Abstract
To demonstrate the mechanisms of the curative effect of Saussurea lappa ethanol extract (SLE) against prostate cancer, we evaluated the effect of SLE on the induction of apoptosis and autophagy and investigated whether SLE-induced autophagy exerts a pro-survival or pro-apoptotic effect in lymph node carcinoma of the prostate (LNCaP) prostate cancer cells. SLE was prepared using 100% ethanol and added to LNCaP cells for 24 hours. Cell viability was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay, and cell apoptosis was evaluated by Tali assay. The expression of apoptosis-related mRNA and proteins was analyzed by quantitative real-time RT-PCR and western blotting. SLE treatment decreased the viability of LNCaP cells and increased Bax expression while suppressing the expression of pro-caspases-8/9/3, PARP, Bid, and Bcl-2, thereby inducing apoptosis in LNCaP cells. Cell proliferation related proteins, including p-Akt, androgen receptor, and prostate-specific antigen, were suppressed by SLE treatment. SLE also induced autophagy in LNCaP cells, and inhibition of autophagy enhanced the apoptosis induced by SLE treatment. These results suggest that SLE exerts anticancer effects through the induction of both cellular apoptosis and autophagy, and apoptotic cell death can be facilitated by blocking autophagy in SLE-treated LNCaP cells. Therefore, SLE might be a potential anticancer agent for the treatment of prostate cancer.
Keywords: apoptosis, autophagy, prostate cancer, Saussurea lappa
1. Introduction
Prostate cancer is the most common cancer among males worldwide. In recent years, the incidence and mortality rate of prostate cancer have increased rapidly in Asian countries.[1] Because prostate cancer is an androgen-sensitive tumor, androgen deprivation therapy (ADT) is usually used for the treatment of early-stage prostate cancer.[2] However, chemotherapy including ADT shows high toxicity and causes serious side effects in cancer patients, and drug resistance and low anticancer efficacy are important clinical problems.[3,4] For these reasons, many studies have investigated various natural products as potential anticancer drug candidates with low toxicity and fewer side effects for the prevention and treatment of prostate cancer.
Apoptosis and autophagy are 2 major routes of programmed cell death that are closely associated with the fate of a tumor.[5] Apoptosis is a regulated cellular suicide mechanism characterized by nuclear condensation, cell shrinkage, membrane blebbing, and DNA laddering and can be initiated through 2 pathways: the mitochondria-controlled intrinsic pathway and the membrane death receptor mediated extrinsic pathway.[6] Autophagy, the major intracellular degradation mechanism, plays an important role in preventing diseases such as cancer, diabetes, liver disease, and infections. Moreover, recent studies have reported that autophagy can inhibit apoptosis by promoting cell survival or may cooperate with apoptosis to induce cell death.[7] Autophagy and apoptosis are commonly activated by a variety of stress stimuli and share multiple molecular switches. The balance between autophagy and apoptosis is key for the prevention and cure of various diseases, including cancers.[8]
Saussurea lappa (SL) has been used as a traditional herbal medicine for asthma,[9] inflammatory disease,[10,11] ulcers,[12] and stomach problems in Korea, China, and Japan. Several previous studies have suggested that SL has anticancer effects in neuroblastoma,[13] lung cancer,[14] hepatocellular carcinoma,[15] gastric cancer,[16,17] and prostate cancer.[18] The hexane extract of SL induced apoptosis in androgen-insensitive human prostate cells[18]; however, the effect of SL extract (SLE) on apoptosis and autophagy in prostate cancer cells has yet to be demonstrated.
In this study, we investigated the anticancer effect of SLE against prostate cancer, focusing on the relationship between apoptosis and autophagy in SLE-mediated cell death in lymph node carcinoma of the prostate (LNCaP) cells.
2. Methods
2.1. Materials
Minimum essential medium (MEM) and fetal bovine serum (FBS) were purchased from Invitrogen (CA). Antibodies specific to Bcl-2, pro-caspase-3, pro-caspase-8, pro-caspase-9, cleaved-caspase-3, Bax, poly ADP-ribose polymerase (PARP), Bid, truncated Bid (t-Bid), androgen receptor (AR), prostate-specific antigen (PSA), phosphatase and tensin homolog (PTEN), anti-microtubule-associated protein light chain-3 (LC3), and beclin1 were purchased from Cell Signaling Technology Inc. (MA). 3-Methyladenine (3-MA) was obtained from Sigma Aldrich (MO). SLE was extracted with 100% ethanol at room temperature for 24 hours in a shaker, and the filtered extracts were concentrated and powdered under reduced pressure. The powder was lyophilized and stored at 4°C.
