Abstract
The study aimed to establish effective nomograms for prediction of tumor regional recurrence and distant recurrence of papillary thyroid carcinoma (PTC) patients after partial or total thyroidectomy.
These nomograms were based on a retrospective study on 1034 patients who underwent partial or total thyroidectomy for PTC. The predictive accuracy and discriminative ability of the nomograms were evaluated by the concordance index (C-index) and calibration curve. In addition, a validation cohort was included at the same institution.
Multivariate analysis demonstrated that family history, maximal tumor diameter, capsular invasion, and lymph node staging were independent risk factors for regional recurrence-free survival; and family history, histological variants, capsular invasion, perineuronal invasion, and vascular invasion were independent risk factors for distant recurrence-free survival. They were selected into the 2 nomograms, respectively, and the C-index for regional recurrence-free survival and distant recurrence-free survival prediction were 0.72 and 0.83, respectively. In the validation cohort, the 2 nomograms displayed a C-index of 0.72 and 0.89, respectively.
The nomograms developed in this study demonstrated their discrimination capability for predicting 3 and 5-year regional recurrence and distant recurrence after partial or total thyroidectomy, and can be used to identify high-risk patients.
Keywords: distant recurrence, nomogram, papillary thyroid carcinoma, regional recurrence
1. Introduction
Papillary thyroid carcinoma (PTC) accounts for more than 80% of all thyroid malignancies that arise from thyroid follicular cells, and in a meta-analysis of 23 studies regarding the application of radioactive iodine (RAI) for PTC, the 10-year disease-specific mortality remained very low at about 1.7%.[1] However, although death rates for differentiated thyroid carcinoma (DTC) are low, tumor relapse rates are relatively high.[2] Therefore, in contrast to most other malignancies, where the endpoint is survival, the focus and endpoint of PTC should be recurrence. Disease relapse occurs in 2 important forms: regional recurrence (residual thyroid, lymph nodes, etc) and distant recurrence (lung, bone, brain, etc). Whereas disease relapse is evidence of biologically aggressive disease that may eventually be life-threatening, especially for distant recurrence, although they are distinctly uncommon compared with regional recurrence, which accounts for less than 10% of PTC disease relapse.[3] Despite relatively mild biological behavior and an excellent prognosis of PTC, and the fear on the part of patients and physicians alike that even tiny number of cancer was very worrisome and required intervention, it is urgent to establish a new method to uncover even miniscule amounts of patients with the potential to relapse during the course of compulsive postoperative disease evaluation and surveillance; consequently, liberal use of more extensive surgery, RAI therapy, or more stringent postoperative thyroid hormone suppressive therapy is required to ablate this modest disease.
In recent years, multiple staging systems have been commonly used for thyroid cancer to predict the risk of death in patients with DTC,[4] such as metastases, age at diagnosis, completeness of resection, invasion, size of the tumor (MACIS) scoring system and American Joint Committee on Cancer (AJCC)/Union for International Cancer Control tumor, node, and metastasis (TNM) staging systems. However, none of the staging systems is designed to predict the risk of recurrence in DTC,[5–8] which seems more important for PTC patients. So the American Thyroid Association (ATA) guidelines of the 2009 version proposed a 3-tiered risk-stratification system that classified patients as having high, intermediate, or low risk of recurrence.[9] High-risk patients had distant metastasis, incomplete tumor removal, gross extrathyroidal extension, or abnormally serum Tg values. Intermediate-risk patients demonstrated cervical lymph node metastasis, microscopic extrathyroidal extension, vessel invasion, iodine intake lesions in the neck outside the thyroid, or aggressive tumor pathological histology. Low-risk patients presented with intrathyroidal DTC with no sign of extrathyroidal extension, vessel invasion, or metastasis. However, the ATA initial risk-stratification system did not specifically address the risk of recurrence associated with family history, histological subtypes, multifocality, extent of vascular invasion, perineuronal invasion, or extent of metastatic lymph node involvement.
A nomogram is a graphical predictive tool that provides the overall probability of a specific outcome for an individual patient,[10] and it has been developed in the majority of cancer types.[11–13] It is more accurate for personalized prognostic prediction compared with the traditional staging systems for many cancers. Currently, it has been proposed as an alternative or even as a new standard.[14–16] However, to date, the application of nomogram for predicting disease-specific regional recurrence and distant recurrence of PTC is still uncommon. In this study, we constructed 2 prognostic nomograms from a primary cohort to predict the likelihood of regional recurrence and distant recurrence in patients with PTC after partial or total thyroidectomy, respectively, and validated the nomograms with validation cohort of PTC patients. To our knowledge, it has never been reported before.
