Supplemental Digital Content is available in the text
Keywords: acute gouty arthritis, delta neutrophil index, differential diagnosis, septic arthritis
Abstract
The most important differential diagnoses of acute monoarticular arthritis are septic arthritis and acute gout attack. Identifying infection is crucial in preventing the devastating outcome of septic arthritis. The delta neutrophil index (DNI) is a value that corresponds to the fraction of circulating immature granulocytes. As DNI reflects the burden of infection, we evaluated this index as a differentiating marker between septic arthritis and acute gout attack.
The medical records of 149 patients with septic arthritis and 194 patients with acute gout attack were reviewed. A specific cell analyzer, ADVIA 2120, was used to measure DNI. Clinical and laboratory markers associated with predicting septic arthritis were assessed by using logistic regression.
Patients with septic arthritis showed higher levels of DNI than those with acute gout attack (3.3 vs 0.6%, P < .001). Similar results were observed in patients without monosodium urate (MSU) crystal confirmation or those with normouricemia (3.3 vs 0.5 and 3.1 vs 0.7%, respectively; P < .001 for both). A DNI level of 1.9% was determined as the cutoff value for predicting septic arthritis. In the multivariate analysis, DNI was the most powerful independent value for predicting septic arthritis (odds ratio 14.003).
This study showed the possibility of using DNI as a differentiating marker between septic arthritis and acute gout attack at the crucial early phase. DNI showed its relevance regardless of confirmation of MSU crystal deposition or serum level of uric acid.
1. Introduction
Gouty arthritis is the most common inflammatory arthritis with its prevalence markedly increased in the last decade.[1] A typical gout attack is monoarticular arthritis characterized by acute, intense pain preferentially involving the lower extremities. Septic arthritis also presents with single joint pain and effusion; however, there has been no clear manner suggested to distinguish septic arthritis from acute gout attack based on physical examination. Differentiating septic arthritis from acute gout attack is the most important step in managing a patient with acute monoarthritis, as septic arthritis is a crucial joint emergency that can be rapidly disabling or life-threatening for patients. However, in real clinical settings, differentiating these 2 disease entities is a challenge, especially when arthrocentesis is contraindicated or when polarizing microscope is not available, including in most primary care units.
Considering the devastating outcome of misdiagnosis, reliable markers to diagnose septic arthritis are critically needed. However, neither serum markers such as white blood cell (WBC) count, C-reactive protein (CRP), or erythrocyte sedimentation rate (ESR) nor radiologic images can exclude the possibility of septic arthritis in a patient with acute monoarticular arthritis.[2] In a study of 75 patients with acute monoarthritis, WBC > 100,000/μ or ESR >30 mm/h or CRP >100 mg/L increased the likelihood of septic arthritis only minimally (likelihood ratio [LR] 1.4, 95% confidence interval [CI] 1.1–1.8; LR 1.3, 95% CI 1.1–1.8; LR 1.6, 95% CI 1.1–2.5, respectively).[3,4] Furthermore, patients with acute gout attack may also present with elevated CRP, indicating that serum levels of acute phase reactants do not give reliable cutoffs for distinguishing between the 2 diseases. Moreover, synovial fluid analysis of both septic arthritis and gouty arthritis can present with a WBC count of >50,000/mm3, and the decreased synovial glucose level has failed to lead to a diagnosis of septic arthritis.[5,6]
The gold standard for diagnosing septic arthritis is to detect the microorganism in the synovial fluid of the infected joint; however, the result requires a few days and a negative finding does not exclude the diagnosis, as the sensitivity of the test may be only 50% to 80%.[7,8] The high serum uric acid level and a history of gout raise the possibility of gouty arthritis; however, septic arthritis can be superimposed on gouty arthritis, implying that physicians should always consider septic arthritis in the differential diagnosis. Therefore, a novel serum marker for distinguishing the 2 diseases would be useful in clinical practice especially in the emergency department.
