Abstract
Rationale:
The treatment of iron-deficiency anemia with oral iron supplements can present side-effects on the GI tract mucosa including necrosis, ulceration, or ischemia. The particular endoscopic findings and the histopathological exam will establish the diagnosis of erosive gastritis with iron deposits in the gastric mucosa.
Patient concerns:
We present the case of a 14-year-old female admitted in our clinic for upper digestive hemorrhage, nausea, melena, and abdominal pain. Her personal history revealed iron deficiency anemia receiving oral iron supplements for approximately 2 weeks.
Diagnosis:
The laboratory tests at the moment of admission pointed out anemia, increased level of serum iron, increased liver transaminases, a decreased level of ferritin, but with normal levels of both total iron-binding capacity and transferrin.
Interventions:
The eso-gastro-duodenoscopy revealed multiple brown deposits on the surface of the gastric mucosa and multiple hemorrhagic lesions, under the aspect of erosions all over the gastric mucosa, but more severe in the antral part, and the histopathological exam confirmed the presence of iron deposits at this level.
Conclusion:
Iron-pill induced gastritis is a rare, under-diagnosed entity that can be present even at pediatric ages with potential severe clinical impact.
Keywords: child, gastritis, oral iron supplements
1. Introduction
Erosive and hemorrhagic gastritis or gastropathy is rather an endoscopic entity than a clinical one which is characterized by the presence of multiple erosions and hemorrhages of the gastric mucosa. Among the causes that can lead to erosive and hemorrhagic gastritis, we can encounter: “stress” gastropathy, neonatal gastropathies, traumatic gastropathy, aspirin, and other non-steroid anti-inflammatory drugs (NSAISs), other drugs, portal hypertensive gastropathy, uremic gastropathy, chronic varioliform gastropathy, bile gastropathy, Henoch-Schonlein gastropathy, corrosive gastropathy, exercise-induced gastropathy and radiation gastropathy.[1] Nevertheless, these causes can also lead to nonerosive gastritis or gastropathy. Even though aspirin and other NSAIDs are the most common class of drugs associated with side-effects on the gastrointestinal (GI) tract, there are also other types of drugs or toxic substances with a similar effect, such as valproic acid, dexamethasone, chemotherapeutic agents, alcohol, potassium chloride, iron, long-term fluoride ingestion, and cysteamine.[1]
Oral iron supplements have been widely used for many years for the treatment of iron-deficiency anemia, a common disorder worldwide that according to the World Health Organization afflicts approximately 25% of the population.[2] Although it is well-documented that high doses of iron can lead to severe injuries of the GI tract such as mucosal necrosis, ulceration, and ischemia, it was also reported that therapeutic doses of iron supplements can present the same effect.[3] Ferrous sulfate tablets are the most common oral iron compounds associated with negative impact on the GI tract.[3] The diagnosis of this rare medical entity is based mainly on the endoscopic findings and the histopathological exam because the clinical manifestations are nonspecific and can be represented by abdominal pain, nausea, vomiting, and in certain cases upper digestive hemorrhage. The mucosal injury caused by iron resembles to that caused by a chemical burn, endoscopically presenting under the aspect of erosion, ulceration, or diffuse gastritis.[4] The histopathological exam will reveal the presence of brown deposits, mainly extracellular, luminal, lying adjacent to the surface epithelium or both.[3] Also, crystalline iron can be encountered at the level of lamina propria, granulation tissue, or in the blood vessels as iron-containing thrombi.[5,6] On the other hand, hemosiderin pigment can be found intracellularly, within superficial epithelial cells, antral glands, fundic glands, and it has a more fine granular aspect.[3,5] This type of gastric injury is produced though a direct effect of iron on the gastric mucosa and it was suggested that its severity depends on the concentration of iron compound; therefore, this effect was not noticed in patients receiving a liquid form of iron supplement.[4]
The aim of this paper is to underline the fact that despite its rarity, this entity of reactive, erosive gastritis induced by oral iron supplements can also be encountered in children, even though most of the cases are reported in adults, and it can lead to potentially fatal complications such as severe upper digestive hemorrhage.
