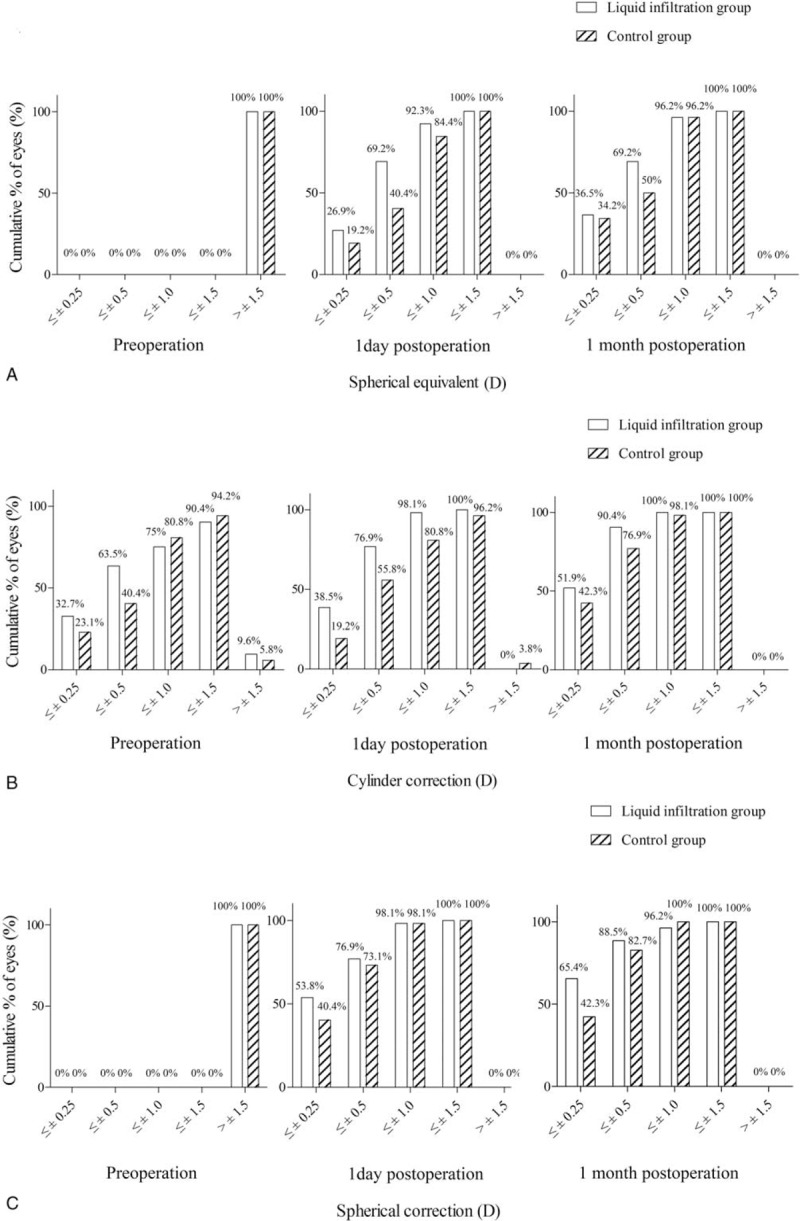
Cumulative percentage of eyes that achieved definite cumulative levels of UDVA over 1 month of follow-up. All eyes in both the groups were included. (A) Spherical equivalent; (B) cylinder; (C) sphere. UDVA = uncorrected distance visual acuity.
3.4. Wavefront aberrations
Wavefront aberrations were also evaluated (Fig. 2). Horizontal comas in the liquid infiltration group were significantly inferior to those in the control group (all P < .05) except 3 months after surgery. Meanwhile, the horizontal comas after surgery were all markedly higher than those before surgery in both groups (all P < .05), except at 3 months after surgery in the liquid infiltration group (Fig. 2A). The vertical coma showed no significant intergroup differences before and after surgery, except 1 month after surgery (P = .04). Moreover, changes in vertical coma were also observed in both the groups at all time points after surgery than before surgery (all P < .05), except at 1 week after surgery in the control group (Fig. 2B). In addition, no intergroup differences were detected in spherical aberration at any examined time points, but the spherical aberrations after surgery in both the groups were markedly higher than those before surgery (all P < .05). Meanwhile, the spherical aberrations at 3 months after surgery in both the groups were significantly lower than those at 1 week and 1 month after surgery (all P < .05, Fig. 2C).
Figure 2.