2.2. Cell culture and cell viability assay
Human prostate carcinoma LNCaP cells were purchased from the American Type Culture Collection (VA) and incubated with MEM containing 10% FBS and 1% penicillin-streptomycin at 37°C with a 5% CO2 atmosphere in a humidified incubator. The effect of SLE on cell viability was determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) colorimetric method. Cells (1 × 104 cells/well) were plated in a 96-well plate and treated with SLE for 24 hours. At the end of treatment, 100 μL MTT solution (5 mg/mL) was added and incubated for a further 4 hours. The medium was removed, and 100 μL dimethyl sulfoxide (DMSO) was added to dissolve the insoluble formazan. Absorbance was measured at 550 nm using an Infinite-M200 spectrophotometer (Tecan, Männedorf, Switzerland).
2.3. Tali image-based cytometric assay
Apoptosis was assessed using a Tali image-based cytometer (Thermo Fisher Scientific, MA). LNCaP cells were seeded in 6-well plates (5 × 105 cells/well). After SLE treatment for 24 hours, cells were stained using the Tali Apoptosis Kit. Apoptotic cells were stained with green Annexin V-Alexa Fluor 488, whereas dead cells were stained with red propidium iodide and green Annexin V-Alexa Fluor 488. The percentages of apoptotic and dead cells were calculated.
2.4. Western blot analysis
LNCaP cells were seeded in 6-well plates (2 × 106 cells/well) and treated with SLE (0, 10, 20, or 40 μg/mL) for 24 hours. The cells were washed with PBS and lysed with RIPA lysis buffer (Invitrogen, CA). Cell lysate (30 μg) was separated by 10% SDS-PAGE gel and transferred to nitrocellulose membranes. The membranes were blocked in skim milk dissolved in TBST buffer for 1 hour and then incubated with primary antibodies overnight at 4°C. The membranes were washed 5 times with TBST buffer and incubated in 5% skim milk/TBST with secondary antibody for 2 hours at room temperature. Target proteins were visualized using an enhanced chemiluminescence method and ImageSaver6 software.
2.5. Real-time quantitative (qRT)-PCR
LNCaP cells were seeded into 6-well plates (5 × 105 cells/well) and treated with 0, 10, or 20 μg/mL SLE for 24 hours. Cells were harvested using TriZol reagent. The quantitative real-time PCR assay was performed by previously described procedure with minor modification.[19] Briefly, RNase-Free DNase (QIAGEN, Venlo, Netherlands) were treated to RNA samples for elimination of DNA contamination. The verification of RNA purity and integrity was performed by measuring absorbance ratios at 260/280 nm employing NanoDrop (ND-1000; NanoDrop Technologies, DE). The cDNA was synthesized using only RNA samples within an absorbance ratio between 1.8 and 2.1. The quantitative real-time (qRT)-PCR was conducted with light cycler system (STRATAGENE, CA) using the cDNA mixed with SYBR Green (TAKARA Co., LTD., Shiga, and TECAN, Männedorf, Switzerland, Japan). Specific primers were as follows: AR forward, 5′-ATGTCCTGGAAGCCATTGAGCCA-3′, reverse, 5′-CAGAAAGGATCTTGGGCACTTGC-3′; PSA forward, 5′- TACCCACTGCATCAGGAACA-3′, reverse, 5′-CCTTGAAGCACACCATTACA-3′; PTEN forward, 5′-ACCTGTTAAGTTTGTATGCAAC-3′, reverse, 5′- TCCAGGAAGAGGAAAGGAAA-3′; GAPDH forward, 5′- CGGAGTGAACGGATTTGGTCGTAT-3′, reverse, 5′-AGCCTTCTCCATGGTGGTGAAGAC-3′. The PCR cycling parameters were set as follows: 94°C for 3 minutes, followed by 45 amplification cycles of 94°C for 30 seconds, 50°C for 30 seconds, and 72°C for 30 seconds. Relative gene expression was calculated with the comparative Ct method using GAPDH as an internal standard.
2.6. Monodansylcadaverine staining
Autophagic vacuoles were measured on the basis of monodansylcadaberine (MDC) staining. LNCaP cells were seeded a density of 2 × 104 cells/well in 96-well plate. The cells were treated with various concentrations of SLE for 24 hours. After then, 50 μM MDC was treated for 30 minutes at 37°C and fixed using 4% paraformaldehyde for 15 minutes. Cells were washed twice with PBS and dissolved using DMSO. The fluorescence was determined at a 380 nm for excitation and 525 nm for emission with Multi-reader. The data were analyzed by MDC/EtBR values compared with control.
2.7. Statistical analyses
Representative data from 3 independent experiments are shown and quantitated, and represented as means ± standard error of the mean (SEM). Statistical significance between control and experimental values was calculated by 2-way analysis of variance (ANOVA), which is performed by Graphpad prism 5 (GraphPad Software, San Diego, CA). Significant values are represented by an asterisk: ∗P < .05.