Infection promotes the production of granulocytes in the bone marrow, resulting in the presence of immature granulocytes (IGs).[9,10] The diagnostic and prognostic significance of elevated peripheral IGs on infection and sepsis has been shown.[11–13] However, manual counting of IGs requires considerable time and effort, and the accuracy is highly dependent on the examiner. The delta neutrophil index (DNI) is a novel index reflecting a circulating fraction of IGs that is calculated automatically by using a cell analyzer which is reported in routine complete blood count with differentials. It is calculated by the formula as follows: delta neutrophil index = (neutrophil [%] + eosinophil [%] – polymorphonuclear neutrophil [PMN][%]). Previous studies have demonstrated that DNI well correlated with IGs and showed its relevance as a predictive and prognostic marker of sepsis. Elevated DNI was well associated with an increased rate of disseminated intravascular coagulation, bacteremia, and mortality in patients with sepsis.[14–16] To date, there are no data on DNI in relation to septic arthritis. On the basis of the background that DNI is associated with infection, we evaluated the usefulness of DNI as a simple index for differentiating septic arthritis from acute gout attack in this study.
2. Patients and methods
2.1. Patients and data collection
We reviewed the data of 411 patients with a diagnosis of septic arthritis and 335 patients with a diagnosis of acute gout attack. All patients had been admitted to Yonsei University Health System, Severance Hospital, from January 2010 to August 2016. A clinical data retrieval system was used to screen patients with the 2 diseases, and we reviewed the medical records to identify their final diagnoses. We finally recruited 149 patients with septic arthritis and 194 patients with acute gout attack based on the inclusion criteria. At the entry of this retrospective study, we enrolled patients with acute gout attack who met the 1977 American College of Rheumatology (ACR) classification criteria for acute arthritis of primary gout,[17] and we included patients with septic arthritis who had a positive synovial fluid culture or those who had definite macroscopic pus discharge as an operative finding.[2] All patients were over 18 years or age and they had their blood test done before antibiotics or anti-gout medication administration within 24 hours after hospitalization.
We excluded 121 patients who concomitantly had other infections such as pneumonia and acute pyelonephritis; 105 patients who received antibiotics or anti-gout medications before the laboratory test; 81 patients who did not fulfill the 1977 ACR criteria; 61 patients who were suspected to have septic arthritis, but fell short of the inclusion criteria; and 35 patients who had hematological diseases or malignancies or who had received chemotherapy or granulocyte colony-stimulating factor. We reviewed the operative findings and pathological evaluation of patients who had undergone surgery, as well as the bacterial cultures from synovial fluid and blood or any other body fluid where infection had been suspected (Supplementary Figure 1). Institutional review board approval was obtained by Yonsei University, College of Medicine (ref. 4-2015-0949).
2.2. Subgroup analysis
We divided 194 patients with acute gout attack into 2 subgroups according to the presence of MSU crystals, to evaluate the usefulness of DNI irrespective of crystal confirmation. A total of 113 of 194 (58.2%) patients were classified as “patients with acute gout attack without MSU confirmation,” defined as patients who did not undergo synovial fluid aspiration. Furthermore, we gathered patients with septic arthritis and acute gout attack whose serum uric acid levels were below 7.0 mg/dL and analyzed the difference of DNI. Of the patients, 140 of 149 (94.0%) were classified as “patients with normouricemic septic arthritis.” Finally, 79 of 194 (40.7%) patients were classified as “patients with acute normouricemic gout attack.”
2.3. DNI measurement
DNI was measured by using a specific automatic cell analyzer (ADVIA 2120 Healthcare Diagnostics, Forchheim, Germany) as in our previous study.[18] This cell analyzer counts WBC in 2 independent channels, myeloperoxidase (MPO) and nuclear lobularity channels. DNI is calculated by using the following formula: DNI (%) = (the neutrophil subfraction and the eosinophil subfraction measured in the MPO channel by cytochemical MPO reaction) – (PMN subfraction measured in the nuclear lobularity channel by the reflected light beam).[19] The DNI is obtained automatically by subtracting the mature PMN leukocytes from the MPO-reactive cells. Our institution provides DNI as a part of routine complete blood count.
2.4. Statistical analysis
IBM SPSS software version 23.0 was used to perform statistical analyses. Data are expressed as means ± standard deviation or absolute and relative frequencies (%). The t-test or chi-square test was used to assess the differences between acute gout attack and septic arthritis. As DNI was non-normally distributed, group differences of DNI were assessed using the Mann–Whitney U test. For the variables which showed significant differences between the 2 groups (WBC count, DNI, ESR, CRP, and uric acid), we used the area under the receiver operator characteristic (AUROC) curve to determine the optimal cutoffs for predicting septic arthritis. We performed multivariate logistic regression analysis to evaluate the independent predictive value for septic arthritis among the variables which showed significant differences in univariate analysis or had clinical implications. A P-value of < .05 was considered statistically significant.