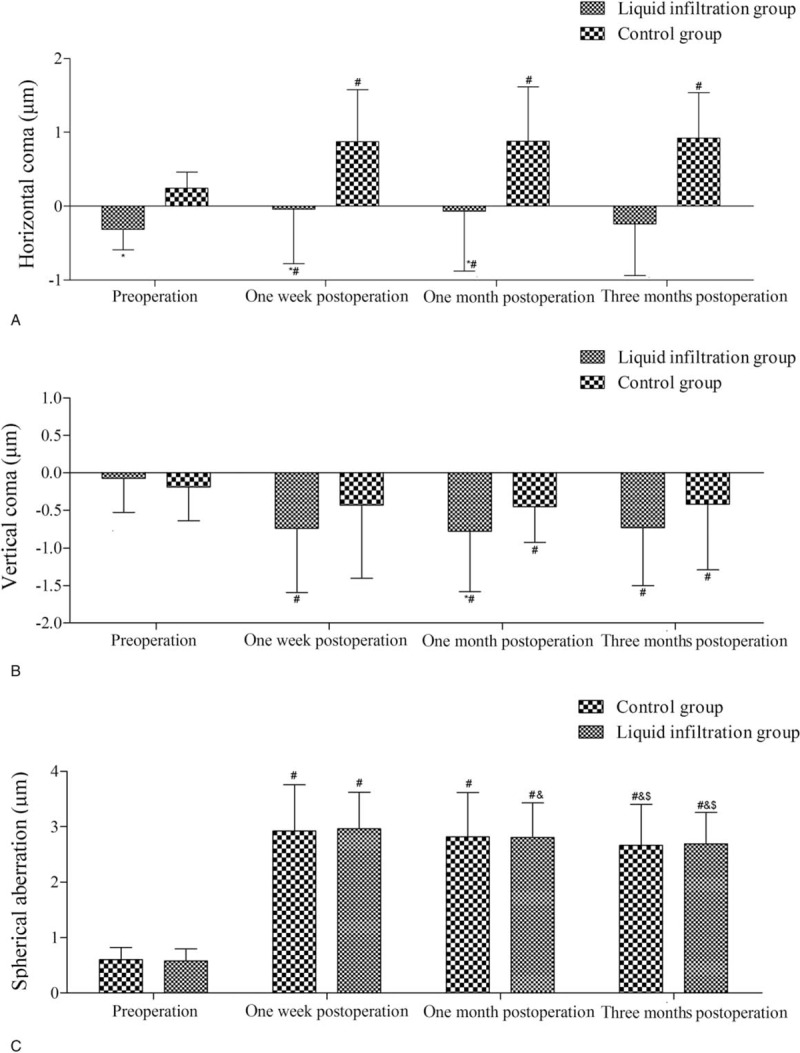
Preoperative and postoperative corneal aberrations of the eyes in both groups. (A) Horizontal coma; (B) vertical coma; (C) spherical aberration. ∗Compared with the control group, P < .05; #compared with preoperative, P < .05; &compared with 1 week postoperatively, P < .05; $compared with 1 month postoperatively, P < .05.
3.5. IOP
The preoperative and postoperative IOPs are shown in Fig. 3. No significant intergroup differences in IOP were identified before surgery (P > .05). After surgery, however, the IOPs in both the group reduced significantly at 1 month after surgery (liquid infiltration group, 12.49 ± 2.61, P < .001; control group, 12.45 ± 2.42, P < .01), but no marked intergroup difference was observed in postoperative IOP (P = .936).
Figure 3.
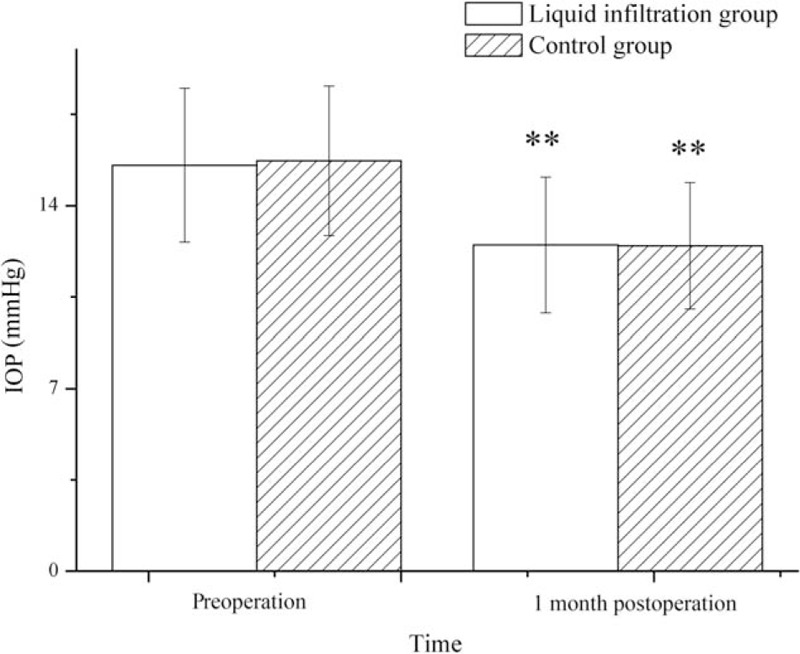
Preoperative and postoperative intraocular pressure in both the groups. IOP = intraocular pressure. ∗∗Compared with preoperative, P < .01.