3. Results
3.1. Cytotoxic effect of SLE on LNCaP cells
To assess the potential anticancer activity of SLE on prostate cancer, LNCaP prostate cancer cells were incubated with 6.3, 12.5, 25, 50, and 100 μg/mL SLE for 24 hours, and the cytotoxicity was evaluated using the MTT assay. As shown in Fig. 1, SLE significantly decreased the viability of LNCaP cells in a dose-dependent manner.
Figure 1.
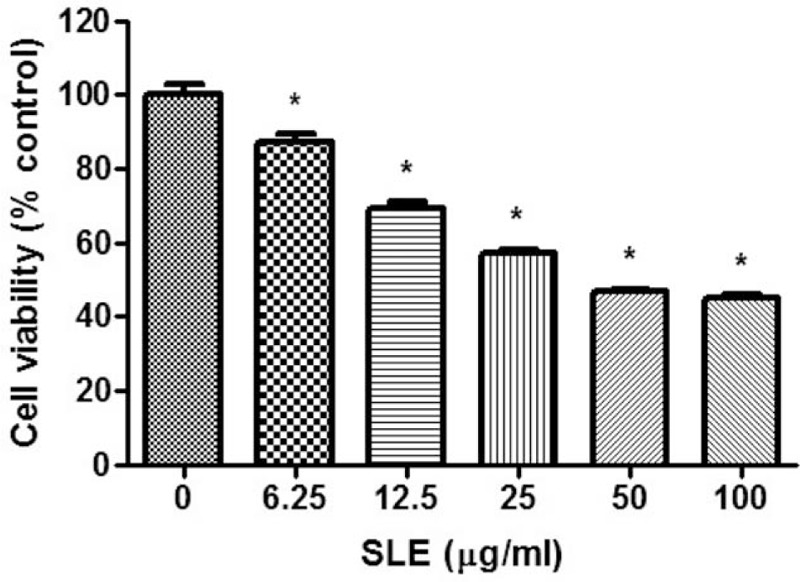
Effect of SLE treatment on viability of LNCaP cells. Cells were treated with SLE for 24 h. The cell viability was measured using the MTT assay. Values are mean ± S.E.M of 3 independent experiments. ∗P < .05, significantly different from untreated cells. LNCaP = lymph node carcinoma of the prostate, MTT = 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
3.2. Effect of SLE on apoptotic cell death in LNCaP cells
We next determined whether the cytotoxic effects of SLE in LNCaP cells were mediated by apoptosis and found that SLE increased apoptotic cell death in a dose-dependent manner (Fig. 2A). To investigate the cellular mechanism of SLE-induced apoptosis, we examined the effect of SLE treatment on the levels of anti-apoptotic and pro-apoptotic proteins. SLE downregulated the expression levels of Bcl-2, pro-caspase 3, pro-caspase 8, pro-caspase 9, and PARP, and upregulated expression of Bax, cleaved caspase-3, cleaved caspase-8, and cleaved PARP compared with the control group (Fig. 2B, C). We also observed a decrease in Bid expression and an increase in the levels of truncated Bid (tBid) in SLE-treated LNCaP cells. Moreover, expression of Fas-associated protein with death domain (FADD), the death receptor-related protein involved in extrinsic apoptosis, was upregulated by SLE treatment in LNCaP cells (Fig. 2D).
Figure 2.
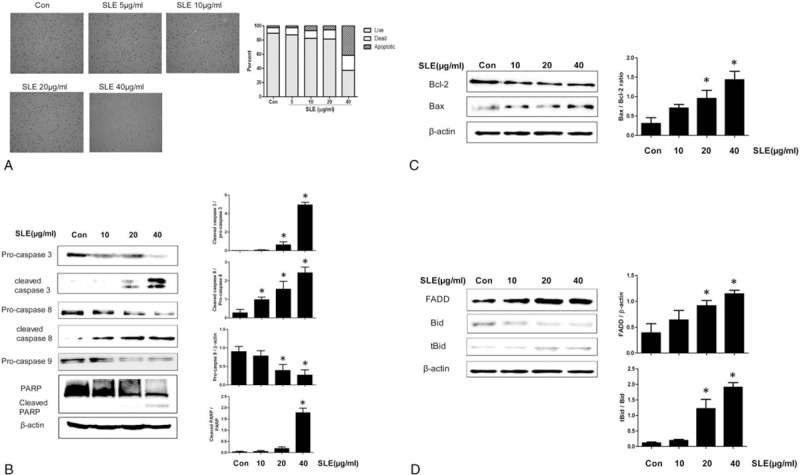
Effect of SLE on the expression of apoptosis-related molecules in LNCaP cells. Cells were treated with SLE for 24 h. (A) Induction of apoptosis in SLE-treated LNCaP cells was analyzed using Tali image-based cytometric assay. (B–D) Expression of pro-caspases 3/8/9 and PARP (B), Bcl-2 and Bax (anti-apoptotic proteins) (C), and tBid, Bid, and FADD (pro-apoptotic proteins) (D) was measured by western blot analysis. β-actin was used as a loading control. Expression of cleaved caspase 3, 8, PARP, pro-caspase 9, BAX, Bcl-2, FADD, and Bid was quantified by scanning densitometry. The relative intensities were expressed as the ratio of cleaved caspase 3, 8, PARP, Bax and tBid to pro-caspase 3, 8, PARP, Bcl-2, and Bid as well as pro-caspase 9 and FADD to β-actin. Representative data from 3 independent experiments are shown and quantitated. Values are mean ± S.E.M of 3 independent experiments. ∗P < .05, significantly different from the control group. LNCaP = lymph node carcinoma of the prostate.
