Supplemental Digital Content is available in the text
Keywords: combined diagnosis, hematocrit, infected pancreatic necrosis, necrotizing pancreatitis, procalcitonin
Abstract
To assess the association between the clinical parameters within 48 hours of admission and the occurrence of infected pancreatic necrosis (IPN) during the late phase of necrotizing pancreatitis (NP).
All patients were divided into 2 groups, the IPN and non-IPN groups. The clinical data were retrospectively analyzed. Univariate and multivariate logistic regression analyses were performed to evaluate the relationship between clinical parameters and IPN secondary to NP. The performance of each independent variable was plotted by the receiver-operating characteristic (ROC) curve. Consequently, the cut-off level of each independent variable with its sensitivity and specificity was calculated.
A total of 215 patients were enrolled in our study. Among them, 87 (40.5%) patients developed IPNs after a median of 13.5 (9.5–23.0) days from admission. Multivariate analysis indicated that the level of hematocrit (HCT) from 40% to 50% (P=.012, odds ratio (OR) = 2.407), HCT over 50% (P < .009, OR = 6.794), blood urea nitrogen (BUN) (P = .040, OR = 1.894), C-reactive protein (CRP) (P = .043, OR = 1.837), and procalcitonin (PCT) (P = .002, OR = 2.559) were independent risk factors of IPN secondary to NP. The ROC cures revealed that the area under the ROC (AUC) of the maximum level of HCT, BUN, CRP, and PCT within 48 hours of admission was 0.687, 0.620, 0.630, and 0.674 respectively. Furthermore, the combination of these 4 individual parameters contributes to a more preferable AUC of 0.789 with a sensitivity of 67.8% and specificity of 77.3%.
The maximum levels of PCT, CRP, HCT, and BUN within 48 hours of admission are independent factors of IPN and their combination might accurately predict the occurrence of IPN secondary to NP.
1. Introduction
Acute pancreatitis (AP) is an inflammatory disorder in pancreas. It leads the most common gastrointestinal problem of hospital admissions with increasing incidence in the United State and other countries.[1] Although it presents a mild and limited course in most of the cases, the manifestation and the etiology is multifactorial and complex. Approximately 15% to 20% of patients develop necrotizing pancreatitis (NP), which is a severe disease accompanied by eventful outcomes during the natural course of AP.[2] NP most commonly manifests as necrosis involving the pancreas and peripancreatic tissues, which can remain sterile or become infected. The development of secondary infection in pancreatic necrosis is associated with an increased morbidity and mortality.[3,4]
The 2012 revised Atlanta Classification represents a global consensus that infected pancreatic necrosis (IPN) serves as a key determinant of the severity in the late phase of AP.[5] As IPN is the major determinants of mortality in AP, determining the best available predictors of this determinant of severity is urgent. An accurate indicator of IPN allows early triage of those patients who require transfer to a referral center, treatment in an intensive care unit (ICU), and/or specific interventions. The Ranson score was the first prognostic clinical score that represents a major advance in evaluating the severity of AP. Other scoring systems, including the acute physiology and chronic health evaluation-II (APACHE-II) score, computed tomography severity index (CTSI) have been explored for predicting the severity of AP. However, none of them could balance the accuracy with simplicity in clinical practice. The major drawback of these systems is the complexity and cumbersome to calculate. Meanwhile, several scoring systems require a relatively extended period (more than 48 hours) and this may miss a potentially therapeutic window. Besides, these systems could not predict the sterile or infected pancreatic necrosis specially.[6]
Several serum laboratory indicators are served as predictors for the disease severity, including C-reactive protein (CRP), procalcitonin (PCT), and others. Although CRP increases slowly and peaks up later than 72 hours after the onset of symptoms, the higher accuracy makes it the most valuable among them.[7] PCT is also an effective predictor for the severity of AP and the risk of developing IPN. Many studies suggest the value of PCT as a predictor for the diagnosis of severity of AP and IPN if following repeated measurements over a 2-week period.[8] Reproducible and routine laboratory parameters including blood urea nitrogen (BUN), hematocrit (HCT), and creatinine (Cr) have been explored as the great potential and having standardized reference ranges for evaluating the severity. Several studies have shown that dynamic increase in these simple and inexpensive biochemical parameters would be of significant association with severe disease.[9,10]
Patients with NP have a higher risk for developing IPN compared with AP. Thus, the best prediction strategy is to find valuable parameters in patients with confirmed pancreatic necrosis rather than in AP patients.[11] Few studies provide simple and practical predictions for the development of IPN.[6,12] The present study aimed to explore the early clinical parameters that were independently associated with IPN secondary to NP.