3. Results
3.1. Comparison of variables between patients with septic arthritis and those with acute gout attack
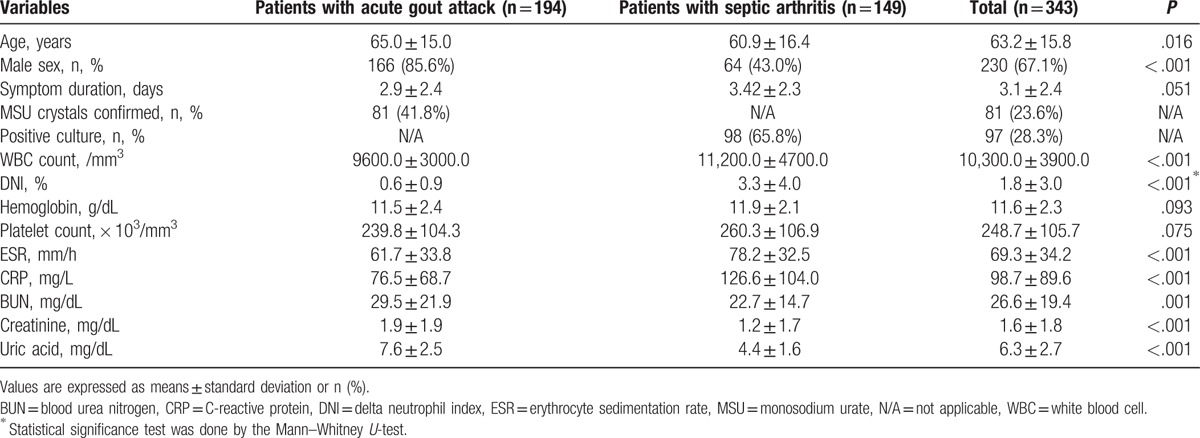
The mean age and male prevalence of patients with acute gout attack were higher than those with septic arthritis. Eighty-one of 194 (41.8%) patients with acute gout attack had MSU crystal confirmation, whereas 98 of 149 (65.8%) patients with septic arthritis had positive synovial cultures. Among 149 patients with septic arthritis, 34 (22.8%) had bacteremia and 136 (91.3%) underwent surgery. Patients with septic arthritis had significantly higher WBC counts, and ESR and CRP levels but lower uric acid levels than those with acute gout attack (P < .01 for all). The mean DNI was significantly higher in patients with septic arthritis than those with acute gout attack (3.3 vs 0.6%; P < .001) (Table 1). To assess the influence of bacterial burden on DNI, we divided the patients with septic arthritis into 3 subgroups: septic arthritis with negative cultures (n = 51), septic arthritis with positive synovial cultures (n = 66), and septic arthritis with bacteremia (n = 34). Patients with acute gout attack had lower DNI than all the subgroups of septic arthritis (0.6% for acute gout attack vs 2.3% for septic arthritis with negative culture results vs 2.4% for septic arthritis with positive synovial culture vs 6.3% for septic arthritis with bacteremia, P < .001). Among patients with septic arthritis, patients with bacteremia showed higher DNI than those without bacteremia, implying that DNI increases as the bacterial burden becomes heavier (Supplementary Figure 2).
Table 1.
Comparison of variables between patients with acute gout attack and those with septic arthritis.