Informed consent was obtained from the patient's mother (legal guardian) for the publication of this case report.
2. Case report
2.1. Presenting concerns
We present the case of a 14-year-old female teenager admitted in our clinic with the following complaints: upper digestive hemorrhage for approximately 24 hours (8 episodes of vomiting with fresh blood, the last one of approximately 100–150 mL), nausea, melena for approximately 5 days, and abdominal pain, especially in the epigastric area. Her personal history revealed that she was diagnosed with iron deficiency anemia 1 month ago for which she was receiving oral iron supplements as ferrous sulfate compounds for approximately 2 weeks, which were administered at approximately 2 hours after meals.
2.2. Clinical findings
The clinical exam performed at the moment of admission revealed the following pathological elements: ailing face, pallor, and diffuse abdominal tenderness.
2.3. Diagnostic focus and assessment
The laboratory tests at the moment of admission pointed out anemia (Hb: 11.2 g/dL, Htc: 35.2%, MEV: 67.4 fL, MEH: 21.4 pg), increased level of serum iron (51.56 μmol/L), increased liver transaminases (AST: 404.8 U/L, ALT: 429.7 U/L, GGT: 52 U/L, LDH: 334 U/L), a decreased level of ferritin (6.7 ng/mL), but with normal levels of both total iron-binding capacity and transferrin. The biomarkers of coagulation were in normal ranges. We excluded an acute liver pathology based on the negative biomarkers for viral hepatitis, normal markers for autoimmune hepatitis, and also by performing serological tests for other etiologies, such as parasitic infection, but they were also normal. Due to the positive history of iron deficiency anemia and increased liver biomarkers, we ruled out also a celiac disease based on the negative antitransglutaminase and antiendomysial antibodies. The abdominal ultrasound did not reveal anything pathological. We performed also an upper digestive endoscopy which revealed brown deposits on the gastric mucosa, edematous gastric folds, and multiple hemorrhagic lesions of the gastric mucosa, under the aspect of erosions all over the gastric mucosa, but more severe in the antral part (Figs. 1 and 2). We took multiple biopsies from both the gastric and duodenal mucosa.
Figure 1.
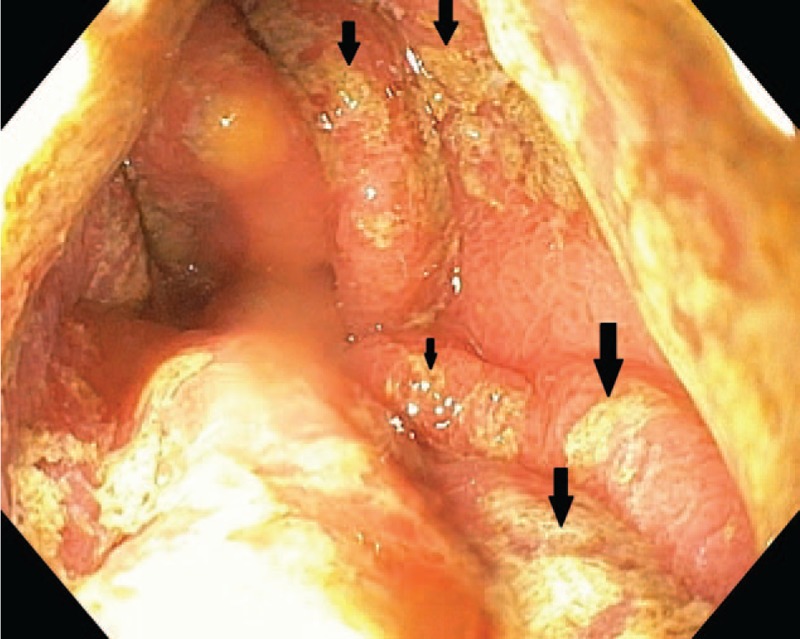
Macroscopic aspect of the gastric folds with brown deposits.
Figure 2.
