Abstract
This study aimed to investigate the effects of epidermal growth factor (EGF) on the proliferation and differentiation of adipose stem cells (ASC) during the repeated passaging and probe the underlying signal pathway. Results showed that the Ki67 positive rate remained at a high level, the number of ASCs in G0/G1 phase reduced significantly, but ASCs in G2/M phase and S phase increased markedly in ASCs treated with EGF when compared with ASCs without EGF treatment, indicating that EGF made more ASCs in proliferation phase. The adipogenic capability of ASCs without EGF was compromised when compared with that of ASCs after EGF treatment, although significant difference was not observed. The osteogenic and chondrogenic potencies increased significantly in ASC with EGF treatment indicating EGF could maintain differentiative capacity of ASCs. Gene Set Enrichment Analysis showed EGF upregulated the expression of molecules in the epithelial mesenchymal transition and G2/M checkpoint signal pathways. GeneMANIA database analysis indicated the network interaction between EGF and STAT. EGF receptor (EGFR) inhibitor and STAT3 inhibitor were independently used to validate the role of both pathways in these effects. After inhibition of EGFR or STAT3, the proliferation of ASCs was significantly inhibited, and Western blotting showed EGF was able to markedly increase the expression of EGFR and STAT3. These findings suggest EGF not only promotes the proliferation of ASCs and delays their senescence, but also maintains the differentiation potency of ASCs, which are related to the EGF-induced activation of STAT signal pathway.
Keywords: adipose stem cells, EGF, signal pathway, STAT
1. Introduction
Adipose stem cells (ASCs) are a group of pluripotent stem cells and differentiate into osteoblasts, chondroblasts, adipogenic cells and neurons.[1,2] ASCs may also secrete different growth factors and exert immunoregulatory effects, which significantly improve the repair of tissues and organs.[3,4] ASCs have wide sources, are easy to collect, and have a wide potential of clinical application. In recent years, ASCs have become a hot topic in researches.[5,6] ASCs with high quality and sufficient amount are crucial for the basic medicine and clinical translation. However, the passaging of ASCs will significantly compromise the proliferation and differentiation of ASCs. Although these can be partially resolved by construction of immortalized cells,[7,8] the immortalized stem cells may cause immune rejection. Thus, it is imperative to develop a method with which the proliferation of stem cells is promoted without the compromised stemness.
There is evidence showing that removal of growth factors in the culture medium will cause senescence and stemness reduction of bone marrow recharge stem cells.[9] Whether addition of growth factors is able to maintain the stemness of ASCs and delay their senescence is still unclear. Epidermal growth factor (EGF) may bind to EGF receptor (EGFR) on cells to activate a variety of signal pathways including MAPK-Erk, PI3K-Akt, and STAT pathways. These pathways play important roles in the cell differentiation and proliferation.[10–12] Thus, this study aimed to investigate the effects of EGF on the proliferation and differentiation of ASCs and explore the potential mechanism, which may improve our understanding about the regulation of proliferation and fate of ASCs.
2. Materials and methods
2.1. Separation and culture of ASCs
Adipose tissues were collected by liposuction. Informed consent was obtained before study, and the whole study was approved by the Ethics Committee of School of Medicine. ASCs were separated according to the method provided by Bunnell et al[5] with modifications. The adipose tissues were incubated with 0.1% type I collagenase at 37°C for 60 minutes. After addition of dulbecco's modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS) to stop digestion, the mixture was filtered through a 100-μm filter, followed by centrifugation at 1500g for 10 minutes. The supernatant was removed, and 5 mL of erythrocyte lysis buffer was added for resuspension. The mixture was allowed to incubate at room temperature for 5 minutes, and then 10 mL of DMEM was added, followed by centrifugation at 1500g for 5 minutes. The supernatant was removed, and cells were rinsed with 10 mL of DMEM. After centrifugation at 1500g for 5 minutes, the supernatant was removed, and 1 mL of complete medium (DMEM + 10% FBS + 1% antibiotics) was added. Then, cells were incubated at 37°C in an environment with 5% CO2.