3.3. Effect of SLE on androgen signaling in LNCaP cells
To study whether the expression of PSA and AR, specific biomarkers for prostate cancer, is affected by SLE, we measured their protein and mRNA expression levels in SLE-treated LNCaP cells. As shown in Fig. 3A, B, SLE treatment significantly reduced both protein and mRNA expression of PSA and AR compared with the control group. We also determined the expression of PTEN, a tumor suppressor that is frequently mutated in prostate cancer, in SLE-treated LNCaP cells using quantitative real-time RT-PCR. Treatment with 20 μg/mL SLE increased the level of PTEN mRNA expression. Furthermore, we demonstrated suppression of AKT phosphorylation by SLE treatment in LNCaP cells (Fig. 3C), suggesting that PTEN inhibits the PI3K/AKT pathway and its downstream functions including cell survival and proliferation in SLE-treated LNCaP cells.
Figure 3.
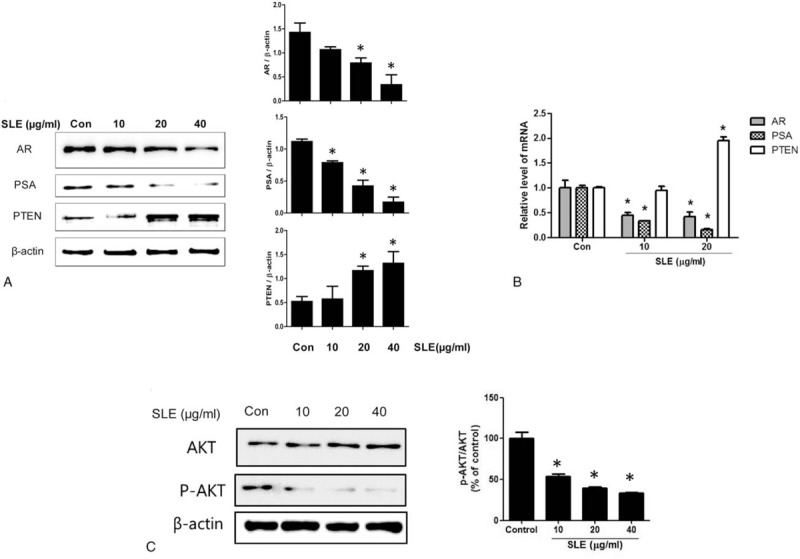
Effect of SLE on androgen signaling in LNCaP cells. Cells were treated with SLE (0, 10, 20, and 40 μg/mL) for 24 h. (A) Expression of AR, PSA, and PTEN was measured by western blot analysis. (B) The mRNA expression of AR, PSA, and PTEN was evaluated using quantitative real-time qRT-PCR. (C) The levels of phosphorylated Akt (p-Akt) and total Akt were measured by western blot analysis. The ratio of p-Akt/Akt is shown in the graph. The data are fold normalized to untreated control. Expression of AR, PSA, and PTEN was quantified by scanning densitometry. The relative intensities were expressed as the ratio of AR, PSA, and PTEN to β-actin. β-actin was used as a loading control. Representative data from 3 independent experiments are shown and quantitated. Values are mean ± S.E.M of 3 independent experiments. ∗P < .05, significantly different from the control group. LNCaP = lymph node carcinoma of the prostate.
3.4. Effect of SLE on autophagic activity in LNCaP cells
Next, we evaluated the effect of SLE treatment on autophagic activity and expression of autophagy-related proteins in LNCaP cells. The autophagy marker microtubule-associated protein 1 light chain 3 (LC3) exists in 2 forms (LC3I and LC3II). LC3I, the precursor form, is converted to LC3-phosphatidylethanolamine conjugate (LC3II), which is involved in the formation of autophagosomal membranes. Therefore, the LC3II/LC3I ratio is used as a marker of autophagy. Our results showed that SLE treatment increased autophagic activity in a dose-dependent manner (Fig. 4A) and upregulated LCI and LC3II levels as well as the ratio of LC3II/LC3I in LNCaP cells (Fig. 4B). Moreover, we showed upregulation of beclin1 and inhibition of mTOR phosphorylation by SLE treatment in LNCaP cells, suggesting that the mTOR pathway that is a key component of cell growth and autophagy regulation might be involved in SLE-induced autophagy in LNCaP cells (Fig. 4B).