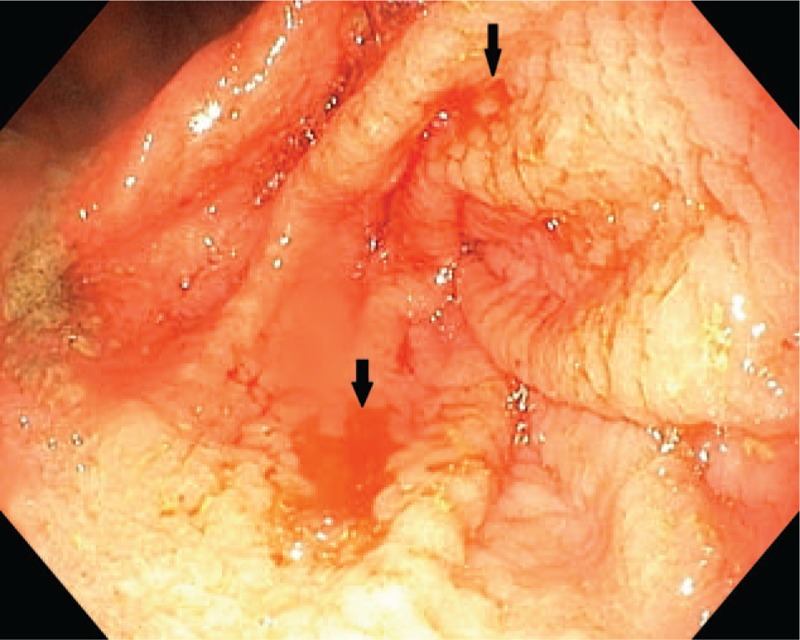
Multiple hemorrhagic lesions of the gastric mucosa.
The histopathological exam of the duodenal biopsies was normal, but that of the gastric mucosa pointed out erosions, numerous intraepithelial neutrophils, in the mucosal chorion with an abundant polymorph inflammatory infiltrate and multiple brown deposits defining the presence of iron at this level (Figs. 3 and 4), therefore establishing the diagnosis of acute hemorrhagic gastritis induced by the oral treatment with iron supplements. The histopathological exam excluded the presence of Helicobacter pylori within the gastric mucosa.
Figure 3.
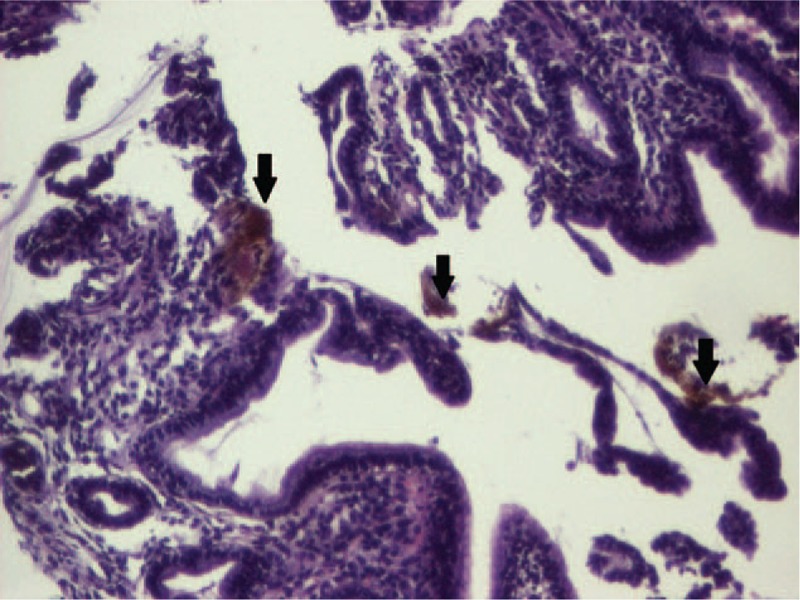
Microscopic aspect of the gastric mucosa (HE, ×10). HE = hematoxylin & eosin.
Figure 4.