2.2. Detection of cell proliferation and addition of inhibitors
To investigate the effects of EGF on the proliferation of ASCs, ASCs were digested, then harvested, and seeded into 6-well plates (1000 cells/well for colony formation assay) and 96-well plates (1000 cells/well for CCK-8 assay). Cells with and without 10 ng/mL of EGF treatment were incubated for 48 hours. In the colony formation assay, crystal violet staining was performed, and the colonies were counted. CCK-8 assay was performed according to the manufacturer's instructions. Absorbance was measured at 450 nm for the evaluation of cell proliferation. To investigate the effects of inhibitor of EGFR signal pathway on the proliferation of ASCs, ASCs were digested, then harvested, and seeded into 6-well plates (100000 cells/well). Cells were treated with 1 or 10 μmol/L of Lapatinib, Pelitinib, or Afatinib for 2 hours, followed by incubation with 10 ng/mL of EGF for another 48 hours.
2.3. Induced differentiation of ASCs
ASCs have multidirectional differentiation ability and can be induced to differentiate into adipocytes, osteoblasts, and chondroblasts. Cells were digested with trypsin and then seeded into 24-well plates (2 × 104 cells/well). When the cell confluence reached 90%, the induced differentiation was performed. Media for adipogenic, osteogenic, and chondrogenic differentiation (Saiye Biotech Co. Ltd., Beijing, China) were independently added to 24-well plates. The medium was refreshed once every 3 days, and induction continued for 14 days. After induced differentiation, cells were fixed in 4% paraformaldehyde (PFA), followed by 0.1% Alizarin red staining after osteogenic differentiation, 0.1% oil red O staining after adipogenic differentiation, and 0.1% toluidine blue staining after chondrogenic differentiation. Alizarin red and toluidine blue were independently dissolved in 200 μL of Release Buffer (10% acetic acid, 20% methanol, and 70% ddH2O), and oil red O was dissolved in 200 μL of isopropyl alcohol. Absorbance was measured at 450 nm for Alizarin red staining, 600 nm for toluidine blue, and 560 nm for oil red O.
2.4. Real-time quantitative polymerase chain reaction
Total RNA was extracted with TRIzol (Invitrogen). The concentration of total RNAs was determined using a Nanodrop 1000 spectrophotometer (Thermo Scientific, Waltham, MA). Then, 1 μg of total RNA was reversely transcribed into cDNA with TIANscript RT Kit. Real-time quantitative polymerase chain reaction (Q-PCR) was performed with SYBR Green. GAPDH served as an internal reference. Experiment was done 3 times, and 2-ΔΔCt method was employed to analyze the gene expression. The primers used for Q-PCR are listed in Table 1.
Table 1.
Primers used for Q-PCR.

2.5. Immunoflurescence staining
Cells were seeded into 24-well plates, followed by incubation for 2 days under different conditions. The medium was removed, and cells were rinsed with 1× phosphate-buffered solution (PBS). After fixation in 4% PFA for 15 minutes, cells were treated with 0.1% phosphate-buffered solution with triton X-100 (PBST) at room temperature for 20 minutes. After blocking in 3% bovine serum albumin at room temperature for 30 minutes, rabbit-anti-human Ki67 was added, followed by incubation at 4°C overnight. Then, Donkey-anti-Rabbit Cy3 was added, followed by incubation. The nucleus was stained with 4,6- diamidino -2-phenylindole. Cells were observed under a fluorescence microscope.
2.6. Western blotting
Cells were lysed in radio immunoprecipitation assay, and total protein was extracted. The protein concentration was determined with BCA method. Total protein was harvested after passaging 4 times (P4), P6 and P8. After separation of proteins by 10% substrate-sodium dodecyl sulfate-polyacrylamide gel electrophoresis, proteins were electrically transferred onto polyvinylidene difluoride membrane which was then blocked in 5% non-fat milk. After addition of primary antibody (1:1000), the membrane was incubated at 4°C overnight. The membrane was washed in tris-buffered saline–tween 20 (TBST) 3 times (10 minutes for each), and then secondary antibody was added (1:5000), followed by incubation at 37°C for 1 hour. After washing TBST thrice (10 minutes for each), visualization was done with enhanced chemiluminescence. The optical density was determined with Quantity One. GAPDH served as an internal reference. The relative expression of target proteins was determined.