2. Materials and methods
2.1. Patient identification and definition
The study protocol was approved by the ethics committee of the First Affiliated Hospital of Harbin Medical University. Consecutive adult patients (>18 years) with a first episode of AP who were admitted to the Department of Pancreatic and Biliary Surgery, First Affiliated Hospital of Harbin Medical University from January 2012 to August 2016 were enrolled. Transferred patients were excluded from this study. The detailed exclusion criteria for patients are shown in a flow chart (Fig. 1). All data were collected from the database, which was established in 2010 and modified according to the revised Atlanta classification, if necessary. The diagnosis of AP followed at least 2 of the following 3 criteria: abdominal pain consistent with AP, serum lipase level (or amylase level) at least 3 times greater than the upper limit of normality, and abdominal imaging findings in accordance with AP. Pancreatic necrosis was diagnosed by lack of pancreatic gland enhancement in patients with available contrast-enhanced computed tomography (CT) of the abdomen. All enrolled patients were followed up for 3 months after discharge. The presence of IPN was suspected by the patients’ clinical courses, including continuous fever and general deterioration, and it was confirmed by the CT evidence of free air within the necrotic tissue or peripancreatic collections. Microbiological confirmations were established with positive cultures of samples obtained by fine needle aspirations under CT/ultrasound guidance and/or samples obtained during invasive therapeutic procedures. We defined IPN as any infection of the necrotic pancreatic parenchyma or peripancreatic collections that developed prior to any invasive interventions. Respiratory, cardiovascular, and renal systems were assessed to define the organ failure. Organ failure was defined as a score of 2 or more for 1 of these 3 organ systems using the modified Marshall scoring system.[5]
Figure 1.
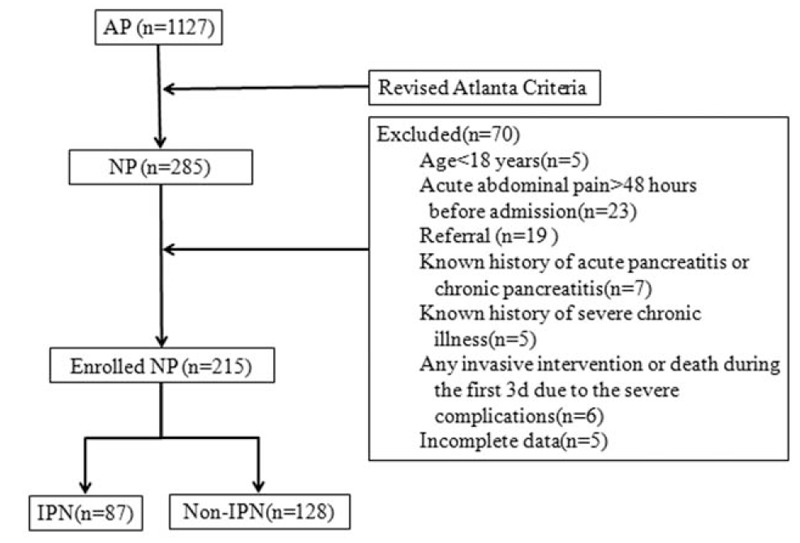
Flow chart of patients with NP between January 2012 and August 2016. AP = acute pancreatitis, NP = necrotizing pancreatitis, IPN = infected pancreatic necrosis.
2.2. Clinical management protocols
All patients received individualized conservative therapy immediately after admission, including intensive resuscitation, fluid and electrolyte monitoring, nutritional support (nasojejunal feeding or total parenteral nutrition), and supportive care.[13] Antibiotics were not administered prophylactically unless indicated by infections in other systems (e.g., biliary tract, urinary, and tract).[14,15] Contrast-enhanced CT was performed on the third day after admission, the modified CTSI and percentage of pancreatic necrosis was calculated. When the temperature was over 38.0°C, a blood culture was drawn. A sequential culture result is important for patients with fever and clinical deterioration. We tried to perform percutaneous catheter drainage (PCD) with a pigtail catheter if patients had persistent fever and/or progressive clinical deterioration in the presence of fluid collection on CT scanning. Retroperitoneal pancreatic necrosectomy and open pancreatic necrosectomy ensued for patients if the infection was not controlled due to inadequate drainage.[16–18] Cultures were collected during all the procedures to confirm the diagnosis of IPN and to guide antibiotic therapies.
2.3. Data collection
The baseline variables were recorded within 48 hours of admission, including demographic data, such as the age, gender, etiology, and body mass index (BMI), and the maximum value of the following clinical data within 48 hours: white blood cell (WBC) count, HCT, platelet (PLT) count, BUN, Cr, D-dimer, CRP, PCT, and heart rate. APACHE-II and Imrie scores were evaluated on the second day after admission. Additionally, the modified Marshall scoring system, sequential organ failure assessment (SOFA) score, and modified CTSI at the end of third day were also documented.
2.4. Statistical analyses
The data were analyzed using SPSS version 22.0 (IBM Corp, Armonk, NY). Quantitative variables are presented as the mean ± standard deviation for normal distributions or as the median (inter quartile range, IQR) in case of non-normal distributions. Categorical variables are presented as absolute numbers and proportions. The variables found to be statistically significant in the univariate logistic regression analysis were introduced into a multivariate logistic analytic model to identify the independent risk factors with odds ratios (ORs) and 95% confidence intervals. Furthermore, utilizing receiver operating characteristic (ROC) curves, the areas under the curves (AUCs) and cut-off values with the associated sensitivities and specificities of the qualified independent risk factors were calculated. A P value less than .05 was considered to indicate statistical significance.