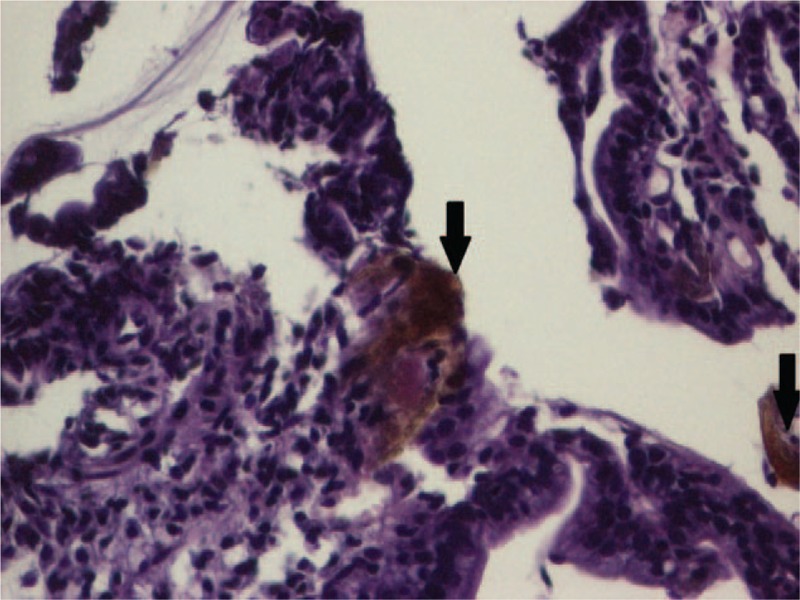
Microscopic aspect of the gastric mucosa (HE, ×20). HE = hematoxylin & eosin.
2.4. Therapeutic focus and assessment
On the day of admission, we performed a gastric lavage with a hemostatic drug (etamsylate), and we initiated treatment with proton pump inhibitors and aminoacids by vein for the acute liver impairment for 5 days. On the first 3 days, we also administered rehydration electrolytes and glucose solution by vein. The evolution was favorable under the administered treatment, and we discharged the patient with the recommendations to continue the proton pump inhibitor treatment and liver protectors orally for 1 month.
2.5. Follow-up and outcome
After 1 month, the laboratory parameters revealed the persistence of iron deficiency anemia, but the liver biomarkers were in normal ranges. We also repeated the upper digestive endoscopy, but the macroscopic aspect of the gastric mucosa was normal (Fig. 5), and the histopathological exam did no longer point out the presence of iron in the gastric mucosa (Fig. 6). In order to correct the iron deficiency anemia, we recommended a liquid-form of iron supplement for 1 month with the correction of hemoglobin and serum iron values.
Figure 5.
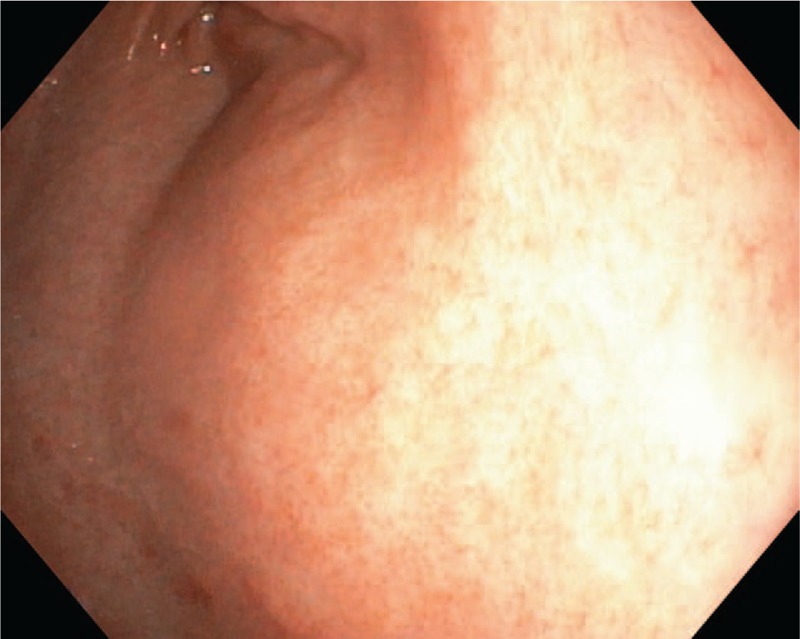
Follow-up macroscopic aspect of the gastric mucosa.