Figure 4.
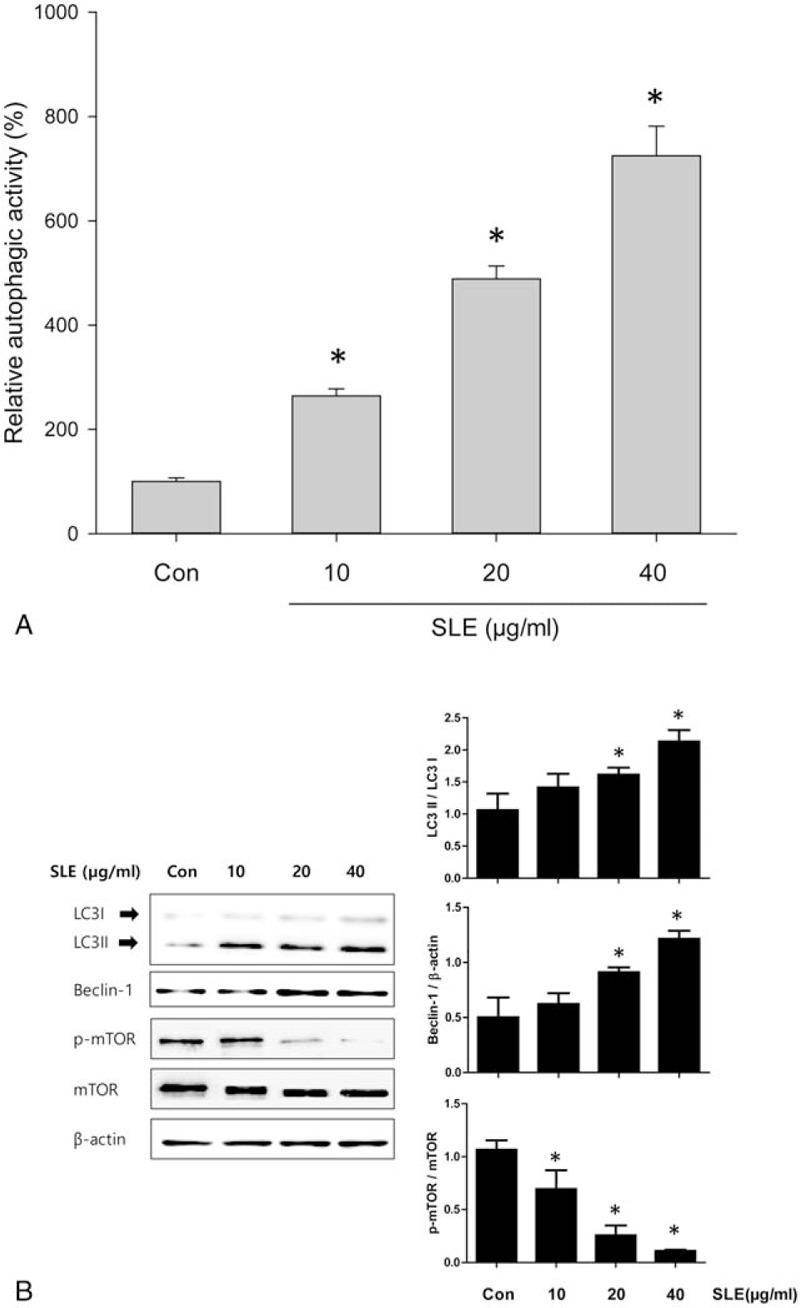
Effect of SLE on autophagic activity in LNCaP cells. LNCaP cells were treated with different concentrations of SLE for 24 h. (A) Autophagic activity were measured using monodansylcadaberine (MDC) staining. (B) The protein levels of LC3, beclin-1, and mTOR in LNCaP cells were measured using western blot analysis. β-actin was used as a loading control. The relative intensities were expressed as the ratio of LC3 II, Beclin-1, and p-mTOR to LC3 I, β-actin, and mTOR. Representative data from 3 independent experiments are shown and quantitated. Values are mean ± S.E.M of 3 independent experiments. ∗P < .05, significantly different from the control group. LNCaP = lymph node carcinoma of the prostate.
3.5. The relationship between apoptotic cell death and autophagic activity in SLE-treated LNCaP cells
To elucidate whether inhibition of autophagic activity affects apoptotic cell death in SLE-treated LNCaP cells, we cotreated cells with the autophagy inhibitor 3-MA and SLE and evaluated the induction of apoptotic cell death. As shown in Fig. 5A, cotreatment with 3-MA and SLE decreased the protein levels of procaspase-3 and Bcl-2 compared with only SLE treatment, whereas Bax expression was increased in cotreated LNCaP cells. In addition, SLE rapidly decreased the viability of LNCaP cells in the presence of 3-MA, suggesting that inhibition of autophagic activity enhanced apoptotic cell death in SLE-treated LNCaP cells (Fig. 5B).