3. Result
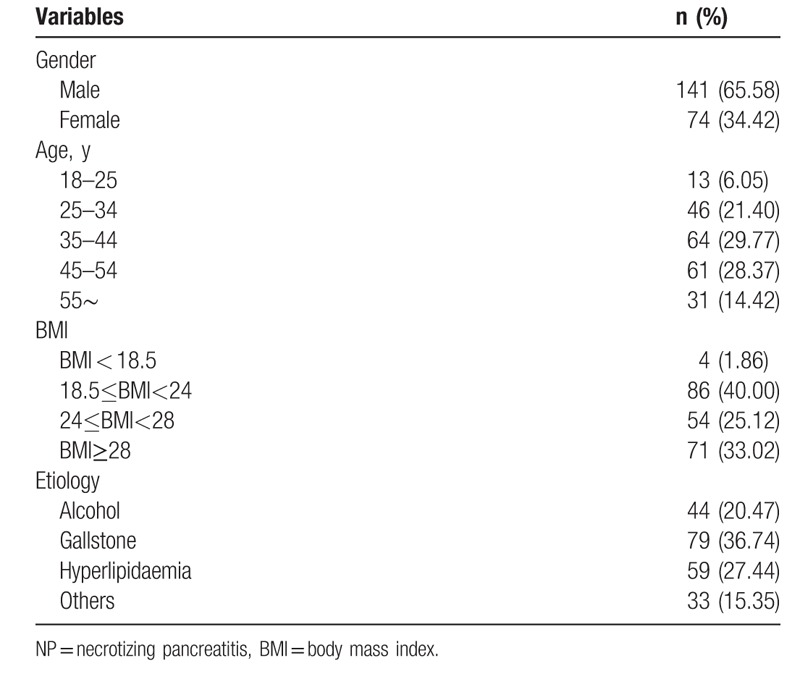
A total of 215 patients with moderate severe acute pancreatitis (MSAP) and severe acute pancreatitis (SAP) were enrolled in our study. The average age was 42.2 ± 11.6 years. Among all patients, there were 141(65.6%) males, 79(36.7%) patients had a biliary etiology, 59 (27.4%) patients had hyperlipidemia, 44 (20.5%) patients had an alcoholic etiology, and 33 (15.4%) patients had other etiology, such as post-ERCP, pancreatic cancer, anatomical abnormalities, and idiopathic. Eighty-seven (40.5%) patients developed into IPN with a median 13.5 (9.5–23.0) days after admission. The overall in-hospital mortality was 8.4% (18/215). The demographic characteristics of both IPN and non-IPN groups are shown in Table 1.
Table 1.
Demographic data of 215 patients with NP.
Among the IPN patients, mixed infections (53, 61.3%) were dominant, which was followed by infections with gram-positive bacteria alone (28, 32.2%) and gram-negative bacteria alone (8, 9.2%). In addition, concomitant fungal infection was found in 17 (19.5%) patients. Among the infectious microbes, Escherichia coli (26, 29.9%) and Staphylococcus (21, 24.1%) were the most commonly isolated. The results of univariate logistic regression analysis showed that the maximum levels of WBC (P = .005), D-dimer (P < .001), HCT (P < .001), BUN (P = .003), CRP (P = .001), and PCT (P < .001) within 48 hours of admission were statistically significant between the IPN and non-IPN groups (Table 2). Furthermore, multivariate logistic regression analysis was performed, and the results indicated that the maximum levels of HCT within 48 hours after admission between 40% and 50% (P = .012, OR = 2.407, 95% CI = 1.214–4.772) as well as more than 50% (P < .009, OR = 6.794, 95% CI = 1.618–28.520) were both the independent risk factors of IPN. Furthermore, the maximum levels of BUN (P = .040, OR = 1.894, 95% CI = 1.03–3.482), CRP (P = .043, OR = 1.837, 95% CI = 1.018–3.314), and PCT (P = .002, OR = 2.559, 95% CI = 1.409–4.649) were also the independent risk factors of IPN (Table 3). We then plotted ROC curves to explore the performance of these predictors and the results indicated that the AUC of the maximum level of HCT within 48 hours of admission was 0.687. The cut-off value of the HCT was 42.86% with sensitivity of 56.3% and specificity of 73.4%. The AUC of the maximum BUN level within 48 hours of admission was 0.620 and the cut-off level was 8.42 mmol/L with a sensitivity of 69.0% and specificity of 54.7%. The AUC of the maximum level of CRP within 48 hours of admission was 0.630, and the cut-off value of CRP was 257.50 mg/L with a sensitivity of 44.8% and specificity of 89.1%. The AUC of the maximum level of PCT within 48 hours of admission was 0.674 and the cut-off value of PCT was 1.39 ng/mL with a sensitivity of 60.9% and specificity of 75.0%. Furthermore, the AUC of combined diagnosis, which consisted of the aforementioned parameters, was 0.789 with a sensitivity of 67.8% and specificity of 77.3%. All the results are shown in Table 4 and Fig. 2.
Table 2.
Univariate analysis of variables with the development of IPN secondary to NP as the endpoint.