2. Patients and methods
2.1. Dataset
A retrospective study was conducted on a primary cohort of patients who underwent partially or totally thyroidectomy for PTC between January 2000 and December 2009 at the Department of Head and Neck Cancer Center of Zhejiang Cancer Hospital. From January 2010 to December 2010, another group of consecutive patients who underwent partially or totally thyroidectomy for PTC in our hospital was prospectively studied, using the same exclusion and inclusion criteria. This group of patients formed the validation cohort of our study. Furthermore, this study was approved by the Ethics Committee of Zhejiang Cancer Hospital. Informed consent was obtained before surgery. The therapeutic strategies for patients with PTC were carried out according to the guidelines of Chinese Thyroid Association.[17] Histopathologic study of the resected specimens and evaluation of pathologic features were carried out by pathologists who specialize in thyroid pathology. The TNM stages of patients were determined according to the 2010 AJCC criteria.[18]
All enrolled patients received postoperation follow-up. Regional recurrence was defined when suspicious lesions in residual thyroid, lymph node, and other less common regional sites were apparent on imaging, and confirmed by pathology after 6 months from the initial surgical treatment. Distant recurrence was defined when suspicious lesions in lung, bone, brain, and other less common sites were apparent on chest radiograph, and/or computed tomography (CT), and/or magnetic resonance imaging (MRI), and further confirmed by radioiodine scan and/or pathology. Regional recurrence-free survival (RRFS) and distant recurrence-free survival (DRFS) were defined as the interval between the first diagnosis to the first regional recurrence and distant recurrence from the disease, respectively. Patients not experiencing the endpoint above were censored at the last date of follow-up.
2.2. Statistical analysis
All variables in this study were transformed into categorical variables. Univariate analyses were used to select the potential risk factors, and multivariate analyses were performed by Cox proportional-hazards regression models, and stepwise regression method was used to define risk factors. The processes of Cox proportional-hazards regression models and nomograms formulation were completed by R version 3.3.1 (http://www.r-project.org) with the package rms. Concordance index (C-index) was used to measure the performance of the nomogram. The larger C-index indicates the more accurate of the prognostic prediction.[19] In all of our analyses, P < .05 was considered as statistically significant.
3. Results