Figure 5.
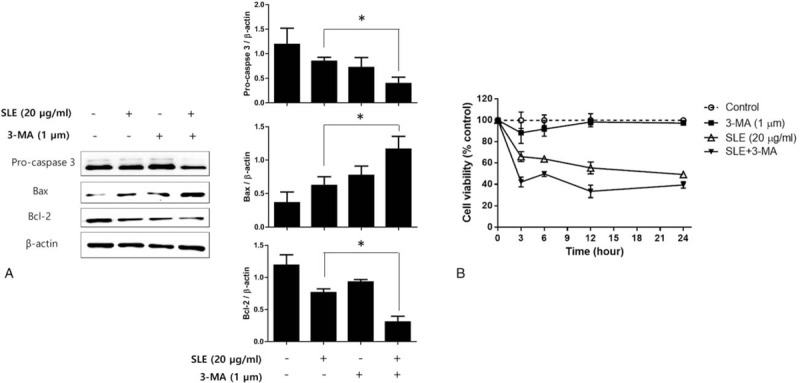
Effect of autophagy inhibition on apoptosis in SLE-treated LNCaP cells. LNCaP cells were cotreated with 1 μM 3-MA and 20 μg/mL SLE, and (A) the expression of caspase 3, Bcl-2, and Bax was measured using western blot analysis, and (B) the cell viability was determined by MTT assay at 3, 6, 12, and 24 h of treatment. β-actin was used as a loading control. Expression of Pro-caspase 3, Bax, and Bcl-2 was quantified by scanning densitometry. The relative intensities were expressed as the ratio of Pro-caspase 3, Bax, and Bcl-2 to β-actin. Representative data from 3 independent experiments are shown and quantitated. Values are mean ± S.E.M of 3 independent experiments. 3-MA = 3-methyladenine. ∗P < .05, significantly different from the control group. LNCaP = lymph node carcinoma of the prostate, MTT = 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
4. Discussion
Several previous studies have demonstrated that isolated components from SL roots induce apoptosis by activation of caspase-3 in leukemia cells,[20] and that extract of SL roots inhibited cell proliferation of gastric cancer cells through cell cycle arrest and apoptosis.[16] In our study, the ethanol extract of SL showed cytotoxic effects via induction of apoptosis and autophagy and suppressed AR and PSA expression in LNCaP cells. As LNCaP cells are androgen-dependent human prostate cancer cells, our results suggested that the anticancer effects of SLE against prostate cancer cell are mediated by suppression of AR and PSA at the transcriptional and translational level.
Two major pathways for the induction of apoptosis have been identified: the mitochondria-mediated intrinsic pathway and the death receptor mediated extrinsic pathway.[6] Our results showed an increase in the levels of cleaved caspase-3, caspase-8, and PARP (intrinsic apoptotic factors), as well as increases in truncated Bid and FADD (extrinsic apoptotic factors); therefore, we suggest that SLE-induced apoptosis might be mediated by either intrinsic or extrinsic apoptotic pathways in LNCaP cells.
Autophagy is an exquisitely regulated process involving the degradation of long-lived proteins or organelles by autophagic vesicles, called autophagosome, to retain cellular vitality.[21,22] In our study, autophagic activity was increased by SLE treatment in a dose-dependent manner and the LC3II/LC3I ratio was significantly increased following SLE treatment of LNCaP cells. Moreover, we found that SLE affected the expression and activation of autophagy-related proteins including beclin-1 and mTOR in LNCaP cells. These results indicate that autophagic activity is involved in SLE-induced cytotoxicity against prostate cancer cells, and that SLE-induced autophagy is regulated through signaling involving Beclin-1 and mTOR.
On the basis of these results, we conclude that the anticancer effect of SLE function against prostate cancer is mediated through the regulation of apoptosis and autophagy. It has been reported that the PI3K/AKT/mTOR pathway is involved in diverse cellular functions, including survival, growth, proliferation, differentiation, metabolism, and angiogenesis,[23] and several studies have suggested that PI3K/AKT/mTOR pathways play a critical role in the regulation of apoptosis and autophagy.[24,25] We showed that SLE suppressed the phosphorylation of AKT and mTOR, suggesting that induction of apoptosis and autophagy by SLE treatment in LNCaP cells might be regulated via the PI3K/AKT/mTOR signaling pathway.