3.6. Optical quality parameters
As shown in Fig. 4A, an obviously higher OSI was observed in the control group than in the liquid infiltration group at 1 day (P = .016), 1 week (P = .004), and 1 month (P = .028) after surgery. However, no remarkable intergroup difference in the OSI was detected at 3 months after surgery (P = .085). The OSIs were markedly reduced at 1 week, 1 month, and 3 months after surgery than at 1 day after surgery in both the group; similar results were obtained in the comparisons between 1 week and 1 month after surgery in both groups (all P < .05).
Figure 4.
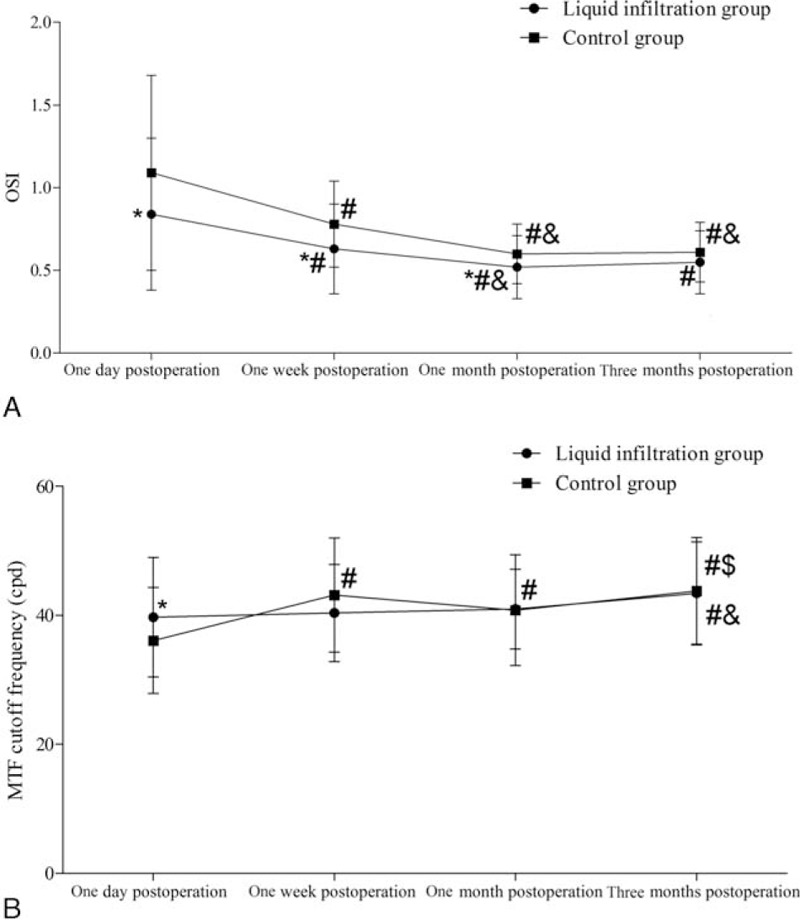
The OSI and MTF cut-off frequency in both the groups. (A) OSI; (B) MTF cut-off frequency. cpd = cycles/degree, MTF = modulation transfer function, OSI = objective scattering index. ∗Compared with the control group, P < .05; #compared with 1 day postoperatively, P < .05; &compared with 1 week postoperatively, P < .05; $compared with 1 month postoperatively, P < .05.
The MTF cut-off frequency was also evaluated (Fig. 4B). However, a significant intergroup difference was only identified at 1 day after surgery (P = .04). Meanwhile, marked changes in the control group were detected at 1 week, 1 month, and 3 months after surgery than at 1 day after surgery (P < .05). Similar results were obtained in the comparison between 1 week and 3 months after surgery (P < .05), but significant intergroup difference was only found in the comparisons between 1 day or 1 week and 3 months after surgery (all P < .05).