2.7. Microarray assay
To further explore the molecular function and pathway affected by EGF and EGFR, we downloaded a microarray dataset GSE18938.[13] In this dataset, MCF10 and HER2+ overexpressed MCF10 (MCF10A-HER2+) cells were cultured in the absence or presence of 20 ng/mL EGF for 1.5 days to 9 days, and then RNA samples were collected and gene expression data were detected by Affymertrix microarray. Classic Student t test was used to screen out genes with fold change >2 and P value <.05, which can be identified as differentially expressed genes (DEGs). DEGs were imported in to Gene Set Enrichment Analysis (GSEA) (http://www.broadinstitute.org/gsea/index.jsp) software to analyze the molecular functions and pathways affected by EGF and EGFRs. Finally, protein-protein interaction (PPI) networks were constructed by GeneMANIA (http://genemania.org/).
2.8. Statistical analysis
Data are expressed as mean ± standard deviation. Statistical analysis was performed with SPSS 17.0 (SPSS Inc, Chicago, IL) and GraphPad Prism 5. One-way analysis of variance (ANOVA) was employed for the comparisons among groups. A value of P < .05 was considered statistically significant.
3. Results
3.1. EGF maintains the proliferation and differentiation of ASCs
ASCs are a group of pluripotent stem cells, but the proliferation of these cells is significantly compromised after passaging 4 times in vitro. In addition, the adipogenic and osteogenic differentiation potencies of ASCs are also significantly reduced with the increase in in-vitro passaging. Thus, it is imperative to develop a method to maintain the proliferation and differentiation potencies of ASCs during the in-vitro passaging. Studies have shown that EGF is an important growth factor and plays a crucial role in the maintenance of proliferation and differentiation of stem cells. Thus, in the present study, EGF was added, aiming to maintain the stemness of ASCs. Ki67 is a cell proliferation-associated antigen; thus, Ki67 immunofluorescence staining was used to detect proliferative cell numbers (Fig. 1A). Results showed the Ki67 positive rate remained at a high level in ASCs treated with EGF (Ki67 positive rate in P8 cells >50%), but that of ASCs without EGF treatment reduced significantly (Ki67 positive rate in P8 cells <10%), and there was significant difference between them (P < .01). Thus, it can be referred that EGF could maintain ASCs in continuous proliferation status. Besides, flow cytometry was performed for the analysis of cell cycle distribution (Fig. 1B). Results showed almost 90% ASCs without EGF treatment were in the G0/G1 phase, suggesting inactive proliferation. However, after EGF treatment, only <70% ASCs were in the G0/G1 phase, and the number of ASCs in G2/M phase and S phase increased significantly (P < .05). This indicated that EGF induced more ASCs into cell generation cycle, suggesting the active proliferation.
Figure 1.
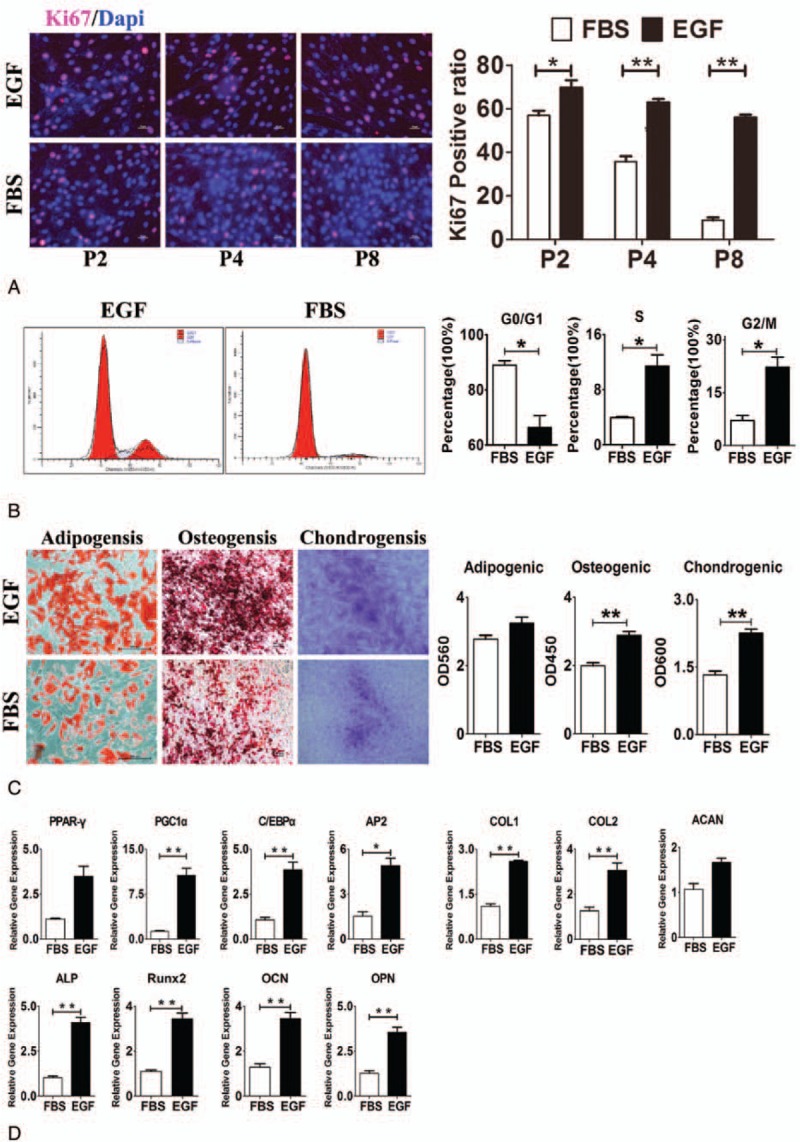
EGF maintains the proliferation and differentiation potencies of ASCs. (A) Ki67 immunofluorescence staining shows the proliferation of ASCs after EGF treatment was more active than that of cells without EGF treatment. (B) Flow cytometry shows the distribution of cell cycle in cells with and without EGF treatment. (c) Differentiation potencies of ASCs with and without EGF treatment into different cells: induced differentiation images and optical density value analysis. (D) Expression of differentiation-related genes in ASCs with and without EGF treatment. Note: EGF, ASCs with EGF treatment; FBS, ASCs without EGF treatment. ∗P < .05, ∗∗P < .01. ASCs = adipose stem cells, EGF = epidermal growth factor, FBS = fetal bovine serum.