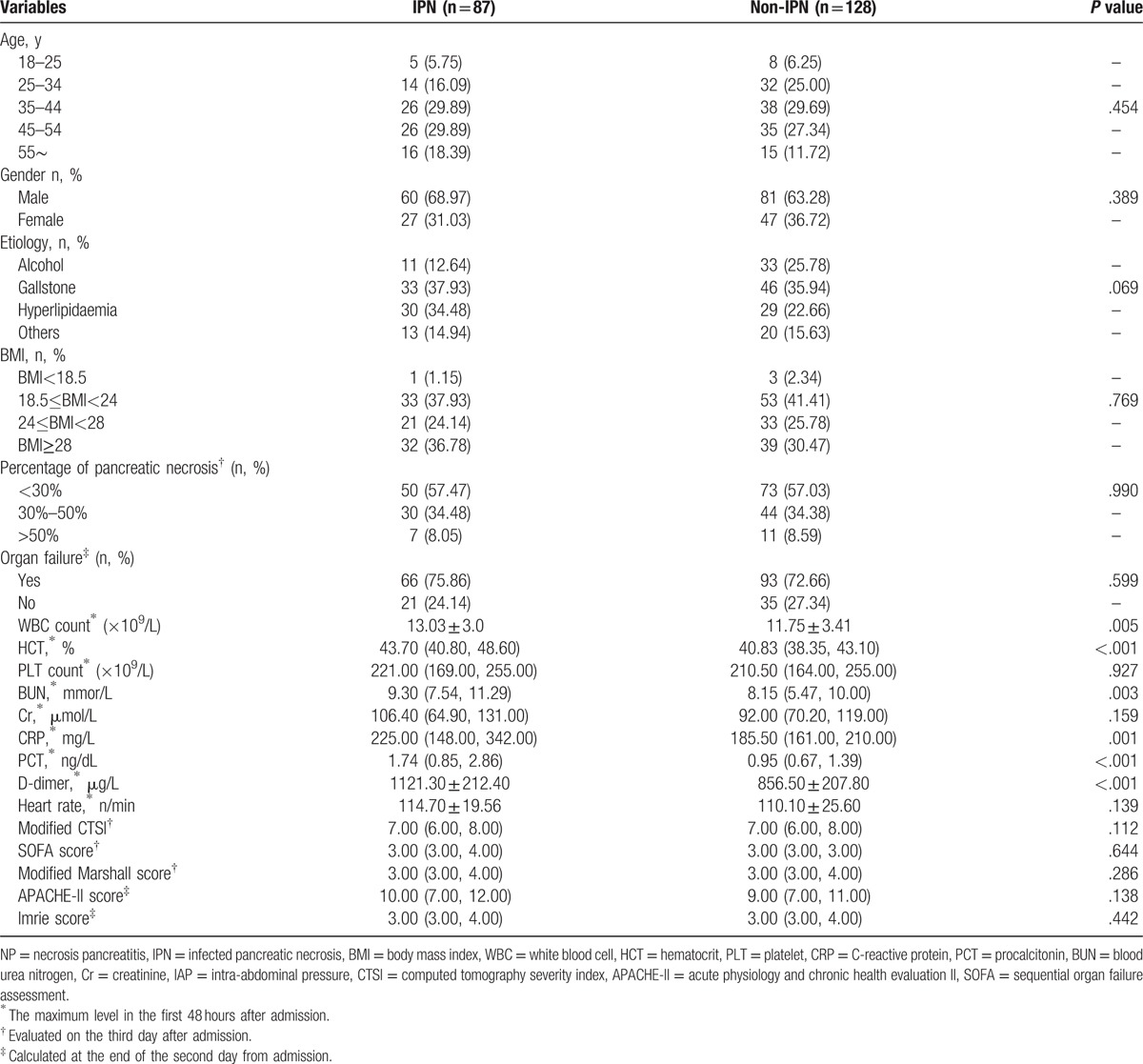
Table 3.
Multivariate analysis of variables with the development of IPN secondary to NP as the endpoint.
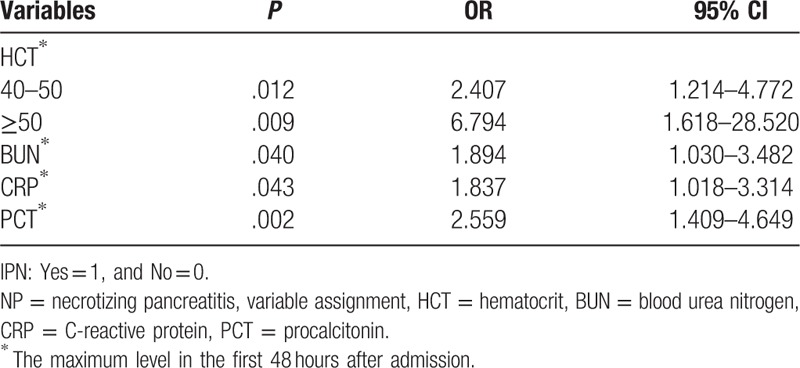
Table 4.
The performances of HCT, BUN, CRP, PCT, and combined diagnosis associated with the development of IPN secondary to NP.
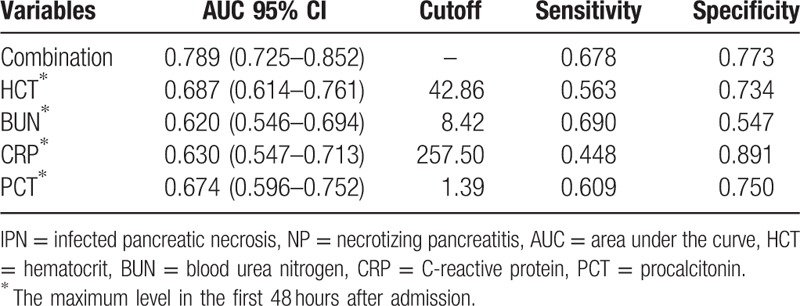
Figure 2.