Autophagy and apoptosis pathways perform overlapping functions and share regulatory components; blockage of autophagic signaling increases apoptosis, whereas inhibition of apoptosis results in autophagic cell death.[25–27] In addition, there is an inverse correlation between malignancy and autophagic activity, and autophagy may lead to either cell survival or autophagic cell death depending on the stage or duration and severity of the tumorigenesis.[8,9] Although the role of autophagy in control of cancer cell survival or death in response to anticancer drugs remain to be defined, it has been reported that sulforaphane induced both autophagy and apoptosis during cancer cell death, and the apoptosis was accelerated by blocking autophagy in prostate cancer cells cotreated with 3-MA and sulforaphane.[28]
In the present study, cotreatment with the 3-MA and SLE highly suppressed expression of apoptotic proteins (Bcl-2) and increased expression of pro-apoptotic protein (Bax), compared with only SLE. The viability of LNCaP cells was also rapidly decreased until 3 hours after SLE treatment, and the decrease in cell viability was greater in the cotreated group than in cells treated with only SLE, indicating that inhibition of autophagy facilitates apoptosis at an early stage in SLE-treated prostate cancer cells. In other words, although the cytotoxic effect of SLE in LNCaP cells was exerted through both apoptosis and autophagy, blockage of autophagic activity greatly increased apoptotic cell death, suggesting that autophagy might not lead to cell death, but acts to inhibit apoptosis for cancer cell survival. Thus, it is possible that SLE could affect to tumor cell fate via similar mechanisms to those of natural molecules, such as sulforaphane, in prostate cancer. Indeed, the further research is required for identification of precise switch factor between autophagy and apoptosis.
In conclusion, our results showed that SLE treatment significantly affects cell viability of prostate cancer cell lines in a dose-dependent manner through the induction of apoptosis and autophagy, and that the expression of AR and PSA was suppressed in SLE-treated LNCaP cells. We also presented evidence that SLE induces autophagy through PI3K/AKT/mTOR signaling. In particular, apoptosis and autophagy induced by SLE appear to play a competitive role in the cytotoxic activity against prostate cancer cells, and further studies are necessary to analyze anticancer efficacy of compounds present in the SLE related to the induction of autophagy or apoptosis, respectively. Our data, together with previous findings, indicate that SLE is a potential anticancer agent against prostate cancer, and that simultaneous autophagy inhibition and SLE treatment could be a novel therapeutic approach for prostate cancer.
Footnotes
Abbreviations: AR = androgen receptor, Bax = Bcl-2-associated X protein, Bcl-2 = B-cell lymphoma 2, Bid = BH3 interacting-domain death agonist, FADD = Fas-associated protein with death domain, LC3 = microtubule-associated protein 1A/1B-light chain 3, mTOR = mechanistic target of rapamycin, PARP = poly (ADP-ribose) polymerase, PSA = prostate-specific antigen, PTEN = phosphatase and tensin homolog, SLE = Saussurea lappa ethanol extract.
XT and HSS contributed equally to this work.
All authors declare no conflict of interest related to this paper.
References
- [1].Chen R, Ren S, Yiu MK, et al. Prostate cancer in Asia: a collaborative report. Asian J Urol 2014;1:15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Edwards J, Bartlett J. The androgen receptor and signal-transduction pathways in hormone-refractory prostate cancer. Part 2: androgen-receptor cofactors and bypass pathways. BJU Int 2005;95:1327–35. [DOI] [PubMed] [Google Scholar]
- [3].Blomberg K, Wengström Y, Sundberg K, et al. Symptoms and self-care strategies during and six months after radiotherapy for prostate cancer: scoping the perspectives of patients, professionals and literature. Eur J Oncol Nurs 2016;21:139–45. [DOI] [PubMed] [Google Scholar]
- [4].Gottesman MM. Mechanisms of cancer drug resistance. Ann Rev Med 2002;53:615–27. [DOI] [PubMed] [Google Scholar]
- [5].Zhang L, Wang H, Xu J, et al. Inhibition of cathepsin S induces autophagy and apoptosis in human glioblastoma cell lines through ROS-mediated PI3K/AKT/mTOR/p70S6K and JNK signaling pathways. Toxicol Lett 2014;228:248–59. [DOI] [PubMed] [Google Scholar]
- [6].Lockshin RA, Zakeri Z. Programmed cell death and apoptosis: origins of the theory. Nat Rev Mol Cell Biol 2001;2:545–50. [DOI] [PubMed] [Google Scholar]