3.2. Optimal cutoffs of variables and multivariate analysis predicting septic arthritis
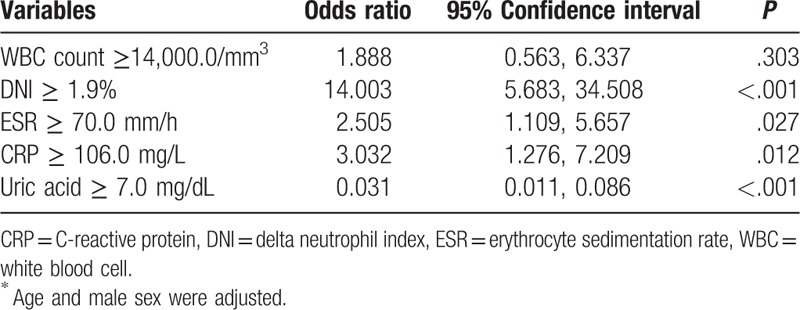
We selected the optimal cutoffs for predicting septic arthritis among variables which showed significant differences between the 2 diseases as follows: WBC ≥ 14,000/mm3 (AUC 0.588, 95% confidence interval [CI] 0.525, 0.650), DNI ≥ 1.9% (AUC 0.784, 95% CI 0.732, 0.836), ESR ≥ 70.0 mm/h (AUC 0.941, 95% CI 0.581, 0.702), CRP ≥ 106.6 mg/L (AUC 0.652, 95% CI 0.593, 0.711), and uric acid of 7.0 mg/dL (the point widely used for hyperuricemia in clinical practice). In multivariate regression analysis, DNI ≥ 1.9% sustained its predictive value for predicting septic arthritis after adjusting for age and sex (OR 14.003, 95% CI 5.683, 34.508, P < .001). Moreover, ESR ≥ 70 mm/h and CRP ≥ 106.0 mg/L showed their value for predicting septic arthritis, but the impact was weaker than that of DNI (OR 2.505, 95% CI 1.109, 5.657, P = .027; OR 3.032, 95% CI 1.276, 7.209, P = .012, respectively). Furthermore, uric acid ≥ 7.0 mg/dL was a negative predictive value for septic arthritis (OR 0.031, 95% CI 0.011, 0.086, P < .001) (Table 2).
Table 2.
Multivariate analysis of the predictive values for septic arthritis in patients with acute gout attack and those with septic arthritis∗.
3.3. Comparison of patients with septic arthritis and those with acute gout attack without MSU confirmation
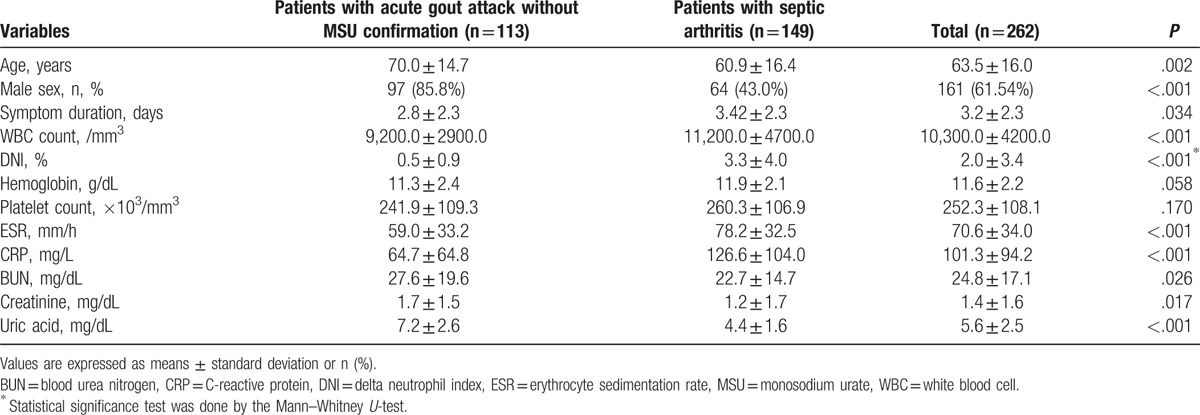
Analyses comparing patients with septic arthritis and those with acute gout attack without MSU confirmation showed similar results. Patients with septic arthritis showed higher WBC counts, ESR, and CRP, but lower serum uric acid level than those with acute gout attack without MSU confirmation (P < .001 for all). The DNI was higher in patients with septic arthritis than in those with acute gout attack without MSU confirmation like the preceding results (3.3 vs 0.5%, P < .001) (Table 3).
Table 3.
Comparison of variables between patients with acute gout attack without monosodium urate confirmation and those with septic arthritis.