3.1. Clinicopathologic characteristics of patients
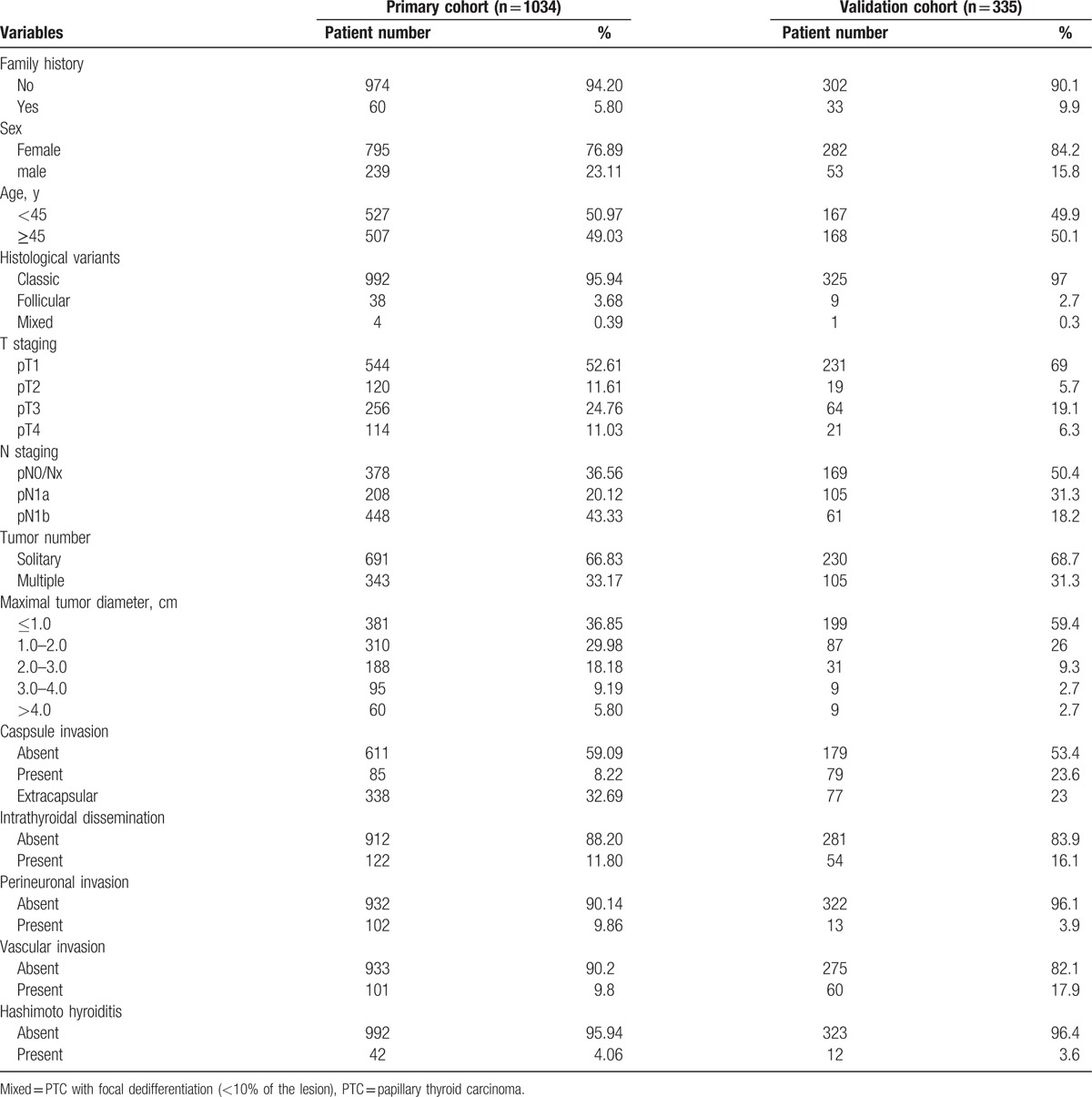
In the primary cohort, 1034 patients with PTC who received partial or total thyroidectomy during the study period met the inclusion criteria, and were entered into our study. For the validation cohort, we studied 335 consecutive patients. The clinicopathologic characteristics of patients are listed in Table 1, and there were no significant differences in the clinicopathologic characteristics between the 2 cohorts of patients.
Table 1.
The demographics and clinicopathologic characteristics of patients with PTC.
3.2. Prognosis of the primary cohort
The median follow-up time was 145.83 months (range 5.00–224.00 months), and the median time to regional recurrence was 140.22 months (range 5.00–223.00 months), and the postoperative 3 and 5-year RRFS rates were 94.87% and 92.75%, respectively. The median time to distant recurrence was 42.22 months (range 5.00–100.00 months), and the 3 and 5- year DRFS rates were 97.78% and 97.29%, respectively. At the time of diagnosis of the first regional or distant recurrence after partial or total thyroidectomy, 120 patients had regional recurrence: 28 patients had residual thyroid recurrence only; 59 patients had lymph node recurrence only; 26 patients had both residual thyroid and lymph node recurrence; and 7 patients had other less common sites of recurrence. Forty-six patients had distant recurrence: 37 patients had lung metastasis recurrence only; 2 patients had bone metastasis recurrence only; 1 patient had brain metastasis recurrence only; 4 patients had both lung and bone metastasis recurrence; and 2 patients had both lung and brain metastasis recurrence.
3.3. Construction of the nomograms
The results of the univariate analyses are showed in Table 2. Multivariate analysis demonstrated that family history, maximal tumor diameter, capsular invasion, and lymph node staging were independent risk factors for RRFS (Table 2), and family history, histological variants, capsular invasion, perineuronal invasion, and vascular invasion were independent risk factors for DRFS (Table 2). The prognostic nomograms that integrated all significant independent factors for RRFS and DRFS in the primary cohort are shown in Fig. 1. The C-index for RRFS and DRFS prediction were 0.72 (95% confidence interval [CI] 0.70–0.75) and 0.83 (95% CI 0.79–0.87), respectively. The calibration plot for the probability of 3 and 5-year regional recurrence and distant recurrence after surgery showed a good agreement between the prediction by nomogram and actual observation (Fig. 2).
Table 2.
Univariate and Multivariate Analysis of the Primary Cohort (n = 1034).