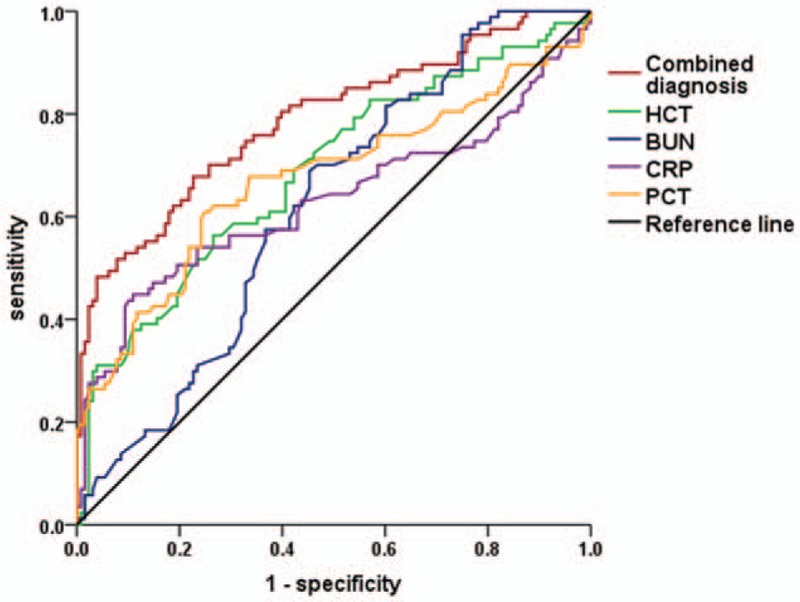
Receiver operating characteristic curves for the maximum PCT, CRP, HCT, and BUN levels within 48 hours after admission and the combined diagnosis of the 4 parameters associated with the development of IPN secondary to NP. IPN = infected pancreatic necrosis, NP = necrotizing pancreatitis, BUN = blood urea nitrogen, CRP = C-reactive protein, HCT = hematocrit, PCT = procalcitonin.
4. Discussion
Although IPN typically occurs in the late phase of AP, it accounts for up to 50% to 80% of mortalities.[19,20] Identifying the subgroups of patients who are prone to IPN facilitates patients’ timely transfer to disease-specific diagnosis, allowing for improvements in individualized treatment.[21,22]
An early and late peak of mortality followed in the dynamic disease process of AP. The late phase is characterized by persistence of systemic and local signs of inflammation which merely occurs in patients with MASP or SAP. Infection is the major cause of death in the late phase of disease.[23] Secondary infection of pancreatic or peripancreatic necrotic tissue, accompanied with gut barrier dysfunction and a series of bacterial translocation are correlated with the development of sepsis.[24] In addition, abdominal infection and sepsis will accordingly aggravate the state of IPN and lead to a higher mortality of 80% to 85% in the late phase.[25] In our study, the mortality of patients with IPN is 13.8% (12/87), which is significantly higher than the mortality of patients with sterile necrosis (4.7%, 6/128). Tenner et al[26] reported that patients with IPN suffered an essential increase in mortality ranging from 14% to 69% due to sepsis and multiple organ failure, compared with patients with sterile necrosis. The improvement of intensive care and supportive treatment of organ function, as well as increased emphasis on early fluid resuscitation result in a lower mortality in the early phase of disease. However, the overall mortality of SAP is still higher. The main reason is that IPN and sepsis contribute to the high mortality and morbidity in the late phase of course.[27] Therefore, accurately predicting the occurrence of IPN in the early course plays a critical role in improving patients’ outcomes.
The HCT is routinely measured at a low cost in every AP case starting at admission. An elevated admission HCT≥44% or failure to decrease the HCT within the first 24 hours of admission is a significant risk factor for developing pancreatic necrosis and organ failure according to a prospective study.[28] However, our data suggested that the increased maximum level of HCT in 48 hours of admission from 40% to 50% to over 50% increases the odds ratio of IPN from 2.407 to 6.794. These findings indicated that the maximum level of the HCT within the first 48 hours of admission could be reliably used to identify the patients who might eventually develop IPN. Previous studies [29] showed hemoconcentration can be used to predict necrosis and mortality rates in AP, which supported our hypothesis that an increase in the HCT could predict the development of IPN. Hemoconcentration and disrupted microcirculation are commonly detected during the course of AP [30] and they are associated with pancreatic tissue perfusion and pancreatic necrosis, which are susceptible to secondary infections.[31] The sensitivity (56%) of the HCT level was relatively lower, while we achieved a relatively higher specificity of 73.4%. Along with the HCT, the BUN is also a convenient, inexpensive, and baseline clinical parameter for predicting IPN secondary to NP. Koutroumpakis et al[32] reported that the elevation of BUN at 48 hours may be the optimal predictor in pancreatic necrosis. Meanwhile, changes in its value at 48 hours from admission may reflect responses to the initial treatment and tailor further management decisions. Additionally, elevation of BUN by 5 mg/dL within 48 hours of admission was associated with developing primary IPN.[33] Our data indicated that the maximum BUN level in the first 48 hours after admission was correlated with the presence or absence of IPN (P = .039). The maximum BUN level was an independent risk factor of IPN secondary to NP (P = .003, OR = 1.894). PCT is the inactive 116 amino acid propeptide of the biologically active hormone calcitonin, which was first described to have significantly increased concentrations in patients with bacterial and fungal infections.[34,35] The PCT level is considered a valuable predictive factor for severity of AP and the risk of developing IPN.[36–39] In a previous study, a cut-off PCT level >0.5 ng/mL seems to be an accurate predictor of severity.[40] However, our data suggested that a maximum level of PCT within 48 hours after admission was an independent risk factor of IPN (P = .002, OR = 2.559, 95% CI = 1.409–4.649) and the cut-off PCT level is 1.39 ng/mL. The heterogeneity among patients likely contributes to the discrepancy in cut-off levels of PCT among these studies. As one of the biggest tertiary pancreatic centers in the northeast of China, our department recruits a larger number of patients in critical condition than primary care center, which may contribute to a relatively higher average PCT. Although the greatest PCT value from serial daily measurements over a long period outperformed the identification of IPN compared with intermittent measurement,[8] a modest timeframe (the first 48 hours after admission) was proposed in our study for the diagnostic timeliness and financial cost. CRP has recently been shown to be a predictor of the development of infected necrosis in AP.