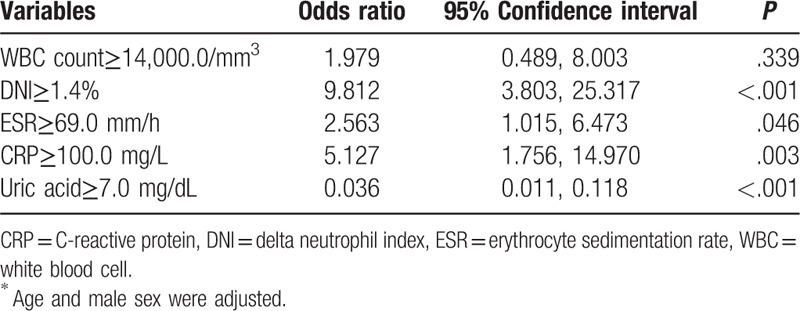
By using AUROC, we determined the optimal cutoff values of the variables which showed significant differences for predicting septic arthritis: WBC ≥ 14,000/mm3 (AUC 0.620, 95% CI 0.553, 0.687), DNI ≥ 1.4% (AUC 0.800, 95% CI 0.746, 0.854), ESR ≥ 69.0 mm/h (AUC 0.664, 95% CI 0.593, 0.734), and CRP ≥ 100.0 mg/L (AUC 0.689, 95% CI 0.624, 0.753). The uric acid level of 7.0 mg/dL was again used as the cutoff value again. The results of the multivariate regression model were similar among all patients. DNI ≥ 1.4% had an influence on predicting septic arthritis (OR 9.812, 95% CI 3.803, 25.317, P < .01) stronger than ESR ≥ 69.0 mm/h (OR 2.563, 95% CI 1.015, 6.473, P = .046) or CRP ≥ 100.0 mg/L (OR 5.127, 95% CI 1.756, 14.970, P = .003). Uric acid ≥ 7.0 mg/dL had a negative predictive value for septic arthritis (OR 0.036, 95% CI 0.011, 0.118, P < .001) (Table 4).
Table 4.
Multivariate analysis of the predictive values for cellulitis in patients with acute gout attack without monosodium urate confirmation and those with septic arthritis∗.
3.4. Comparison of patients with normouricemic septic arthritis and those with acute normouricemic gout attack
Similarly, patients with normouricemic septic arthritis showed higher WBC counts, ESR, and CRP, but lower serum uric acid level than those with acute normouricemic gout attack (P < .005 for all). The DNI was higher in patients with septic arthritis than in those with acute gout attack (3.1 vs 0.7%, P < .001) (Table 5).
Table 5.
Comparison of variables between patients with acute normouricemic gout attack and those with normouricemic septic arthritis.
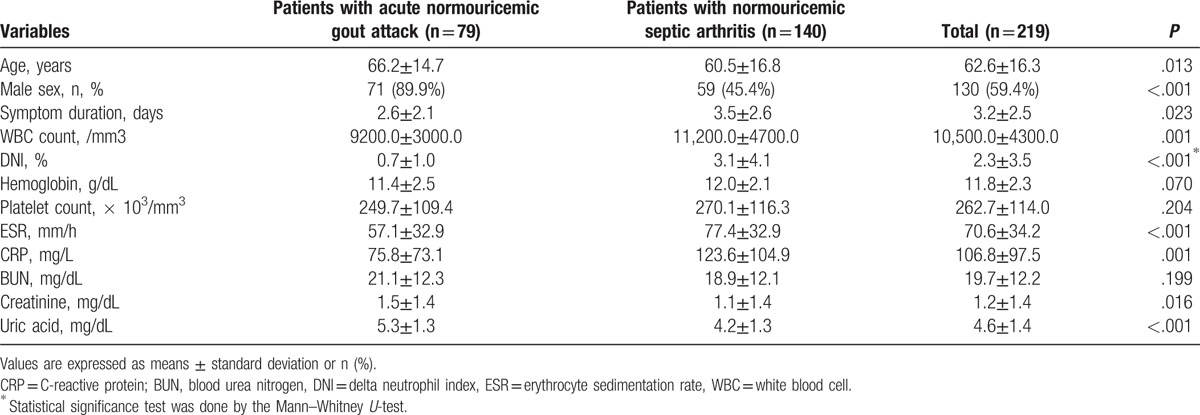
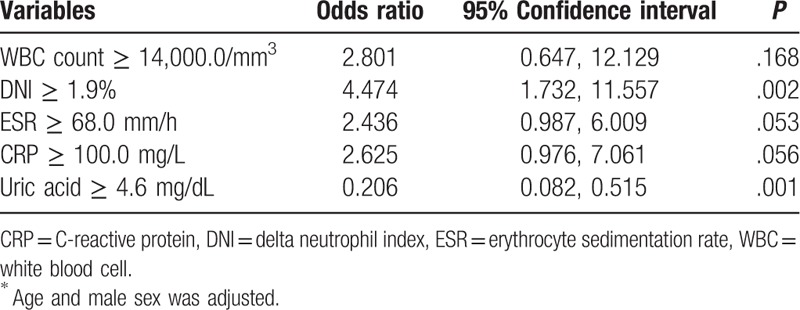
The optimal cutoff values of variables having significant differences between the 2 diseases were assessed by using AUROC: WBC ≥ 14,000/mm3 (AUC 0.616, 95% CI 0.542, 0.691), DNI ≥ 1.9% (AUC 0.757, 95% CI 0.694, 0.820), uric acid ≥ 4.6 mg/dL (AUC 0.268, 95% CI 0.196, 0.340), and CRP ≥ 100.0 mg/L (AUC 0.645, 95% CI 0.570, 0.721). Among these variables which showed statistical differences, DNI ≥ 1.9% had an independent predictive value for septic arthritis (OR 4.474, 95% CI 1.732, 11.557, P = .002) (Table 6).