In this study, the effects of EGF on the 3 differentiation capacities of ASCs were analyzed. Oil red O staining (Fig. 1C) showed the adipogenic capability of ASCs without EGF was a little bit weaker than that of ASCs after EGF treatment, although significant difference was not observed (P > .05), whereas the osteogenic and chondrogenic potencies increased significantly in ASCs with EGF treatment as compared with cells without EGF treatment (P < .01). They were also confirmed by the semiquantification of expression of differentiation-related genes. Q-PCR showed (Fig. 1D), after EGF treatment, the expression of genes related to differentiation was significantly higher than in cells without EGF treatment. Adipogenic capability-related genes were PPAR-γ, PGC1α, C/EBPα, and AP2, osteogenic capability-related genes were ALP, Runx2, OCN, and OPN, and chondrogenic capability-related genes were COL1, COL2, and ACAN.
Taken together, EGF is able to maintain the proliferation and differentiation potencies of ASCs during the in-vitro passaging.
3.2. EGF acts on G2/M checkpoint to promote the proliferation of ASCs
To investigate the influence of EGF on the potential signal pathways related to cell proliferation, microassay data about the influence of EGF on MCF10A cells with Her2 over-expression and on normal MCF10A cells were obtained from Gene Expression Omnibus database (http://www.ncbi.nlm.nih.gov/geo/). t Test was employed to screen 3000 differentially expressed genes after EGF treatment. GSEA of 3000 genes was performed with GSEA v2.2.3 (http://www.broadinstitute.org/gsea/index.jsp) (Fig. 2A). GSEA showed EGF significantly affected the epithelial mesenchymal transition signal pathway of MCF10A cells (P < .001). In addition, EGF also influenced G2/M Checkpoint signal pathway, although significant difference was not observed (P = .8). These pathways were closely related to cell proliferation and pluripotency. Heatmap analysis of GSEA indicated the expression of several genes in the epithelial mesenchymal transition and G2/M checkpoint pathways was significantly upregulated. It has been confirmed that epithelial mesenchymal transition signal pathway and G2/M checkpoint signal pathway play important roles in the cell proliferation, differentiation, and apoptosis. GeneMANIA was employed to analyze the potential interaction relationship between EGF and STAT3 (Fig. 2C). Based on the GSEA analysis and PPI analysis, real-time PCR was employed to confirm these results (Fig. 2B). Results of Q-PCR were consistent with those from GSEA and PPI. Compared with ASCs without EGF treatment, the expression of genes related to cell cycle progression (CCND1, CDK2, CDK6, E2F1, and HMGA1) was significantly higher in ASCs after EGF treatment (P < .05), and the expression of genes related to the inhibition of cell cycle progression (p53 and p16) decreased significantly in ASCs after EGF treatment (P < .01). Compared with ASCs without EGF treatment, the expression of genes related to STAT signal pathway (STAT3, STAT5, and ERBB2) was significantly higher in ASCs after EGF treatment (P < .05), and the expression of genes related to cell adherence and apoptosis (PTK2B, MAP3K5, and WNT2) decreased significantly in ASCs after EGF treatment (P < .05). According to above findings, we speculated that EGF might affect Stat3, Stat5, and other key proteins to further regulate downstream target proteins such as PTK2B and MAPK in ASCs, which finally influenced the proliferation and differentiation of ASCs.