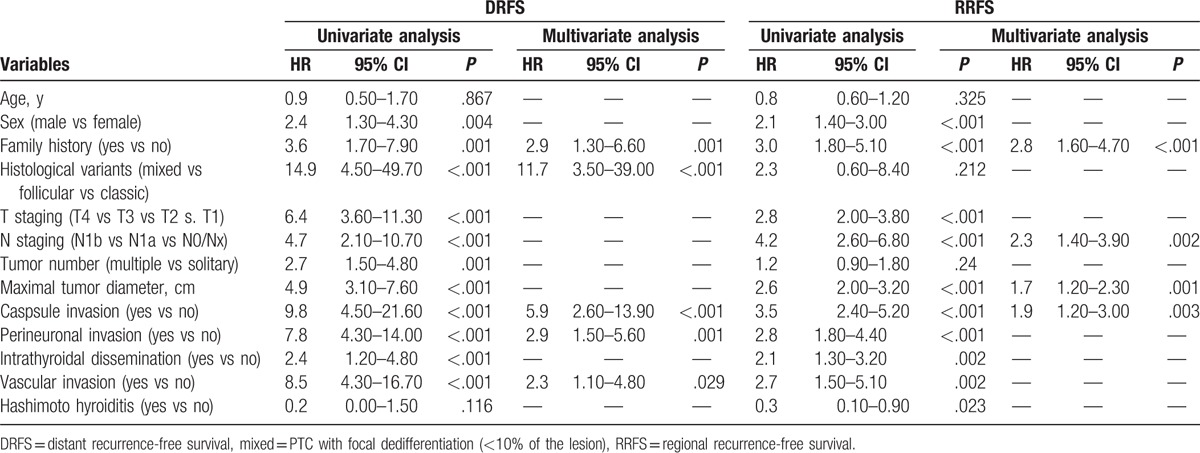
Figure 1.
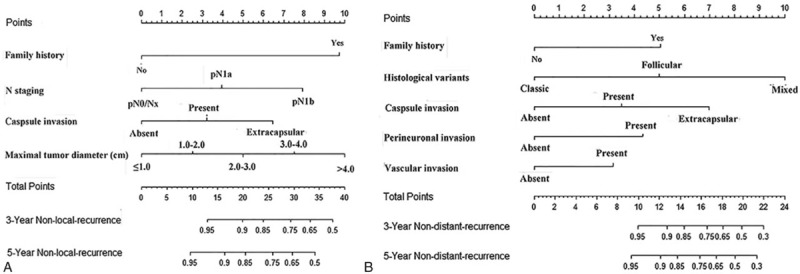
PTC regional recurrence nomogram (A) and PTC distant recurrence nomogram (B). PTC = papillary thyroid carcinoma.
Figure 2.
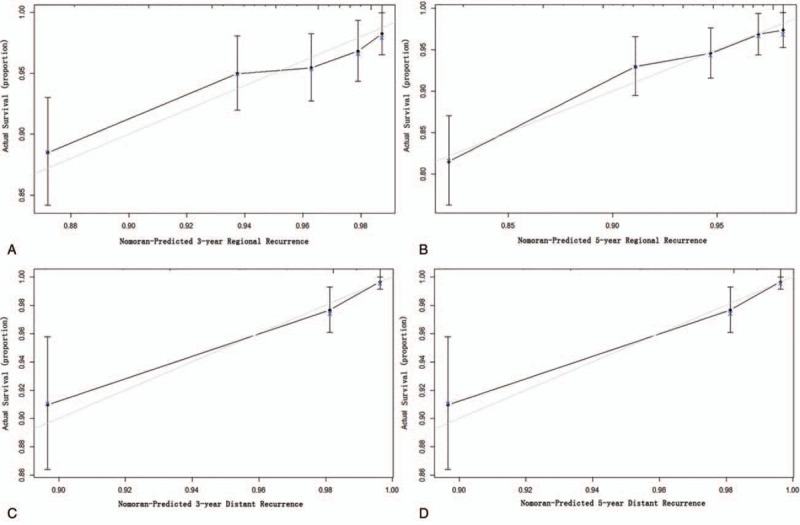
Calibration curve of the nomogram model in primary cohort. The x-axis and y-axis represent the nomogram-predicted recurrence of thyroid cancer and actual probabilities. (A) Calibration curve of 3-year regional recurrence probability. (B) Calibration curve of 3-year regional recurrence probability. (C) Calibration curve of 3-year distant recurrence probability. (D) Calibration curve of 3-year distant recurrence probability.