Table 6.
Multivariate analysis of the predictive values for cellulitis in patients with acute normouricemic gout attack and those with normouricemic septic arthritis∗.
4. Discussion
The most important differential diagnoses in patients with acute monoarthritis are septic arthritis and crystal-induced arthritis, because their treatment strategies differ. This study presents a novel serum marker that differentiates septic arthritis from acute gout attack. To date, there has been no single serum marker which can discriminate these 2 disease entities. Commonly used acute-phase reactants, such as WBC counts with differentials, ESR, and CRP, have been considered for the differential diagnosis; however, there are no proper cutoff values to distinguish septic arthritis from acute gout attack.[20] We have suggested cutoffs for WBC counts, ESR, and CRP in the present study; however, after multivariate regression, WBC count lost its predictive value for septic arthritis and DNI had the most significant power in comparison with ESR or CRP. Similar results were found when patients without MSU confirmation were analyzed, which showed that WBC count lost its predictive value for septic arthritis and DNI was the most powerful predictive value compared with ESR or CRP. Furthermore, among patients with normouricemia, along with WBC counts, ESR and CRP also lost their predictive value after multiple regression analyses. Only DNI maintained its predictive value for predicting septic arthritis, implying that DNI was the most potent factor for predicting septic arthritis.
To date, synovial fluid analysis with obtaining gram stain and culture results is the gold standard test for diagnosing septic arthritis in clinical practice. However, as it usually takes more than 2 days to confirm the results, diagnosis of septic arthritis cannot be made promptly enough to minimize the subsequent devastating consequences. Moreover, synovial WBC counts and PMN cell counts are helpful for diagnosing septic arthritis, in which the likelihood of the disease increases as the synovial fluid WBC counts increases.[21,22] Nonetheless, these counts are insufficient to differentiate septic arthritis from acute gout attack because gouty arthritis also often presents with synovial fluid WBC count of >50,000/mm3 and PMN dominance. In fact, in our present population, 19 of 149 (12.8%) patients with septic arthritis presented with synovial WBC count less than 50,000/mm3 and 32 of 128 (25%) patients with acute gout attack who undergone synovial fluid analysis showed synovial WBC count more than 50,000/mm3. Therefore, there is a need for a laboratory modality complementary to traditionally used methods for diagnosing of septic arthritis.
Hyperuricemia is the key pathophysiology of gout; however, it does not rule out septic arthritis because two-thirds or more patients with hyperuricemia are asymptomatic and not presenting with gout.[23] In our data, the uric acid level showed a reasonable potential for diagnosing acute gout attack. However, ruling out septic arthritis is crucial in managing acute arthritis, and the possibility of the coexistence of the 2 diseases should always be considered. A previous study by Lenski and Scherer[6] also showed similar results presenting a significant difference of uric acid level between septic arthritis and acute gout attack. However, the authors concluded that the uric acid level may help in distinguishing acute gout attack from other inflammatory arthritis but is insufficient to rule out septic arthritis.