Figure 2.
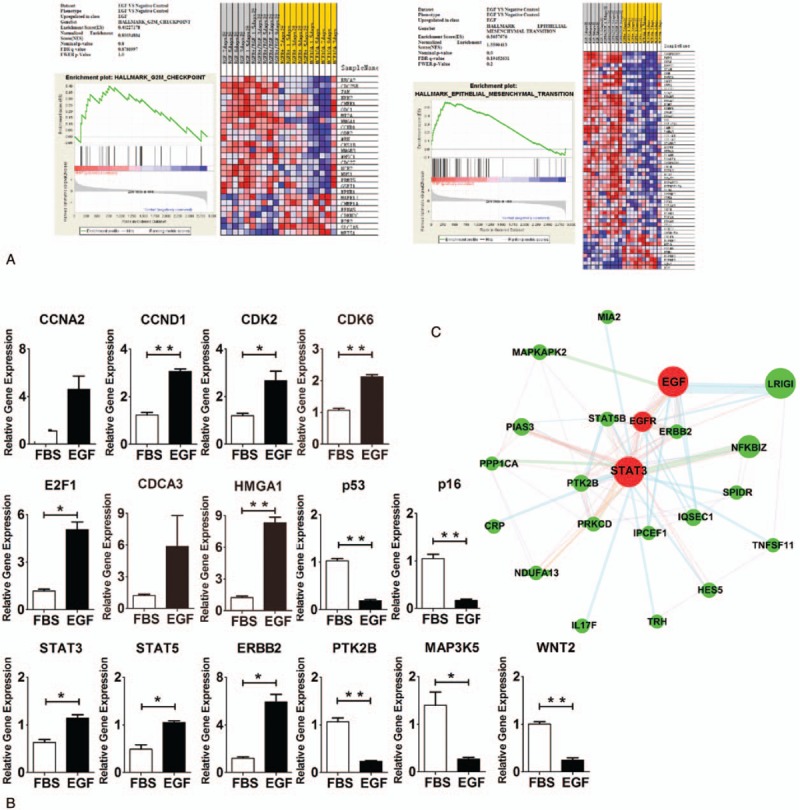
EGF maintains the proliferation and differentiation of ASCs via regulating G2/M checkpoint and STAT signal pathway. (A) GSEA showed EGF significantly influenced epithelial mesenchymal transition (P < .001) and upregulated G2/M checkpoint signal pathway, although significant difference was not observed. Heatmap analysis showed the expression of genes related to epithelial mesenchymal transition and G2/M checkpoint was upregulated, but that of other genes was downregulated. (B) Q-PCR was employed to detect the gene expression in ASCs with and without EGF treatment. (C) PPI analysis showed there was gene interaction network between EGF and STAT signal pathway. Notes: EGF, ASCs with EGF treatment; FBS, ASCs without EGF treatment. ∗P < .05, ∗∗P < .01. ASCs = adipose stem cells, EGF = epidermal growth factor, FBS = fetal bovine serum, GSEA = Gene Set Enrichment Analysis, Q-PCR = real-time quantitative polymerase chain reaction, PPI = protein-protein interaction.