3.4. Validation of the nomograms
In the validation cohort, the median follow-up time of patients was 71.00 months (range 6.00–78.00 months), and the median time to regional recurrence was 23.00 months (range 6.00–63.00 months), and the postoperative 3 and 5-year RRFS rates were 92.54% and 89.55%, respectively. The median time to distant recurrence was 23.00 months (range 9.00–70.00 months), and the 3 and 5-year DRFS rates were 97.61% and 96.43%, respectively. At the time of diagnosis of the first regional or distant recurrence after partial or total thyroidectomy, 36 patients had regional recurrence: 9 patients had residual thyroid recurrence only; 20 patients had lymph node recurrence only; 6 patients had both residual thyroid and lymph node recurrence; and 1 patient had other less common sites recurrence. Twelve patients had distant recurrence: 7 patients had lung metastasis recurrence only; 1 patient had bone metastasis recurrence only; 1 patient had brain metastasis recurrence only; 2 patients had both lung and bone metastasis recurrence; and 1 patient had both lung and brain metastasis recurrence. The 2 nomograms displayed a C-index of 0.72 (95% CI 0.64–0.81) and 0.89 (95% CI 0.82–0.97), respectively, presenting a good calibration curve for predicting the 3 and 5-year rate of RRFS and DRFS (Fig. 3).
Figure 3.
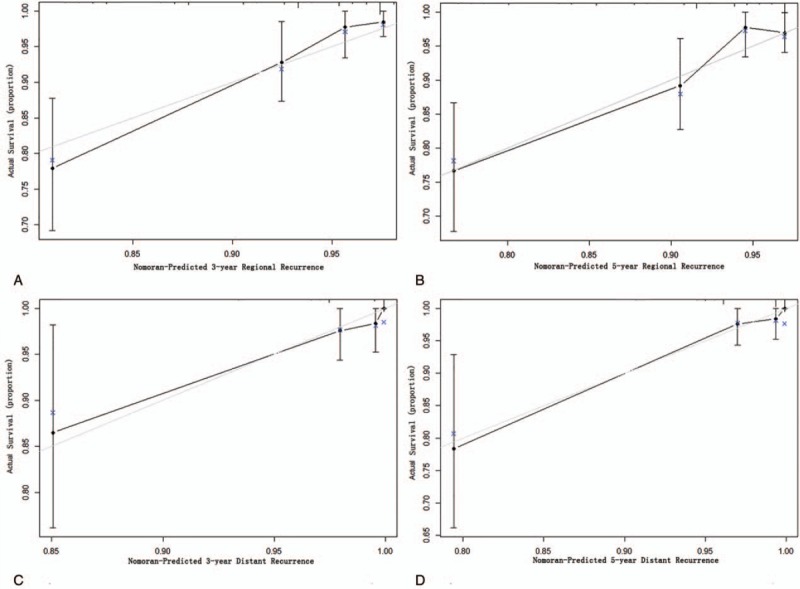
Calibration curve of the nomogram model in validation cohort. The x-axis and y-axis represent the nomogram-predicted recurrence of thyroid cancer and actual probabilities. (A) Calibration curve of 3-year regional recurrence probability. (B) Calibration curve of 3-year regional recurrence probability. (C) Calibration curve of 3-year distant recurrence probability. (D) Calibration curve of 3-year distant recurrence probability.
4. Discussion
Nomogram is 1 of statistical prediction models that has been developed and shown to be more favorable than the traditional staging systems,[14] primarily because of its ability to reduce statistical predictive models into a simple graphical representation that generates a numerical probability of a clinical event, such as recurrence or death, that is tailored to the individual patient.[20] Recently, some nomograms were developed and validated for predicting 10-year disease-specific death and recurrence in PTC,[21–24] and a latest nomogram was developed by Lang et al.[24] In the study, factors included in the prognostic nomogram for predicting 10-year disease-specific death were age at diagnosis, extrathyroidal invasion, tumor size, nodal status, and distant metastases, and the prognostic nomogram for predicting recurrence from PTC including factors of sex, age at diagnosis, extrathyroidal invasion, lymphovascular invasion, tumor size, and nodal status. They had excellent discriminatory ability and accuracy in predicting 10-year disease-specific death and recurrence from PTC. However, these nomograms did not specifically address the risk of recurrence associated with family history, histological variants, and perineuronal invasion, and they also did not differentiate the 2 types of recurrences: regional recurrence and distant recurrence, and in some sense, they were completely different. Thus, we built 2 prognostic nomograms for PTC patients, to predict regional recurrence and distant recurrence, respectively. It has never been done previously.