As DNI is the value reflecting the fraction of IGs, it is well correlated with sepsis severity, detection rate by blood cultures, disseminated intravascular coagulation scores, and mortality.[14–16] The present data also supported the findings that DNI increase becomes higher as the infection becomes more severe. The highest DNI was 6.1% in patients with bacteremia among those with septic arthritis. This result was well reproduced, similar to previously published studies, showing DNI levels of 5.2% to 6.5% as the cutoff values for bacteremia.[18,24] The mean DNI value was lower in patients with septic arthritis without bacteremia than in those with bacteremia; however, it was significantly higher than in patients with acute gout attack. Therefore, the present study demonstrated the possibility of using DNI as a differentiating marker between septic arthritis and acute gout attack.
Acute gout attack differs from septic arthritis in that acute gout attack is self-limited despite the presence of severe pain. Previous studies have observed increased levels of transforming growth factor beta-1 and interleukin-1 receptor antagonist in the synovial fluid when serially measured.[25,26] These anti-inflammatory cytokines might be a mechanism of spontaneous resolution of acute gout attack. On the other hand, septic arthritis needs immediate proper antibiotics, unless it devastates the affected joint and even leads to sepsis. This is the point where we suggest a hypothesis for the mechanism by which DNI differentiates between acute gout attack and septic arthritis. Without a self-limiting process such as acute gout attack, inflammation in patients with septic arthritis will sustain and activate the bone marrow continuously, resulting in increased DNI.
Several limitations of this study should be mentioned. First, this study was performed at a single center and the results were analyzed retrospectively; thus, further prospective analyses with a larger population are needed to confirm the value and accurately measure the sensitivity and specificity. Second, a specific type of cell analyzer is needed to measure the DNI, thus restricting its clinical application. Third, the variation of DNI according to time is unknown; thus, the most precise time point to measure DNI after the onset of symptoms is unclear. Last, there was no patient in this present study who had superimposed septic arthritis in a patient who previously had gout. Since it is critical to identify superimposed infection in a patient with gout, future researches would clarify these limitations and contribute to the utilization of DNI in real clinical practice.
In conclusion, this study demonstrates the relevance of DNI as a predictive value for septic arthritis in patients who are suspected as having acute gout attack. We propose that in patients with a DNI of ≥1.9%, the possibility of septic arthritis should always be considered with a high priority, especially in patients whose diagnosis is unclear between acute gout attack and septic arthritis.
Supplementary Material
Footnotes
Abbreviations: ACR = American College of Rheumatology, AUROC = area under the receiver operator characteristic, CI = confidence interval, CRP = C-reactive protein, DNI = delta neutrophil index, ESR = erythrocyte sedimentation rate, IGs = immature granulocytes, LR = likelihood ratio, MPO = myeloperoxidase, MSU = monosodium urate, PMN = polymorphonuclear neutrophil, WBC = white blood cell.
Funding: This study was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health and Welfare, Republic of Korea (HI14C1324).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Kuo CF, Grainge MJ, Zhang W, et al. Global epidemiology of gout: prevalence, incidence and risk factors. Nat Rev Rheumatol 2015;11:649–62. [DOI] [PubMed] [Google Scholar]
- [2].Margaretten ME, Kohlwes J, Moore D, et al. Does this adult patient have septic arthritis? JAMA 2007;297:1478–88. [DOI] [PubMed] [Google Scholar]
- [3].Jeng GW, Wang CR, Liu ST, et al. Measurement of synovial tumor necrosis factor-alpha in diagnosing emergency patients with bacterial arthritis. Am J Emerg Med 1997;15:626–9. [DOI] [PubMed] [Google Scholar]
- [4].Soderquist B, Jones I, Fredlund H, et al. Bacterial or crystal-associated arthritis? Discriminating ability of serum inflammatory markers. Scand J Infect Dis 1998;30:591–6. [DOI] [PubMed] [Google Scholar]