3.3. EGF maintains the differentiation and proliferation potencies of ASCs via activating STAT signal pathway
Based on GSEA analysis and PPI analysis, the STAT3 and EGFR protein expression was detected in P4, P6, and P8 ASCs with and without EGF treatment (Fig. 3A). Results of western blotting showed the expression of STAT3 and EGFR expression in EGF-treated P4 and P6 ASCs was significantly higher than that in ASCs without EGF treatment (P < .05), indicating that the maintenance of proliferation and differentiation capacity of EGF was related with STAT signal pathway. EGFR-specific inhibitor and STAT3-specific inhibitor were used to investigate whether ASCs’ proliferation was related with STAT signal pathway. If related, the effects of EGF on ASCs’ proliferation would be inhibited after adding EGFR specific inhibitor or STAT3 specific inhibitor. If not related, EGF would also maintain ASCs proliferation after adding EGFR-specific inhibitor or STAT3-specific inhibitor. Results of CCK-8 experiment (Fig. 3B) showed that EGFR-specific inhibitor or STAT3-specific inhibitor decreased the ASCs’ proliferation speed significantly (P < .01), suggesting the effects of EGF on promoting proliferation were inhibited. ASCs were treated with STAT3-specific inhibitor S3I-201 for 2 days, and adipogenic, osteogenic, and chondrogenic differentiations of ASCs were evaluated. Results (Fig. 3) showed that the adipogenic capacity was mildly inhibited in ASCs with EGF and S3I-201 treatment (P > .05), whereas the osteogenic and chondrogenic differentiations of ASCs were significantly inhibited in ASCs with EGF and S3I-201 treatment (P < .05), when compared with ASCs with EGF treatment only. In the presence of EGF, STAT3-specific inhibitor significantly inhibited the adipogenic, osteogenic, and chondrogenic differentiations of ASCs, which were similar to those observed in ASCs without EGF treatment (P > .05). Thus, we speculate that EGF induced ASCs’ proliferation and maintained ASCs’ differentiation through STAT signal pathway by activating EGFR (Fig. 3).
Figure 3.
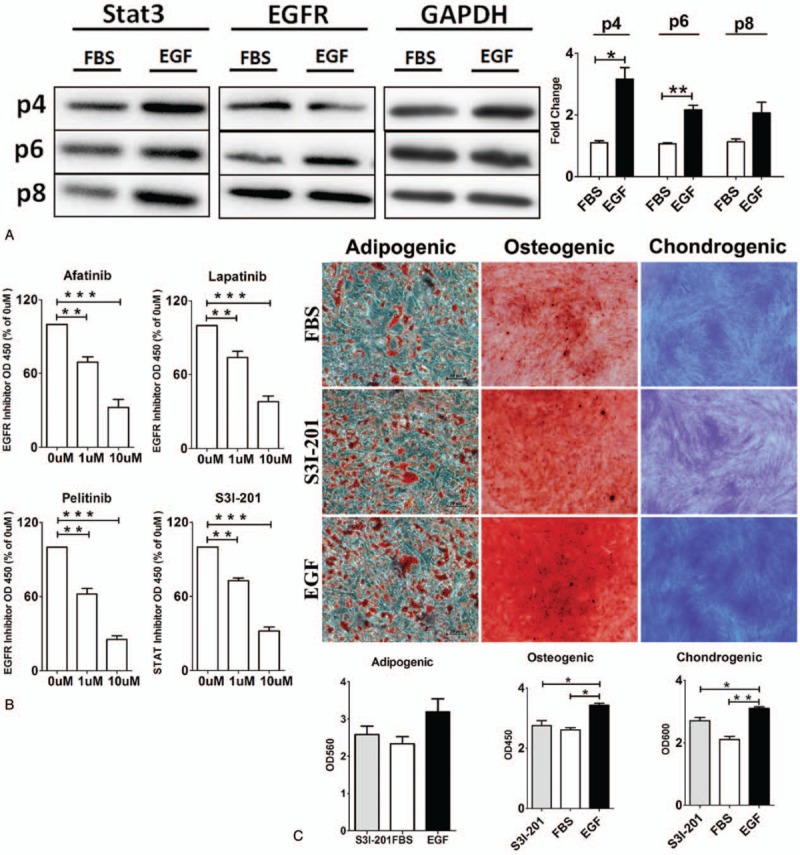
EGF affects the proliferation and differentiation of ASCs via activating STAT signal pathway. (A) The expression of proteins in STAT3 signal pathway and EGFR was detected in ASCs after passaging several times. (B) Cells were treated with different EGFR inhibitors and STAT3 inhibitors. Lapatinib, Pelitinib, and Afatinib were EGFR inhibitors, and S3I-201 was STAT3 inhibitor. (C) STAT3-specific inhibitor S3I-201 was able to inhibit the differentiation of ASCs. Notes: EGF, ASCs with EGF treatment; FBS, ASCs without EGF treatment; S31–201: ASCs with EGF and S31–201 treatment. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001. ASCs = adipose stem cells, EGF = epidermal growth factor, EGFR = epidermal growth factor receptor, FBS = fetal bovine serum.