- [7].Meijer AJ, Codogno P. Regulation and role of autophagy in mammalian cells. Int J Biochem Cell Biol 2004;36:2445–62. [DOI] [PubMed] [Google Scholar]
- [8].Jiang P, Mizushima N. Autophagy and human diseases. Cell Res 2014;24:69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zahara K, Tabassum S, Sabir S, et al. A review of therapeutic potential of Saussurea lappa: an endangered plant from Himalaya. Asian Pac J Trop Med 2014;7:S60–9. [DOI] [PubMed] [Google Scholar]
- [10].Cho JY, Baik KU, Jung JH, et al. In vitro anti-inflammatory effects of cynaropicrin, a sesquiterpene lactone, from Saussurea lappa. Eur J Pharmacol 2000;398:399–407. [DOI] [PubMed] [Google Scholar]
- [11].Gokhale A, Damre A, Kulkarni K, et al. Preliminary evaluation of anti-inflammatory and anti-arthritic activity of S. lappa, A. speciosa and A. aspera. Phytomedicine 2002;9:433–7. [DOI] [PubMed] [Google Scholar]
- [12].Yoshikawa M, Hatakeyama S, Inoue Y, et al. Saussureamines A, B, C, D, and E, new anti-ulcer principles from Chinese Saussureae Radix. Chem Pharm Bull (Tokyo) 1993;41:214–6. [DOI] [PubMed] [Google Scholar]
- [13].Tabata K, Nishimura Y, Takeda T, et al. Sesquiterpene lactones derived from Saussurea lappa induce apoptosis and inhibit invasion and migration in neuroblastoma cells. J Pharmacol Sci 2015;127:397–403. [DOI] [PubMed] [Google Scholar]
- [14].Hung J-Y, Hsu Y-L, Ni W-C, et al. Oxidative and endoplasmic reticulum stress signaling are involved in dehydrocostuslactone-mediated apoptosis in human non-small cell lung cancer cells. Lung Cancer 2010;68:355–65. [DOI] [PubMed] [Google Scholar]
- [15].Hsu Y-L, Wu L-Y, Kuo P-L. Dehydrocostuslactone, a medicinal plant-derived sesquiterpene lactone, induces apoptosis coupled to endoplasmic reticulum stress in liver cancer cells. J Pharmacol Exp Ther 2009;329:808–19. [DOI] [PubMed] [Google Scholar]
- [16].Ko SG, Kim H-P, Jin D-H, et al. Saussurea lappa induces G2-growth arrest and apoptosis in AGS gastric cancer cells. Cancer Lett 2005;220:11–9. [DOI] [PubMed] [Google Scholar]
- [17].Ko S-G, Koh S-H, Jun C-Y, et al. Induction of apoptosis by Saussurea lappa and Pharbitis nil on AGS gastric cancer cells. Biol Pharm Bull 2004;27:1604–10. [DOI] [PubMed] [Google Scholar]
- [18].Kim EJ, Lim SS, Park SY, et al. Apoptosis of DU145 human prostate cancer cells induced by dehydrocostus lactone isolated from the root of Saussurea lappa. Food Chem Toxicol 2008;46:3651–8. [DOI] [PubMed] [Google Scholar]
- [19].Esmaeili MA, Farimani MM. Inactivation of PI3K/Akt pathway and upregulation of PTEN gene are involved in daucosterol, isolated from Salvia sahendica, induced apoptosis in human breast adenocarcinoma cells. South Afr J Botany 2014;93:37–47. [Google Scholar]
- [20].Oh G, Pae H, Chung H, et al. Dehydrocostus lactone enhances tumor necrosis factor-(-induced apoptosis of human leukemia HL-60 cells. Immunopharmacol Immunotoxicol 2004;26:163–75. [DOI] [PubMed] [Google Scholar]
- [21].Gozuacik D, Kimchi A. Autophagy and cell death. Curr Topics Dev Biol 2007;78:217–45. [DOI] [PubMed] [Google Scholar]
- [22].Yang X, Yu D-D, Yan F, et al. The role of autophagy induced by tumor microenvironment in different cells and stages of cancer. Cell Biosci 2015;5:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kornasio R, Riederer I, Butler-Browne G, et al. (-hydroxy-(-methylbutyrate (HMB) stimulates myogenic cell proliferation, differentiation and survival via the MAPK/ERK and PI3K/Akt pathways. BBA-Mol Cell Res 2009;1793:755–63. [DOI] [PubMed] [Google Scholar]
- [24].Ling Y-H, Aracil M, Zou Y, et al. PM02734 (elisidepsin) induces caspase-independent cell death associated with features of autophagy, inhibition of the Akt/mTOR signaling pathway, and activation of death-associated protein kinase. Clin Cancer Res 2011;17:5353–66. [DOI] [PubMed] [Google Scholar]
- [25].Zhang H, Luo X, Ke J, et al. Procyanidins, from Castanea mollissima Bl. shell, induces autophagy following apoptosis associated with PI3K/AKT/mTOR inhibition in HepG2 cells. Biomed Pharmacother 2016;81:15–24. [DOI] [PubMed] [Google Scholar]
- [26].Jain K, Paranandi KS, Sridharan S, et al. Autophagy in breast cancer and its implications for therapy. Am J Cancer Res 2013;3:251–65. [PMC free article] [PubMed] [Google Scholar]
- [27].Jin S, White E. Role of autophagy in cancer: management of metabolic stress. Autophagy 2007;3:28–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Herman-Antosiewicz A, Johnson DE, Singh SV. Sulforaphane causes autophagy to inhibit release of cytochrome C and apoptosis in human prostate cancer cells. Cancer research 2006;66:5828–35. [DOI] [PubMed] [Google Scholar]