4. Discussion
In the present study, we performed a modified SMILE procedure with balanced salt solution infiltration. We also compared the outcomes of traditional and modified SMILE.
CDVA alteration is a direct result of SMILE. In this study, the logMAR UDVA in the control group was markedly higher than that in liquid infiltration group at 1 day after surgery, but no obvious intergroup difference was identified at 1 month after surgery. This indicates that both the modified and traditional SMILE procedure could be used for vision correction; however, for immediate recovery and better surgical outcomes, the modified procedure, that is, liquid infiltration, should be preferred. Similarly, the balanced salt solution before lenticule separation can also be considered a corneal wetting agent during SMILE procedure, and it may be help reduce damage to the corneal epithelium during surgery.[14,15] Moreover, warm balanced salt solution can be used for clearing tear film lipids.[16] Tear film stability is known to be associated with visual quality.[17,18] Furthermore, Chen et al[19] have reported that the balanced salt solution could maintain optical clarity during surgery, and this would improve the efficacy of SMILE for correcting myopia and myopic astigmatism.
Remarkable intergroup differences were detected at 1 day and 1 month after surgery in the spherical equivalent and cylinder, but not in the sphere. This result indicated that the liquid infiltration during SMILE could significantly improve the correction of myopia and astigmatism in patients. Previously, the balanced salt solution has been used as a corneal wetting agent during routine cataract extraction and lens implantation,[20] and it may also perform the same protective function during SMILE for correcting myopia and astigmatism. Therefore, the balanced salt solution infiltration may play a role in protecting cornea and maintaining tear film stability. This protection might produce a better outcome in term of the stability of recovered vision in a short term. In addition, Leitritz et al[21] have found balanced salt solution could prevent early postoperative changes on the foveal surface of epiretinal membranes during macular surgery, which may be the main reason for the better early visual quality in the liquid infiltration group than in the control group. In terms of the OSI and MTF cut-off frequency, better outcomes were also observed in the liquid infiltration group than in the control group at 1 day, 1 week, and 1 month after surgery but not at 3 months after surgery. Furthermore, the changes in horizontal coma were less remarkable in the liquid infiltration group than in the control group. These results indicated that the speed of visual recovery was faster in the liquid infiltration group than in the control group. Thus, we believe that the visual outcomes were better in the liquid infiltration group than in the control group because less corneal epithelial damage and better tear film stability resulting from the using the balanced salt solution.
Nonetheless, this study has some limitations. First, the preoperative horizontal coma was significantly different between the 2 groups, and this may have affected the stability and reliability of the study results. Second, the sample size was small and hence, further studies with larger sample sizes are need to verify our results. Third, the follow-up time after surgery was also short and long-term follow-up is necessary to assess the stability of the clinical outcomes in both the groups. In addition, further studies are needed to evaluate corneal sensitivity,[22,23] which may affect the refractive safety, efficacy, and stability.
5. Conclusions
Balanced salt solution infiltration dissection is a promising medical technique for treating myopia and astigmatism by using SMILE, which might produce better visual outcomes and faster recovery than traditional lenticule dissection does.
Footnotes
Abbreviations: BBS = balanced buffer solution, CDVA = corrected distance visual acuity, IOP = intraocular pressure, LASIK = laser-assisted in situ keratomileusis, logMAR = logarithm of the minimum angle of resolution, MTF = modulation transfer function, OSI = objective scattering index, SD = standard deviation, SMILE = small-incision lenticule extraction, UDVA = uncorrected distance visual acuity.
The authors have no conflicts of interest to disclose.