Figure 6.
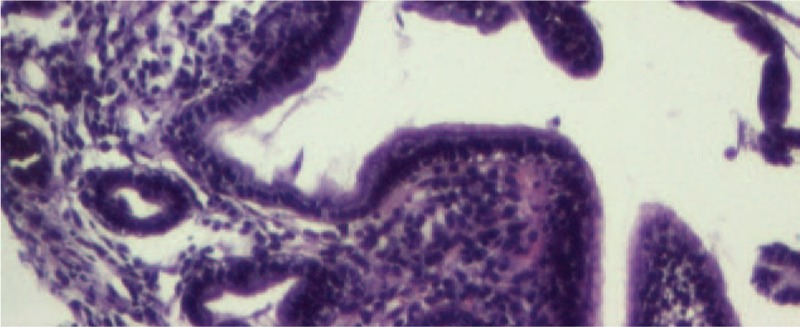
Follow-up microscopic aspect of the gastric mucosa (HE, ×10). HE = hematoxylin & eosin.
3. Discussions
Oral iron supplementation as ferrous sulfate compound in the pill or tablet form is a widely prescribed treatment for iron deficiency anemia, a common condition worldwide. Among the common side-effect of iron supplements, we encounter: constipation, dark stools, and nausea due to gastrointestinal irritation.[7] It is well-known that overdoses of iron can lead to GI impairment, but the effect of therapeutic dosage is still not clearly identified. In a study on 160 patients investigated for iron deficiency anemia, Kaye et al[8] aimed to identify the effect of oral iron supplements on the GI tract. They underlined that out of 16 patients with iron deposits in the gastric mucosa confirmed by the histopathological exam, 6 presented macroscopic erosions at this level without any differences regarding age, gender, and other drug administration, such as aspirin or NSAIDs. In the same study, the authors also included a retrospective analysis on 59 patients encountered with iron deposits at biopsy. Of these 59 subjects, 58 were administered oral iron supplements, and 31 were proved to present erosions and ulceration at endoscopy. The impact of the iron supplementation length on the development of the GI complications is not clearly established. Nevertheless, a study published by Laine et al[9] suggested that the severity of the GI injuries increases with the length of iron supplementation. The study included healthy volunteers without previous iron supplementation and they were divided into 2 groups. The first group included subjects who were administered oral ferrous sulfate 3 times a day for 2 weeks, and the second one with the same dosage, but only for 1 week. All individuals included in this study underwent endoscopy before and after the iron therapy, and the findings revealed that the pathological changes of the gastric mucosa were much more severe in those with a longer length of iron supplementation. Also, of all subjects included in the previously mentioned study, 2 developed gastric ulceration. Thus, based on all these findings, it is not very clear the exact relationship between the length of the therapy and the clinical manifestations. In the above presented case, our patient had been also receiving oral supplements for 2 weeks before presenting with upper digestive hemorrhage.