Family history is defined as when the patient has 1 or more first-degree relatives diagnosed with PTC, which was a significant independent factor for both RRFS and DRFS in our study. Until now, whether or not patients with family history are more aggressive than patients without family history has not been thoroughly studied. In PTC, most,[17,25–28] but not all,[29] studies demonstrate that family history is associated with worse clinical outcomes. In addition, patients in the second generation may exhibit the “genetic anticipation” phenomenon, which demonstrates a genetic disorder at an earlier age and worse prognosis in successive generations.[17,27] Capsular invasion was another significant independent factor for both RRFS and DRFS in our study. Although capsular invasion is well known as a risk of poor outcomes for PTC, it is still important to differentiate the clinical significance of microscopic invasion of the thyroid capsule and macroscopic invasion of the surrounding structures. Because the risk of recurrence in patients with microscopic extrathyroidal extension ranges from 3% to 9%,[30–34] whereas the risk of recurrence associated with gross extrathyroidal extension ranges from 23% to 40%.[6,30,32–35] However, there are also studies suggesting that presence of capsular invasion did not adversely influence biological behavior or prognosis of PTC.[36,37] Interestingly, except for family history and capsule invasion, most factors were not significant for both RRFS and DRFS. For example, lymph node staging and maximal tumor diameter were only associated with RRFS, but not DRFS. Whereas histological variants, perineuronal invasion, and vascular invasion were only associated with DRFS, but not RRFS. It is crucial to stress that the identification of a risk factor of recurrence does not necessarily imply that more aggressive treatments (such as more radical surgery, radioiodine therapy, aggressive postoperative thyroid hormone suppressive therapy) will have a important impact on prognosis. Similarly, the absence of a clinicopathologic risk factor does not mean that more aggressive treatments are not indicated. The 2 new nomograms we developed are considered more practical and clinician-friendly because many of the clinicopathologic risk factors can be easily achieved, and these nomograms allow for a more individualized prediction of tumor outcome by utilizing multiple clinical variables. Thus, nomogram is a powerful clinical tool, which can be used to guide therapeutic intervention (additional therapies or a more conservative management approach) and follow-up management decisions such as the frequency and type of imaging and biochemical testing.
In addition, this study has its own limitations: Firstly, although these 2 nomograms were based on competing risk analysis for the probability of postoperative regional recurrence and distant recurrence, it still lacks sufficient power to identify some true prognostic variables, and at present, we still cannot conclude that these 2 nomograms are absolutely specific for RRFS and DRFS. However, these 2 nomograms are relatively specific for RRFS and DRFS, because the C-indices of the nomogram in predicting regional recurrence were 0.72 (95% CI 0.70–0.75) and 0.72 (95% CI 0.64–0.81) in the primary and validation cohorts, and the C-indices of the nomogram in predicting distant recurrence were 0.83 (95% CI 0.79–0.87) and 0.89 (95% CI 0.82–0.97) in the primary and validation cohorts, presenting a good calibration curve for predicting the 3 and 5-year rate of RRFS and DRFS (Figs. 2 and 3). Secondly, these nomograms were purely based on clinicopathologic factors, perhaps adding some sensitive parameters for thyroid function, such as postsurgical thyroid-stimulating hormone and thyroglobulin, and/or the mutational status of BRAF, and potentially other mutations such as TERT into these nomograms may further improve discrimination and accuracy. Finally, these 2 nomograms were established based on data obtained from a single institution in China, and whether these nomograms can be applied to PTC patients from other sources remains to be tested in future studies.
5. Conclusions
In conclusion, these nomograms, as proposed in this study, objectively and accurately predicted 3 and 5-year regional recurrence and distant recurrence from PTC. The nomograms demonstrate that PTC patients with family history, a higher lymph node staging, capsule invasion, and larger tumor diameter have a higher risk of developing regional recurrence; and PTC patients with family history, nonclassical PTC histological variants, caspsule invasion, perineuronal invasion, and vascular invasion have a higher risk of developing distant recurrence. Additional studies are required to determine whether it can be applied to an external cohort, and with progress, models with higher specificity and accuracy than our nomograms may be established in the future.
Footnotes
Abbreviations: ATA = American Thyroid Association, C-index = concordance index, CT = computed tomography, DRFS = distant recurrence-free survival, DTC = differentiated thyroid carcinoma, MRI = magnetic resonance imaging, PTC = papillary thyroid carcinoma, RAI = radioactive iodine, RRFS = regional recurrence-free survival.
M-HG, JC, and J-YW contributed equally to this study.