[41] Our data revealed that the maximum CRP levels within the first 48 hours were associated with the presence or absence of IPN (P = .012), and these levels are significantly associated with the development of IPN (P = .043, OR = 1.837, 95% CI 1.018–3.314). Although the specificity (89.1%) of the maximum level of CRP within the 48 hours of admission was high, the sensitivity (44.8%) was not preferable. This appears to be a common problem with most of the potential predictors.[6,42] In this context, a high specificity seems to be more meaningful, which indicates that in the absence of an increased level of CRP within 48 hours of admission, the likelihood to develop IPN is low.
Furthermore, these 4 independent risk factors were incorporated into a combined diagnostic manner. Our results revealed that the AUC of combined diagnosis increased to 0.789 with the highest sensitivity (67.8%) compared with all the other single parameters, and the specificity was relatively high (77.3%), which performed better than the PCT (75%), HCT (73.4%), and BUN (54.7%), respectively. The highest Youden index (0.451) of combined diagnosis supported that a notable effect of predicting the development of IPN benefits from the combination of the maximum level of the HCT, BUN, PCT, and CRP within 48 hours of admission. To the best of our knowledge, this study is among the very few that had used all these 4 common parameters to indicate the disease dynamics for predicting the subsequent development of IPN within 48 hours of admission. The predictive value of each single parameter was relatively confined. We attribute this to the timing and method of parameters measurement. First, the observation was set to the first 48 hours of admission because we preferred to timely and effectively react the condition.[43] Early severity assessment is important, especially in the first 48 hours of admission with respect to the window of opportunity for using interventions to prevent necrosis.[44] However, some parameters, such as the CRP, need more than 48 hours to reach a peak value, which may influence the diagnostic accuracy. Even with its delayed increase and peak, CRP remains useful and widely available.[42,45] Ferreira et al[46] suggested that although the maximum serum concentration of CRP is achieved after 72 hours, it is able to discriminate severe cases from mild cases of AP within the first 24 hours. Second, the highest PCT value from serial daily measurements over a long time period better identifies IPN than intermittent measurements. However, measurements of full serial serum parameters can pose financial and logistical challenges, especially for PCT. Therefore, we proposed the use of the maximum level in the first 48 hours after admission to predict the development of IPN. The combined diagnosis of the maximum level of the PCT, CRP, HCT, and BUN in the first 48 hours after admission performed satisfactorily with a high sensitivity and specificity, which may offset the diagnostic deficiency. Our previous study suggested that the maximum D-dimer level was an independent risk factor of IPN secondary to SAP.[47] However, we found that D-dimer was not independent risk factor in this study. We attribute this discrepancy to the increased number of patients and the change of the predictive timeliness.
There are some limitations of this study. First, the limited numbers of patient involved and retrospective nature of the study result in a lower sensitivity and specificity of each individual parameter. Second, the definition of IPN was based on the microbiologically proven infection. We may miss a small proportion of cases that responded to the conservative management. Finally, our database was established in 2010 and modified by the revised Atlanta classification. Other vital parameters relevant to the development of IPN may be missed.
5. Conclusion
In this study, we have shown that the maximum level of the HCT, BUN, PCT, and CRP within 48 hours of admission is an independent factor for IPN. Furthermore, the combined diagnosis with these 4 parameters might accurately predict the occurrence of IPN secondary to NP within the time frame of 48 hours since admission.
Supplementary Material
Footnotes
Abbreviations: APACHE = acute physiology and chronic health evaluation, AUC = area under the ROC, BMI = body mass index, BUN = blood urea nitrogen, Cr = creatinine, CRP = C-reactive protein, CT = computed tomography, CTSI = computed tomography severity index, HCT = hematocrit, ICU = intensive care unit, IPN = infected pancreatic necrosis, MSAP = moderate serve acute pancreatitis, OR = odds ratio, PCD = percutaneous catheter drainage, PCT = procalcitonin, PLT = platelet, ROC = receiver-operating characteristic curve, SAP = severe acute pancreatitis, SOFA = sequential organ failure assessment, WBC = white blood cell.