4. Discussion
ASCs have limited life span and may become senescent and show compromised differentiation potency with the increase in passaging, which significantly limits the clinical application of ASCs. This may be partially resolved by constructing immortalized cell lines. However, the gene expression profile and metabolite profile of immortalized cells are significantly different from those of primary ASCs. For example, immortalized CD34+ or CD34-mADSChTERT cells almost have no interleukin (IL)-6 secretion. Thus, it is imperative to develop a method with which the proliferation of ASCs is promoted without affecting their stemness.
Stem cells are sensitive to the surrounding microenvironment and can express some receptors and proteins to affect the microenvironment, such as growth factor receptor, cytokine receptor, adhesion protein, and immune-related protein.[14,15] EGFR may bind to the EGFR on stem cells to activate a lot of signal pathways, including MAPK-Erk, PI3K-Akt, and STAT signal pathway. Our results showed the proliferation of ASCs was significantly inhibited in the absence of EGF in the medium. At P4, the colony formation capability of ASCs was reduced by 1-fold, and their proliferation was decreased by >40%. After addition of EGF, the proliferation of ASCs was markedly accelerated and their senescence was also delayed. In addition, our results showed the number of SA-β-Gal-positive ASCs at P8 after EGF treatment was reduced by 8-fold as compared to ASCs without EGF treatment, and the expression of senescence-related genes was also significantly reduced. Previous studies also indicated that growth factors not only promoted the proliferation of stem cells, but also maintained the differentiation potency of stem cells.[16,17] Similar findings were also obtained in this study: EGF not only accelerates the proliferation of ASCs and delays their senescence, but also maintains the differentiation potency of ASCs.
STAT3 may be activated by different factors and play vital roles in a variety of cell responses. Togi et al[18] found STAT3 activation was essential for the maintenance of stemness of embryonic stem cells and played a crucial role in the embryonic development. STAT3 may be activated by IL-6 to induce the production of IL-17 in IL-17-producing helper T cells.[19] The relationship between EGF and STAT3 was first identified in mouse liver,[20] which is often observed in the tumorogenesis.[21] In our study, EGF was found to regulate Stat3 expression via EGFR, affecting the proliferation and differentiation of ASCs. When EGFR inhibitor or STAT inhibitor was added, the proliferation of ASCs was significantly inhibited. Western blotting also indicated that EGF treatment significantly increased the expression of EGFR and STAT3. Taken together, we speculate that the EGF-induced proliferation and differentiation maintenance of ASCs is related to the EGFR-mediated activation of STAT signal pathway.
Our study shows EGF not only accelerates the proliferation of ASCs and delays their senescence, but also maintains the differentiation potency of ASCs. GSEA and PPI analysis, use of EGFR inhibitor or STAT inhibitor, and Western blotting confirm that the EGF-induced proliferation and differentiation maintenance of ASCs is associated with EGFR-mediated activation of STAT signal pathway.
Footnotes
Abbreviations: ASCs = adipose stem cells, EGF = epidermal growth factor, EGFR = EGF receptor, PPI = protein-protein interaction.
The authors report no conflicts of interest.
GA and XS contributed equally to this work.