This study was funded by National Natural Science Foundation of China (No: 81550003 and No: 81672642).
The authors have no conflicts of interest to disclose.
References
- [1].Sawka AM, Thephamongkhol K, Brouwers M, et al. Clinical review 170: A systematic review and meta-analysis of the effectiveness of radioactive iodine remnant ablation for well-differentiated thyroid cancer. J Clin Endocrinol Metab 2004;89:3668–76. [DOI] [PubMed] [Google Scholar]
- [2].Kloos RT, Mazzaferri EL. A single recombinant human thyrotropin-stimulated serum thyroglobulin measurement predicts differentiated thyroid carcinoma metastases three to five years later. J Clin Endocrinol Metab 2005;90:5047–57. [DOI] [PubMed] [Google Scholar]
- [3].Grant CS. Recurrence of papillary thyroid cancer after optimized surgery. Gland Surg 2015;4:52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sherman SI. Toward a standard clinicopathologic staging approach for differentiated thyroid carcinoma. Semin Surg Oncol 1999;16:12–5. [DOI] [PubMed] [Google Scholar]
- [5].Orlov S, Orlov D, Shaytzag M, et al. Influence of age and primary tumor size on the risk for residual/recurrent well-differentiated thyroid carcinoma. Head Neck 2009;31:782–8. [DOI] [PubMed] [Google Scholar]
- [6].Baek SK, Jung KY, Kang SM, et al. Clinical risk factors associated with cervical lymph node recurrence in papillary thyroid carcinoma. Thyroid 2010;20:147–52. [DOI] [PubMed] [Google Scholar]
- [7].Tuttle RM, Tala H, Shah J, et al. Estimating risk of recurrence in differentiated thyroid cancer after total thyroidectomy and radioactive iodine remnant ablation: using response to therapy variables to modify the initial risk estimates predicted by the new American Thyroid Association staging system. Thyroid 2010;20:1341–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Vaisman F, Momesso D, Bulzico DA, et al. Spontaneous remission in thyroid cancer patients after biochemical incomplete response to initial therapy. Clin Endocrinol (Oxf) 2012;77:132–8. [DOI] [PubMed] [Google Scholar]
- [9].Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009;19:1167–214. [DOI] [PubMed] [Google Scholar]
- [10].Kattan MW, Scardino PT. Evidence for the usefulness of nomograms. Nat Clin Pract Urol 2007;4:638–9. [DOI] [PubMed] [Google Scholar]
- [11].Bochner BH, Kattan MW, Vora KC. Postoperative nomogram predicting risk of recurrence after radical cystectomy for bladder cancer. J Clin Oncol 2006;24:3967–72. [DOI] [PubMed] [Google Scholar]
- [12].Karakiewicz PI, Briganti A, Chun FK, et al. Multi-institutional validation of a new renal cancer-specific survival nomogram. J Clin Oncol 2007;25:1316–22. [DOI] [PubMed] [Google Scholar]
- [13].Wierda WG, O’Brien S, Wang X, et al. Prognostic nomogram and index for overall survival in previously untreated patients with chronic lymphocytic leukemia. Blood 2007;109:4679–85. [DOI] [PubMed] [Google Scholar]
- [14].Sternberg CN. Are nomograms better than currently available stage groupings for bladder cancer? J Clin Oncol 2006;24:3819–20. [DOI] [PubMed] [Google Scholar]
- [15].Mariani L, Miceli R, Kattan MW, et al. Validation and adaptation of a nomogram for predicting the survival of patients with extremity soft tissue sarcoma using a three-grade system. Cancer 2005;103:402–8. [DOI] [PubMed] [Google Scholar]
- [16].Wang L, Hricak H, Kattan MW, et al. Prediction of organ-confined prostate cancer: incremental value of MR imaging and MR spectroscopic imaging to staging nomograms. Radiology 2006;238:597–603. [DOI] [PubMed] [Google Scholar]
- [17].Cao J, Chen C, Chen C, et al. Clinicopathological features and prognosis of familial papillary thyroid carcinoma: a large-scale, matched, case-control study. Clin Endocrinol (Oxf) 2016;84:598–606. [DOI] [PubMed] [Google Scholar]
- [18].Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471–4. [DOI] [PubMed] [Google Scholar]
- [19].Huitzil-Melendez FD, Capanu M, O’Reilly EM, et al. Advanced hepatocellular carcinoma: which staging systems best predict prognosis? J Clin Oncol 2010;28:2889–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Iasonos A, Schrag D, Raj GV, et al. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol 2008;26:1364–70. [DOI] [PubMed] [Google Scholar]