References
- [1].Wen D, McAlinden C, Flitcroft I, et al. Post-operative efficacy, predictability, safety and visual quality of laser corneal refractive surgery: a network meta-analysis. Am J Ophthalmol 2017;178:65–78.28336402 [Google Scholar]
- [2].Lee JK, Chuck RS, Park CY. Femtosecond laser refractive surgery: small-incision lenticule extraction vs. femtosecond laser-assisted LASIK. Curr Opin Ophthalmol 2015;26:260–4. [DOI] [PubMed] [Google Scholar]
- [3].Demirok A, Agca A, Ozgurhan EB, et al. Femtosecond lenticule extraction for correction of myopia: a 6 month follow-up study. Clin Ophthalmol 2013;7:1041–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Raoof D, Pineda R, editors. Dry Eye after Laser In-Situ Keratomileusis. Seminars in Ophthalmology; 2014. [DOI] [PubMed] [Google Scholar]
- [5].Denoyer A, Landman E, Trinh L, et al. Dry eye disease after refractive surgery: comparative outcomes of small incision lenticule extraction versus LASIK. Ophthalmology 2015;122:669–76. [DOI] [PubMed] [Google Scholar]
- [6].Reinstein DZ, Gobbe M, Gobbe L, et al. Optical zone centration accuracy using corneal fixation-based SMILE compared to eye tracker-based femtosecond laser-assisted LASIK for myopia. J Refract Surg 2015;31:586–92. [DOI] [PubMed] [Google Scholar]
- [7].Park DH, Kim IT. A case of accidental macular injury by Nd: YAG laser and subsequent 6 year follow-up. Korean J Ophthalmol 2009;23:207–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Meltendorf C, Burbach GJ, Bühren J, et al. Corneal femtosecond laser keratotomy results in isolated stromal injury and favorable wound-healing response. Invest Ophthalmol Vis Sci 2007;48:2068–75. [DOI] [PubMed] [Google Scholar]
- [9].Karim MN, Riau AK, Lwin NC, et al. Early corneal nerve damage and recovery following small incision lenticule extraction (SMILE) and laser in situ keratomileusis (LASIK). Invest Ophthalmol Vis Sci 2014;55:1823–34. [DOI] [PubMed] [Google Scholar]
- [10].Blum M, Kunert K, Schröder M, et al. Femtosecond lenticule extraction for the correction of myopia: preliminary 6-month results. Graefes Arch Clin Exp Ophthalmol 2010;248:1019–27. [DOI] [PubMed] [Google Scholar]
- [11].Shah R, Shah S, Sengupta S. Results of small incision lenticule extraction: all-in-one femtosecond laser refractive surgery. J Cataract Refract Surg 2011;37:127–37. [DOI] [PubMed] [Google Scholar]
- [12].Artal P, Benito A, Pérez GM, et al. An objective scatter index based on double-pass retinal images of a point source to classify cataracts. PLoS ONE 2010;6:e16823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Martínez-Roda JA, Vilaseca M, Ondategui JC, et al. Optical quality and intraocular scattering in a healthy young population. Clin Exp Optom 2010;94:223–9. [DOI] [PubMed] [Google Scholar]
- [14].Chen YT, Tseng SH, Ma MC, et al. Corneal epithelial damage during LASIK: a review of 1873 eyes. J Refract Surg 2007;23:916–23. [DOI] [PubMed] [Google Scholar]
- [15].Mccally RL, Bargeron CB. Corneal epithelial injury thresholds for multiple-pulse exposures to Tm:YAG laser radiation at 2.02 microm. Health Phys 2003;85:420–7. [DOI] [PubMed] [Google Scholar]
- [16].Otto CS, Mcmann MA, Parmley VC, et al. Warm balanced salt solution for clearing tear film precipitation during cataract surgery. J Cataract Refract Surg 2002;28:1318–9. [DOI] [PubMed] [Google Scholar]
- [17].Chen S, Wang IJ. Effect of tear film stability on fluctuation of vision after photorefractive keratectomy. J Refract Surg 1999;15:668–72. [DOI] [PubMed] [Google Scholar]
- [18].Gonzalez-Garcia M, Lopez-De-La-Rosa A, Martin V, et al. Analysis of visual function and tear film stability in contact lens wearers with one-day disposable contact lenses. Cont Lens Anterior Eye 2015;38:e29–30. [Google Scholar]
- [19].Chen YA, Hirnschall N, Findl O. Comparison of corneal wetting properties of viscous eye lubricant and balanced salt solution to maintain optical clarity during cataract surgery. J Cataract Refract Surg 2011;37:1806–8. [DOI] [PubMed] [Google Scholar]
- [20].Arshinoff SA, Khoury E. HsS versus a balanced salt solution as a corneal wetting agent during routine cataract extraction and lens implantation. J Cataract Refract Surg 1997;23:1221–5. [DOI] [PubMed] [Google Scholar]
- [21].Leitritz MA, Ziemssen F, Voykov B, et al. Early postoperative changes of the foveal surface in epiretinal membranes: comparison of 23-gauge macular surgery with air vs. balanced salt solution. Graefes Arch Clin Exp Ophthalmol 2014;252:1213–9. [DOI] [PubMed] [Google Scholar]
- [22].Li M, Zhao J, Shen Y, et al. Comparison of dry eye and corneal sensitivity between small incision lenticule extraction and femtosecond LASIK for myopia. PLoS ONE 2013;8:e77797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Li M, Zhou Z, Shen Y, et al. Comparison of corneal sensation between small incision lenticule extraction (SMILE) and femtosecond laser-assisted LASIK for myopia. J Refract Surg 2014;30:94–100. [DOI] [PubMed] [Google Scholar]