This study was funded by the National Nature Scientific Foundation of China (Nos 81372613, 81370565, 81470887, 81670583), National High Technology Research and Development Program of China (2014AA020609), and Wei-Han Yu Scientific Foundation of Harbin Medical University.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology 2013;144:1252–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bruno MJ. Dutch Pancreatitis Study Group. Improving the outcome of acute pancreatitis. Dig Dis 2016;34:540–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Werge M, Novovic S, Schmidt PN, et al. Infection increases mortality in necrotizing pancreatitis: a systematic review and meta-analysis. Pancreatology 2016;16:698–707. [DOI] [PubMed] [Google Scholar]
- [4].Guo Q, Li A, Xia Q, et al. The role of organ failure and infection in necrotizing pancreatitis: a prospective study. Ann Surg 2014;259:1201–7. [DOI] [PubMed] [Google Scholar]
- [5].Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis-2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013;62:102–11. [DOI] [PubMed] [Google Scholar]
- [6].Papachristou GI, Muddana V, Yadav D, et al. Comparison of BISAP, Ranson's, APACHE-II, and CTSI scores in predicting organ failure, complications, and mortality in acute pancreatitis. Am J Gastroenterol 2010;105:435–41. [DOI] [PubMed] [Google Scholar]
- [7].Cardoso FS, Ricardo LB, Oliveira AM, et al. C-reactive protein prognostic accuracy in acute pancreatitis: timing of measurement and cutoff points. Eur J Gastroenterol Hepatol 2013;25:784–9. [DOI] [PubMed] [Google Scholar]
- [8].Mofidi R, Suttie SA, Patil PV, et al. The value of procalcitonin at predicting the severity of acute pancreatitis and development of infected pancreatic necrosis: systematic review. Surgery 2009;146:72–81. [DOI] [PubMed] [Google Scholar]
- [9].Wu BU, Bakker OJ, Papachristou GI, et al. Blood urea nitrogen in the early assessment of acute pancreatitis: an international validation study. Arch Intern Med 2011;171:669–76. [DOI] [PubMed] [Google Scholar]
- [10].Papachristou GI, Muddana V, Yadav D, et al. Increased serum creatinine is associated with pancreatic necrosis in acute pancreatitis. Am J Gastroenterol 2010;105:1451–2. [DOI] [PubMed] [Google Scholar]
- [11].Yang CJ, Chen J, Phillips AR, et al. Predictors of severe and critical acute pancreatitis: a systematic review. Dig Liver Dis 2014;46:446–51. [DOI] [PubMed] [Google Scholar]
- [12].Petrov MS. Predicting the severity of acute pancreatitis: choose the right horse before hitching the cart. Dig Dis Sci 2011;56:3402–4. [DOI] [PubMed] [Google Scholar]
- [13].Forsmark CE, Baillie J. AGA Institute Clinical Practice and Economics Committee; AGA Institute Governing Board. AGA Institute technical review on acute pancreatitis. Gastroenterology 2007;132:2022–44. [DOI] [PubMed] [Google Scholar]
- [14].Wittau M, Mayer B, Scheele J, et al. Systematic review and meta-analysis of antibiotic prophylaxis in severe acute pancreatitis. Scand J Gastroenterol 2011;46:261–70. [DOI] [PubMed] [Google Scholar]
- [15].Trikudanathan G, Navaneethan U, Vege SS. Intra-abdominal fungal infections complicating acute pancreatitis: a review. Am J Gastroenterol 2011;106:1188–92. [DOI] [PubMed] [Google Scholar]
- [16].van Santvoort HC, Besselink MG, Bakker OJ, et al. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med 2010;362:1491–502. [DOI] [PubMed] [Google Scholar]
- [17].Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13(4 suppl 2):e1–15. [DOI] [PubMed] [Google Scholar]
- [18].Babu RY, Gupta R, Kang M, et al. Predictors of surgery in patients with severe acute pancreatitis managed by the step-up approach. Ann Surg 2013;257:737–50. [DOI] [PubMed] [Google Scholar]
- [19].Sakorafas GH, Lappas C, Mastoraki A, et al. Current trends in the management of infected necrotizing pancreatitis. Infect Disord Drug Targets 2010;10:9–14. [DOI] [PubMed] [Google Scholar]
- [20].Bakker OJ, van Santvoort HC, Besselink MG, et al. Prevention, detection, and management of infected necrosis in severe acute pancreatitis. Curr Gastroenterol Rep 2009;11:104–10. [DOI] [PubMed] [Google Scholar]
- [21].Sun B, Li HL, Gao Y, et al. Factors predisposing to severe acute pancreatitis: evaluation and prevention. World J Gastroenterol 2003;9:1102–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sun B, Li HL, Gao Y, et al. Analysis and prevention of factors predisposing to infections associated with severe acute pancreatitis. Hepatobiliary Pancreat Dis Int 2003;2:303–7. [PubMed] [Google Scholar]
- [23].Occhionorelli S, Morganti L, Cultrera R, et al. Acute necrotizing pancreatitis: can tigecycline be included in a therapeutic strategy? G Chir 2015;36:15–20. [PMC free article] [PubMed] [Google Scholar]