- [21].Yang L, Shen W, Sakamoto N. Population-based study evaluating and predicting the probability of death resulting from thyroid cancer and other causes among patients with thyroid cancer. J Clin Oncol 2013;31:468–74. [DOI] [PubMed] [Google Scholar]
- [22].Pathak KA, Mazurat A, Lambert P, et al. Prognostic nomograms to predict oncological outcome of thyroid cancers. J Clin Endocrinol Metab 2013;98:4768–75. [DOI] [PubMed] [Google Scholar]
- [23].Lang BH, Wong CK. Validation and comparison of nomograms in predicting disease-specific survival for papillary thyroid carcinoma. World J Surg 2015;39:1951–8. [DOI] [PubMed] [Google Scholar]
- [24].2016;Lang BH, Wong CK, Yu HW, et al. Postoperative nomogram for predicting disease-specific death and recurrence in papillary thyroid carcinoma. 38(suppl 1):E1256–63. [DOI] [PubMed] [Google Scholar]
- [25].Lee YM, Yoon JH, Yi O, et al. Familial history of non-medullary thyroid cancer is an independent prognostic factor for tumor recurrence in younger patients with conventional papillary thyroid carcinoma. J Surg Oncol 2014;109:168–73. [DOI] [PubMed] [Google Scholar]
- [26].Park YJ, Ahn HY, Choi HS, et al. The long-term outcomes of the second generation of familial nonmedullary thyroid carcinoma are more aggressive than sporadic cases. Thyroid 2012;22:356–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hillenbrand A, Varhaug JE, Brauckhoff M, et al. Familial nonmedullary thyroid carcinoma-clinical relevance and prognosis. A European multicenter study. ESES Vienna presentation. Langenbecks Arch Surg 2010;395:851–8. [DOI] [PubMed] [Google Scholar]
- [28].Uchino S, Noguchi S, Kawamoto H, et al. Familial nonmedullary thyroid carcinoma characterized by multifocality and a high recurrence rate in a large study population. World J Surg 2002;26:897–902. [DOI] [PubMed] [Google Scholar]
- [29].Pinto AE, Silva GL, Henrique R, et al. Familial vs sporadic papillary thyroid carcinoma: a matched-case comparative study showing similar clinical/prognostic behaviour. Eur J Endocrinol 2014;170:321–7. [DOI] [PubMed] [Google Scholar]
- [30].Riemann B, Kramer JA, Schmid KW, et al. Risk stratification of patients with locally aggressive differentiated thyroid cancer. Results of the MSDS trial. Nuklearmedizin 2010;49:79–84. [DOI] [PubMed] [Google Scholar]
- [31].Nixon IJ, Ganly I, Patel S, et al. The impact of microscopic extrathyroid extension on outcome in patients with clinical T1 and T2 well-differentiated thyroid cancer. Surgery 2011;150:1242–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Jukkola A, Bloigu R, Ebeling T, et al. Prognostic factors in differentiated thyroid carcinomas and their implications for current staging classifications. Endocr Relat Cancer 2004;11:571–9. [DOI] [PubMed] [Google Scholar]
- [33].Ito Y, Tomoda C, Uruno T, et al. Prognostic significance of extrathyroid extension of papillary thyroid carcinoma: massive but not minimal extension affects the relapse-free survival. World J Surg 2006;30:780–6. [DOI] [PubMed] [Google Scholar]
- [34].Radowsky JS, Howard RS, Burch HB, et al. Impact of degree of extrathyroidal extension of disease on papillary thyroid cancer outcome. Thyroid 2014;24:241–4. [DOI] [PubMed] [Google Scholar]
- [35].Fukushima M, Ito Y, Hirokawa M, et al. Prognostic impact of extrathyroid extension and clinical lymph node metastasis in papillary thyroid carcinoma depend on carcinoma size. World J Surg 2010;34:3007–14. [DOI] [PubMed] [Google Scholar]
- [36].Furlan JC, Bedard YC, Rosen IB. Significance of tumor capsular invasion in well-differentiated thyroid carcinomas. Am Surg 2007;73:484–91. [PubMed] [Google Scholar]
- [37].Jeon YW, Ahn YE, Chung WS, et al. Radioactive iodine treatment for node negative papillary thyroid cancer with capsular invasion only: results of a large retrospective study. Asia Pac J Clin Oncol 2016;12:e167–73. [DOI] [PubMed] [Google Scholar]