The absorption of iron at the level of GI tract is not fully understood, but it seems that the iron metabolism cycle is regulated during the absorption in the duodenum and jejunum.[10,11] If this physiological system is disturbed by high iron levels (local or systemic), the uptake of iron will be realized very fast, in a passive concentration-dependent manner leading to an iron excess in the body, which will be initially stored as hemosiderin in the liver,[5,10] leading to the impairment of liver function. In our case, the patient presented high serum iron levels and increased liver transaminases suggesting the impairment of liver function. It is well known that the accumulation of iron in different organs will lead to the formation of reactive oxygen metabolits and highly reactive radicals due to the catalytic function of ferrous and ferric ions, like in the case of iron accumulation in the gastric mucosa, also known as gastric siderosis.[10,12]
Recently, there were described 3 main patterns of iron deposition in the gastric mucosa, also called gastric siderosis: type A, which consists in iron deposition in the macrophages, stroma, and epithelium; type B with iron depositions mostly extracellular, with small amount of iron deposits in the blood vessels, macrophages, and epithelium; and type C comprising iron deposits mostly in glandular epithelium of antral and fundic cells.[10,13] These patterns were correlated with the patient's clinical data and it was concluded that 9% of those with pattern A received oral ferrous sulfate tablets, in pattern B, all patients received oral ferrous sulfate tablets, and none of the patients with pattern A underwent this treatment.[10] Therefore, pattern A was also called “nonspecific gastric siderosis” associated with gastric inflammation, ulceration or prior hemorrhages; pattern B—“iron pill gastritis” related to intake of oral solid iron supplements; and type C—“gastric glandular siderosis” encountered in systemic iron overload from hereditary hemochromatosis, multiple blood transfusions, or possibly portal hypertension and cirrhosis.[10,13] The typical macroscopic aspect in patients with iron pill-induced gastritis consists in erosions, erythema, and yellowish-brown discoloration of the mucosa, whereas the histopathological exam reveals iron deposits in the epithelium, lamina propria, in glandular lumen, even micro-thrombi or giant cell reaction.[13] Similarly, in our patient, we found multiple erosions and yellowish-brown deposits on the gastric mucosa due to the presence of iron at this level identified by the histopathological exam.
Except for oral iron supplements, other conditions have also been reported to be associated with gastric siderosis, such as hemochromatosis, alcohol abuse, blood transfusions, hepatic cirrhosis, and spontaneous portacaval shunt with esophageal varices.[10] Nevertheless, there were also reported 2 cases with GS and without any of these previously mentioned factors.[12] A particular case of this condition was reported by Hashash et al[4] regarding 59-year-old male with an oral iron therapy, but also with other comorbidities associated: Child–Pugh B cirrhosis, diabetes mellitus, hypertension, dyslipidemia, and chronic kidney disease. The patients presented by us did not present any other identified comorbidities. Another case reported in the literature involved a 76-year-old male with iron deficiency anemia for which he received oral ferrous sulfate tablets for 4 years, and the histopathological exam revealed brown crystalline deposits in the lamina propria of the gastric mucosa associated with fibrosis, chronic inflammation, and foreign body reaction.[14] In our case, the brown deposits encountered in the gastric mucosa were also associated with an inflammatory infiltrate, but without signs of chronic inflammation. Due to the concentration-dependent action mechanism on the gastric mucosa, the side effect induced by iron solve if the pill or tablet form of supplementation is replaced by a liquid form.[4,15] Among the solid compounds of oral iron, the most dangerous for the gastrointestinal tract is ferrous sulfate, followed by ferrous gluconate, ferrous succinate, and least ferrous carbonate.[3,5] Therefore, iron-pill induced gastritis should be avoided by choosing a solid iron supplement less damaging for the gastrointestinal tract or an oral iron liquid compound or, even a parenteral one, and its treatment consists in immediate cessation of the oral solid iron supplement, symptomatic treatment, and initiation of an oral liquid iron compound. Similarly, our case presented a favorable evolution of anemia after the initiation of an oral liquid compound of iron supplement.
Nevertheless, this rare entity has been well documented in adults, the studies, and case reports of children with iron-pill induced gastritis available in the literature are vague, old and very few.[16,17] Therefore, it is essential to take into account that despite its rarity, iron-pill induced gastritis can be encountered in children and it can lead to life-threatening complications such as severe upper digestive hemorrhage.
4. Conclusions
Gastritis induced by oral iron supplements, also known as iron-pill induced gastritis is a rare clinical entity, under-diagnosed that can carry a severe clinical impact with potentially life-threatening complications, such as upper digestive hemorrhage. Therefore, it is essential for every gastroenterologist to be aware of this condition and to recognize its endoscopic features which can be present even in pediatric ages.