References
- [1].Mizuno H, Tobita M, Uysal AC. Concise review: adipose-derived stem cells as a novel tool for future regenerative medicine. Stem Cells 2012;30:804–10. [DOI] [PubMed] [Google Scholar]
- [2].Skiles ML, Sahai S, Rucker L, et al. Use of culture geometry to control hypoxia-induced vascular endothelial growth factor secretion from adipose-derived stem cells: optimizing a cell-based approach to drive vascular growth. Tissue Eng Part A 2013;19:2330–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].He X, Zhang Y, Zhu A, et al. Suppression of interleukin 17 contributes to the immunomodulatory effects of adipose-derived stem cells in a murine model of systemic lupus erythematosus. Immunol Res 2016;64:1157–67. [DOI] [PubMed] [Google Scholar]
- [4].Poloni A, Maurizi G, Ciarlantini M, et al. Interaction between human mature adipocytes and lymphocytes induces T-cell proliferation. Cytotherapy 2015;17:1292–301. [DOI] [PubMed] [Google Scholar]
- [5].Bunnell BA, Flaat M, Gagliardi C, et al. Adipose-derived stem cells: isolation, expansion and differentiation. Methods 2008;45:115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ren ML, Peng W, Yang ZL, et al. Allogeneic adipose-derived stem cells with low immunogenicity constructing tissue-engineered bone for repairing bone defects in pigs. Cell Transplant 2012;21:2711–21. [DOI] [PubMed] [Google Scholar]
- [7].Darimont C, Zbinden I, Avanti O, et al. Reconstitution of telomerase activity combined with HPV-E7 expression allow human preadipocytes to preserve their differentiation capacity after immortalization. Cell Death Differ 2003;10:1025–31. [DOI] [PubMed] [Google Scholar]
- [8].Kim JH, Choi SC, Park CY, et al. Transplantation of immortalized CD34+ and CD34− adipose-derived stem cells improve cardiac function and mitigate systemic pro-inflammatory responses. PLoS One 2016;11:e0147853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Eom YW, Oh JE, Lee JI, et al. The role of growth factors in maintenance of stemness in bone marrow-derived mesenchymal stem cells. Biochem Biophys Res Commun 2014;445:16–22. [DOI] [PubMed] [Google Scholar]
- [10].Jorissen RN, Walker F, Pouliot N, et al. Epidermal growth factor receptor: mechanisms of activation and signalling. Exp Cell Res 2003;284:31–53. [DOI] [PubMed] [Google Scholar]
- [11].Quesnelle KM, Boehm AL, Grandis JR. STAT-mediated EGFR signaling in cancer. J Cell Biochem 2007;102:311–9. [DOI] [PubMed] [Google Scholar]
- [12].Roberts LE, Fini MA, Derkash N, et al. PD98059 enhanced insulin, cytokine, and growth factor activation of xanthine oxidoreductase in epithelial cells involves STAT3 and the glucocorticoid receptor. J Cell Biochem 2007;101:1567–87. [DOI] [PubMed] [Google Scholar]
- [13].Pradeep CR, Kostler WJ, Lauriola M, et al. Modeling ductal carcinoma in situ: a HER2-Notch3 collaboration enables luminal filling. Oncogene 2012;31:907–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Grabiec M, Hribkova H, Varecha M, et al. Stage-specific roles of FGF2 signaling in human neural development. Stem Cell Res 2016;17:330–41. [DOI] [PubMed] [Google Scholar]
- [15].Rattigan Y, Hsu JM, Mishra PJ, et al. Interleukin 6 mediated recruitment of mesenchymal stem cells to the hypoxic tumor milieu. Exp Cell Res 2010;316:3417–24. [DOI] [PubMed] [Google Scholar]
- [16].Cocola C, Molgora S, Piscitelli E, et al. FGF2 and EGF are required for self-renewal and organoid formation of canine normal and tumor breast stem cells. J Cell Biochem 2017;118:570–84. [DOI] [PubMed] [Google Scholar]
- [17].Khan S, Villalobos MA, Choron RL, et al. Fibroblast growth factor and vascular endothelial growth factor play a critical role in endotheliogenesis from human adipose-derived stem cells. J Vasc Surg 2016;65:1483–92. [DOI] [PubMed] [Google Scholar]
- [18].Togi S, Muromoto R, Hirashima K, et al. A New STAT3-binding Partner, ARL3, Enhances the Phosphorylation and Nuclear Accumulation of STAT3. J Biol Chem 2016;291:11161–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yang XO, Panopoulos AD, Nurieva R, et al. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem 2007;282:9358–63. [DOI] [PubMed] [Google Scholar]
- [20].Zhong Z, Wen Z, Darnell JE., Jr Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science 1994;264:95–8. [DOI] [PubMed] [Google Scholar]
- [21].Chan KS, Carbajal S, Kiguchi K, et al. Epidermal growth factor receptor-mediated activation of Stat3 during multistage skin carcinogenesis. Cancer Res 2004;64:2382–9. [DOI] [PubMed] [Google Scholar]