- [5].Carpenter CR, Schuur JD, Everett WW, et al. Evidence-based diagnostics: adult septic arthritis. Acad Emerg Med 2011;18:781–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lenski M, Scherer MA. Analysis of synovial inflammatory markers to differ infectious from gouty arthritis. Clin Biochem 2014;47:49–55. [DOI] [PubMed] [Google Scholar]
- [7].Krey PR, Bailen DA. Synovial fluid leukocytosis. A study of extremes. Am J Med 1979;67:436–42. [DOI] [PubMed] [Google Scholar]
- [8].Schlapbach P, Ambord C, Blochlinger AM, et al. Bacterial arthritis: are fever, rigors, leucocytosis and blood cultures of diagnostic value? Clin Rheumatol 1990;9:69–72. [DOI] [PubMed] [Google Scholar]
- [9].Cornbleet PJ. Clinical utility of the band count. Clin Lab Med 2002;22:101–36. [DOI] [PubMed] [Google Scholar]
- [10].Seebach JD, Morant R, Ruegg R, et al. The diagnostic value of the neutrophil left shift in predicting inflammatory and infectious disease. Am J Clin Pathol 1997;107:582–91. [DOI] [PubMed] [Google Scholar]
- [11].Borowitz MJ, Kickler TS, Ansari-Lari MA. Immature granulocyte measurement using the sysmex XE-2100 relationship to infection and sepsis. Am J Clin Pathol 2003;120:795–9. [DOI] [PubMed] [Google Scholar]
- [12].Nigro KG, O’Riordan M, Molloy EJ, et al. Performance of an automated immature granulocyte count as a predictor of neonatal sepsis. Am J Clin Pathol 2005;123:618–24. [DOI] [PubMed] [Google Scholar]
- [13].Mare TA, Treacher DF, Shankar-Hari M, et al. The diagnostic and prognostic significance of monitoring blood levels of immature neutrophils in patients with systemic inflammation. Crit Care 2015;19:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Nahm CH, Choi JW, Lee J. Delta neutrophil index in automated immature granulocyte counts for assessing disease severity of patients with sepsis. Ann Clin Lab Sci 2008;38:241–6. [PubMed] [Google Scholar]
- [15].Park BH, Kang YA, Park MS, et al. Delta neutrophil index as an early marker of disease severity in critically ill patients with sepsis. BMC Infect Dis 2011;11:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kim HW, Ku S, Jeong SJ, et al. Delta neutrophil index: could it predict mortality in patients with bacteraemia? Scand J Infect Dis 2012;44:475–80. [DOI] [PubMed] [Google Scholar]
- [17].Wallace SL, Robinson H, Masi AT, et al. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum 1977;20:895–900. [DOI] [PubMed] [Google Scholar]
- [18].Pyo JY, Park JS, Park YB, et al. Delta neutrophil index as a marker for differential diagnosis between flare and infection in febrile systemic lupus erythematosus patients. Lupus 2013;22:1102–9. [DOI] [PubMed] [Google Scholar]
- [19].Kratz A, Maloum K, O’Malley C, et al. Enumeration of nucleated red blood cells with the ADVIA 2120 Hematology System: an International Multicenter Clinical Trial. Lab Hematol 2006;12:63–70. [DOI] [PubMed] [Google Scholar]
- [20].Genes N, Chisolm-Straker M. Monoarticular arthritis update: current evidence for diagnosis and treatment in the emergency department. Emerg Med Pract 2012;14:1–9. quiz 19-20. [PubMed] [Google Scholar]
- [21].Shmerling RH, Delbanco TL, Tosteson AN, et al. Synovial fluid tests. What should be ordered? JAMA 1990;264:1009–14. [PubMed] [Google Scholar]
- [22].Kortekangas P, Aro HT, Tuominen J, et al. Synovial fluid leukocytosis in bacterial arthritis vs. reactive arthritis and rheumatoid arthritis in the adult knee. Scand J Rheumatol 1992;21:283–8. [DOI] [PubMed] [Google Scholar]
- [23].Hall AP, Barry PE, Dawber TR, et al. Epidemiology of gout and hyperuricemia. A long-term population study. Am J Med 1967;42:27–37. [DOI] [PubMed] [Google Scholar]
- [24].Shin DH, Kim EJ, Kim SJ, et al. Delta neutrophil index as a marker for differential diagnosis between acute graft pyelonephritis and acute graft rejection. PloS One 2015;10:e0135819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Scanu A, Oliviero F, Ramonda R, et al. Cytokine levels in human synovial fluid during the different stages of acute gout: role of transforming growth factor beta1 in the resolution phase. Ann Rheum Dis 2012;71:621–4. [DOI] [PubMed] [Google Scholar]
- [26].Chen YH, Hsieh SC, Chen WY, et al. Spontaneous resolution of acute gouty arthritis is associated with rapid induction of the anti-inflammatory factors TGFbeta1, IL-10 and soluble TNF receptors and the intracellular cytokine negative regulators CIS and SOCS3. Ann Rheum Dis 2011;70:1655–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