- [24].Fishman JE, Levy G, Alli V, et al. The intestinal mucus layer is a critical component of the gut barrier that is damaged during acute pancreatitis. Shock 2014;42:264–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Yong FJ, Mao XY, Deng LH, et al. Continuous regional arterial infusion for the treatment of severe acute pancreatitis: a meta-analysis. Hepatobiliary Pancreat Dis Int 2015;14:10–7. [DOI] [PubMed] [Google Scholar]
- [26].Tenner S, Baillie J, DeWitt J, et al. American College of Gastroenterology. American College of Gastroenterology Guidelines: management of acute pancreatitis. Am J Gastroenterol 2013;108:1400–15. [DOI] [PubMed] [Google Scholar]
- [27].van Santvoort HC, Bakker OJ, Bollen TL, et al. A conservative and minimally invasive approach to necrotizing pancreatitis improves outcome. Gastroenterology 2011;141:1254–63. [DOI] [PubMed] [Google Scholar]
- [28].Brown A, Orav J, Banks PA. Hemoconcentration is an early marker for organ failure and necrotizing pancreatitis. Pancreas 2000;20:367–72. [DOI] [PubMed] [Google Scholar]
- [29].Baillargeon JD, Orav J, Ramagopal V, et al. Hemoconcentration as an early risk factor for necrotizing pancreatitis. Am J Gastroenterol 1998;93:2130–4. [DOI] [PubMed] [Google Scholar]
- [30].Gomercic C, Gelsi E, Van Gysel D, et al. Assessment of D-dimers for the early prediction of complications in acute pancreatitis. Pancreas 2016;45:980–5. [DOI] [PubMed] [Google Scholar]
- [31].Pitchumoni CS, Patel NM, Shah P. Factors influencing mortality in acute pancreatitis: can we alter them? J Clin Gastroenterol 2005;39:798–814. [DOI] [PubMed] [Google Scholar]
- [32].Koutroumpakis E, Wu BU, Bakker OJ, et al. Admission hematocrit and rise in blood urea nitrogen at 24 h outperform other laboratory markers in predicting persistent organ failure and pancreatic necrosis in acute pancreatitis: a post hoc analysis of three large prospective databases. Am J Gastroenterol 2015;110:1707–16. [DOI] [PubMed] [Google Scholar]
- [33].Talukdar R, Nechutova H, Clemens M, et al. Could rising BUN predict the future development of infected pancreatic necrosis? Pancreatology 2013;13:355–9. [DOI] [PubMed] [Google Scholar]
- [34].Rau B, Steinbach G, Gansauge F, et al. The potential role of procalcitonin and interleukin-8 in the prediction of infected necrosis in acute pancreatitis. Gut 1997;41:832–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Assicot M, Gendrel D, Carsin H, et al. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet 1993;341:515–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Pavlidis TE, Pavlidis ET, Sakantamis AK. Advances in prognostic factors in acute pancreatitis: a mini-review. Hepatobiliary Pancreat Dis Int 2010;9:482–6. [PubMed] [Google Scholar]
- [37].Lee KJ, Kim HM, Choi JS, et al. Comparison of predictive systems in severe acute pancreatitis according to the revised Atlanta classification. Pancreas 2016;45:46–50. [DOI] [PubMed] [Google Scholar]
- [38].Bezmarevic M, Mirkovic D, Soldatovic I, et al. Correlation between procalcitonin and intra-abdominal pressure and their role in prediction of the severity of acute pancreatitis. Pancreatology 2012;12:337–43. [DOI] [PubMed] [Google Scholar]
- [39].Rau BM, Kemppainen EA, Gumbs AA, et al. Early assessment of pancreatic infections and overall prognosis in severe acute pancreatitis by procalcitonin (PCT) a prospective international multicenter study. Ann Surg 2007;245:745–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Olah A, Belagyi T, Issekutz A, et al. Value of procalcitonin quick test in the differentiation between sterile and infected forms of acute pancreatitis. Hepatogastroenterology 2005;52:243–5. [PubMed] [Google Scholar]
- [41].Cardoso FS, Ricardo LB, Oliveira AM, et al. C-reactive protein prognostic accuracy in acute pancreatitis: timing of measurement and cutoff points. Eur J Gastroenterol Hepatol 2013;25:784–9. [DOI] [PubMed] [Google Scholar]
- [42].Mounzer R, Langmead CJ, Wu BU, et al. Comparison of existing clinical scoring systems to predict persistent organ failure in patients with acute pancreatitis. Gastroenterology 2012;142:1476–82. [DOI] [PubMed] [Google Scholar]
- [43].Fisher JM, Gardner TB. The“golden hours” of management in acute pancreatitis. Am J Gastroenterol 2012;107:1146–50. [DOI] [PubMed] [Google Scholar]
- [44].Afghani E, Pandol SJ, Shimosegawa T, et al. Acute pancreatitis-progress and challenges: a report on an International Symposium. Pancreas. 2015;44:1195–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Otsuki M, Takeda K, Matsuno S, et al. Criteria for the diagnosis and severity stratification of acute pancreatitis. World J Gastroenterol 2013;19:5798–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ferreira A, Bartelega JA, Urbano HC, et al. Acute pancreatitis gravity predictive factors: which and when to use them? Arq Bras Cir Dig 2015;28:207–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Ji L, Lv J-C, Song ZF, et al. Risk factors of infected pancreatic necrosis secondary to severe acute pancreatitis. Hepatobiliary Pancreat Dis Int 2016;15:428–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