Footnotes
Abbreviations: ALT = alanine-aminotransferase, AST = aspartate-aminotransferase, GGT = gamma-glutamyltransferase, GI tract = gastrointestinal tract, Hb = hemoglobin, Htc = hematocrit, LDH = lactate dehydrogenase, MEH = medium erythrocyte hemoglobin, MEV = medium erythrocyte volume, NSAIDs = nonsteroid anti-inflammatory drugs.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Dohil R, Hassal E. Gastritis, gastropathy and ulcer disease. Pediatric Gastrointestinal and Liver Disease 2006;Philadelphia: Elsevier, 373–407. [Google Scholar]
- [2].WHO | Worldwide prevalence on anaemia 1993–2005. WHO. Available at: http://www.who.int/vmnis/anaemia/prevalence/en/. Accessed April 9, 2017. [Google Scholar]
- [3].Ji H, Yardley JH. Iron medication-associated gastric mucosal injury. Arch Pathol Lab Med 2004;128:821–2. [DOI] [PubMed] [Google Scholar]
- [4].Hashash JG, Proksell S, Kuan S-F, et al. Iron pill-induced gastritis. ACG Case Rep J 2013;1:13–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Abraham SC, Yardley JH, Wu TT. Erosive injury to the upper gastrointestinal tract in patients receiving iron medication: an underrecognized entity. Am J Surg Pathol 1999;23:1241–7. [DOI] [PubMed] [Google Scholar]
- [6].Eckstein RP, Symons P. Iron tablets cause histopathologically distinctive lesions in mucosal biopsies of the stomach and esophagus. Pathology (Phila) 1996;28:142–5. [DOI] [PubMed] [Google Scholar]
- [7].Ferrous Sulfate: Uses, Dosage & Side Effects. Drugs.com. Available at: https://www.drugs.com/ferrous_sulfate.html. Accessed April 9, 2017. [Google Scholar]
- [8].Kaye P, Abdulla K, Wood J, et al. Iron-induced mucosal pathology of the upper gastrointestinal tract: a common finding in patients on oral iron therapy. Histopathology 2008;53:311–7. [DOI] [PubMed] [Google Scholar]
- [9].Laine LA, Bentley E, Chandrasoma P. Effect of oral iron therapy on the upper gastrointestinal tract. A prospective evaluation. Dig Dis Sci 1988;33:172–7. [DOI] [PubMed] [Google Scholar]
- [10].Marginean EC, Bennick M, Cyczk J, et al. Gastric siderosis: patterns and significance. Am J Surg Pathol 2006;30:514–20. [DOI] [PubMed] [Google Scholar]
- [11].Griffiths WJH. Haemochromatosis Med (Baltimore) 2011;39:597–601. [Google Scholar]
- [12].Kothadia JP, Arju R, Kaminski M, et al. Gastric siderosis: an under-recognized and rare clinical entity. SAGE Open Med 2016;4:2050312116632109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].De Petris G, Gatius Caldero S, Chen L, et al. Histopathological changes in the gastrointestinal tract due to drugs: an update for the surgical pathologist (part I of II). Int J Surg Pathol 2014;22:120–8. [DOI] [PubMed] [Google Scholar]
- [14].Zhang X, Ouyang J, Wieczorek R, et al. Iron medication-induced gastric mucosal injury. Pathol Res Pract 2009;205:579–81. [DOI] [PubMed] [Google Scholar]
- [15].Jones TA, Parmar SC. Oral mucosal ulceration due to ferrous sulphate tablets: report of a case. Dent Update 2006;33:632–3. [DOI] [PubMed] [Google Scholar]
- [16].Saunders JR, Ferguson AW. Ferrous sulphate: danger to children. Br Med J 1977;1:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mariano D, Cabrerizo S, Docampo PC. [Ferrous sulfate: acute poisoning with a frequent use drug]. Arch Argent Pediatr 2011;109:1–3. [DOI] [PubMed] [Google Scholar]


